Evaluation of the Effect of Limosilactobacillus fermentum CECT5716 on Gastrointestinal Infections in Infants: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
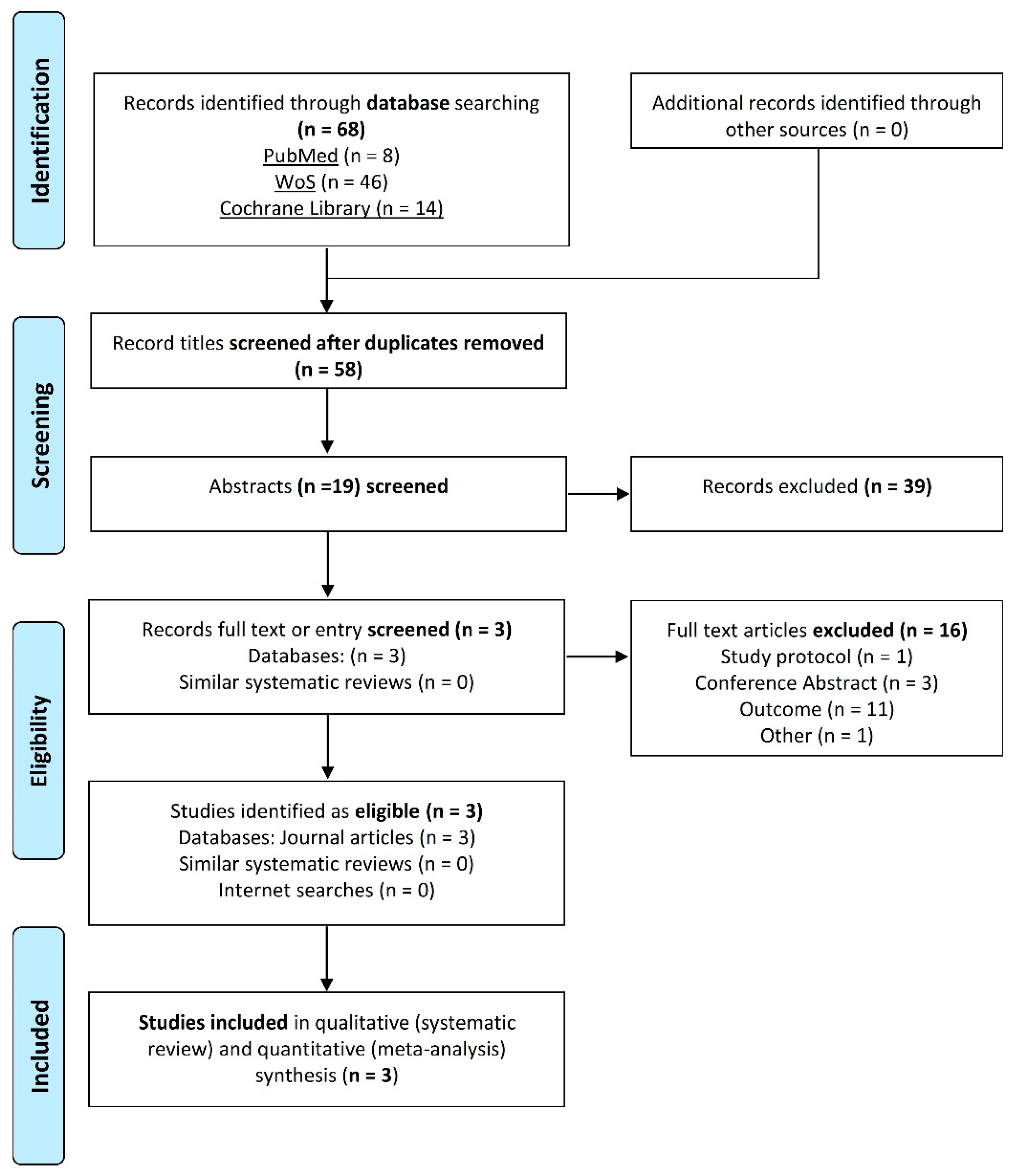
2.3. Study Selection
2.4. Data Extraction and Assessment of the Risk of Bias
2.5. Data Synthesis
2.6. Quality of the Evidence
3. Results
3.1. Study Characteristics
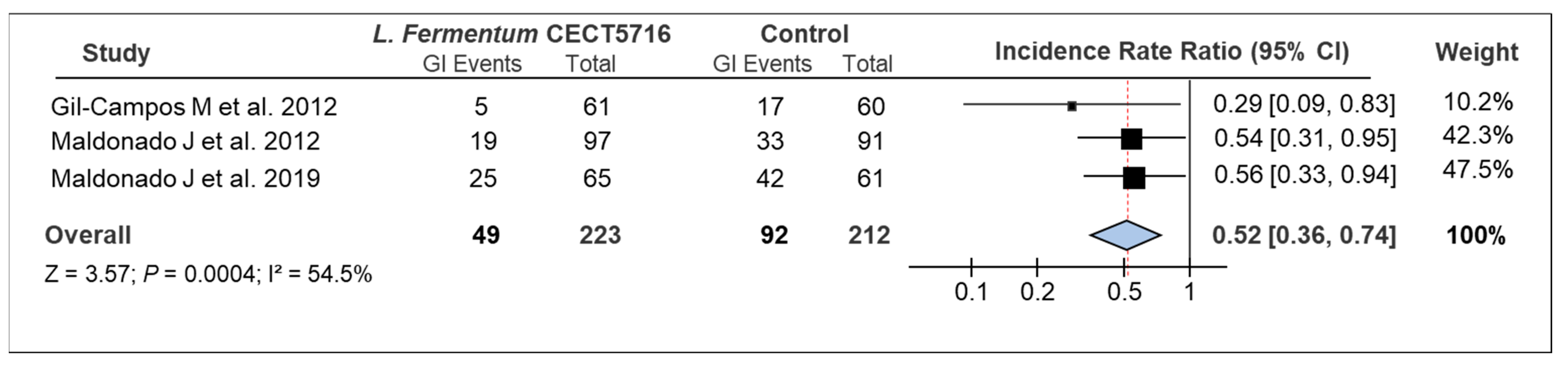
3.2. Overall Results
3.3. Risk of Bias Assessment
3.4. GRADE Assessment
4. Discussion
5. Strengths and Limitations
6. Implications
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Cacho, N.T.; Lawrence, R.M. Innate immunity and breast milk. Front. Immunol. 2017, 8, 584. [Google Scholar] [CrossRef] [Green Version]
- Martín, R.; Langa, S.; Reviriego, C.; Jimínez, E.; Marín, M.L.; Xaus, J.; Fernández, L.; Rodríguez, J.M. Human milk is a source of lactic acid bacteria for the infant gut. J. Pediatr. 2003, 143, 754–758. [Google Scholar] [CrossRef]
- Harmsen, H.J.M.; Wildeboer-Veloo, A.C.M.; Raangs, G.C.; Wagendorp, A.A.; Klijn, N.; Bindels, J.G.; Welling, G.W. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J. Pediatr. Gastroenterol. Nutr. 2000, 30, 61–67. [Google Scholar] [CrossRef]
- Gil-Campos, M.; López, M.Á.; Rodriguez-Benítez, M.V.; Romero, J.; Roncero, I.; Linares, M.D.; Maldonado, J.; López-Huertas, E.; Berwind, R.; Ritzenthaler, K.L.; et al. Lactobacillus fermentum CECT 5716 is safe and well tolerated in infants of 1–6 months of age: A Randomized Controlled Trial. Pharmacol. Res. 2012, 65, 231–238. [Google Scholar] [CrossRef]
- Bahl, R.; Frost, C.; Kirkwood, B.R.; Edmond, K.; Martines, J.; Bhandari, N.; Arthur, P. Infant feeding patterns and risks of death and hospitalization in the first half of infancy: Multicentre cohort study. Bull. World Health Organ. 2005, 83, 418–426. [Google Scholar]
- Duijts, L.; Jaddoe, V.W.V.; Hofman, A.; Moll, H.A. Prolonged and exclusive breastfeeding reduces the risk of infectious diseases in infancy. Pediatrics 2010, 126, e18–e25. [Google Scholar] [CrossRef] [Green Version]
- Bond, D.M.; Morris, J.M.; Nassar, N. Study protocol: Evaluation of the probiotic Lactobacillus fermentum CECT5716 for the prevention of mastitis in breastfeeding women: A randomised controlled trial. BMC Pregnancy Childbirth 2017, 17, 148. [Google Scholar] [CrossRef] [Green Version]
- Wolvers, D.; Antoine, J.M.; Myllyluoma, E.; Schrezenmeir, J.; Szajewska, H.; Rijkers, G.T. Guidance for substantiating the evidence for beneficial effects of probiotics: Prevention and management of infections by probiotics. J. Nutr. 2010, 140, 698S–712S. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- López-Huertas, E. Safety and efficacy of human breast milk Lactobacillus fermentum CECT 5716. A mini-review of studies with infant formulae. Benef. Microbes 2015, 6, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Lara-Villoslada, F.; Sierra, S.; Díaz-Ropero, M.P.; Rodríguez, J.M.; Xaus, J.; Olivares, M. Safety assessment of Lactobacillus fermentum CECT5716, a probiotic strain isolated from human milk. J. Dairy Res. 2009, 76, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Olivares, M.; Díaz-Ropero, M.P.; Martín, R.; Rodríguez, J.M.; Xaus, J. Antimicrobial potential of four Lactobacillus strains isolated from breast milk. J. Appl. Microbiol. 2006, 101, 72–79. [Google Scholar] [CrossRef]
- Díaz-Ropero, M.P.; Martín, R.; Sierra, S.; Lara-Villoslada, F.; Rodríguez, J.M.; Xaus, J.; Olivares, M. Two Lactobacillus strains, isolated from breast milk, differently modulate the immune response. J. Appl. Microbiol. 2007, 102, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Peran, L.; Camuesco, D.; Comalada, M.; Nieto, A.; Concha, A.; Adrio, J.L.; Olivares, M.; Xaus, J.; Zarzuelo, A.; Galvez, J. Lactobacillus fermentum, a probiotic capable to release glutathione, prevents colonic inflammation in the TNBS model of rat colitis. Int. J. Colorectal Dis. 2006, 21, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Peran, L.; Sierra, S.; Comalada, M.; Lara-Villoslada, F.; Bailón, E.; Nieto, A.; Concha, Á.; Olivares, M.; Zarzuelo, A.; Xaus, J.; et al. A comparative study of the preventative effects exerted by two probiotics, Lactobacillus reuteri and Lactobacillus fermentum, in the trinitrobenzenesulfonic acid model of rat colitis. Br. J. Nutr. 2007, 97, 96. [Google Scholar] [CrossRef] [Green Version]
- Arroyo, R.; Martín, V.; Maldonado, A.; Jiménez, E.; Fernández, L.; Rodríguez, J.M. Treatment of Infectious Mastitis during Lactation: Antibiotics versus Oral Administration of Lactobacilli Isolated from Breast Milk. Clin. Infect. Dis. 2010, 50, 1551–1558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maldonado-Lobón, J.A.; Díaz-López, M.A.; Carputo, R.; Duarte, P.; Díaz-Ropero, M.P.; Valero, A.D.; Sañudo, A.; Sempere, L.; Ruiz-López, M.D.; Bañuelos, Ó.; et al. Lactobacillus fermentum CECT 5716 Reduces Staphylococcus Load in the Breastmilk of Lactating Mothers Suffering Breast Pain: A Randomized Controlled Trial. Breastfeed. Med. 2015, 10, 425–432. [Google Scholar] [CrossRef]
- Hurtado, J.A.; Maldonado-Lobón, J.A.; Díaz-Ropero, M.P.; Flores-Rojas, K.; Uberos, J.; Leante, J.L.; Affumicato, L.; Couce, M.L.; Garrido, J.M.; Olivares, M.; et al. Oral Administration to Nursing Women of Lactobacillus fermentum CECT5716 Prevents Lactational Mastitis Development: A Randomized Controlled Trial. Breastfeed. Med. 2017, 12, 202–209. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Cochrane Handbook for Systematic Reviews of Interventions|Cochrane Training. Available online: https://training.cochrane.org/handbook/current (accessed on 24 April 2021).
- Chapter 8: Assessing Risk of Bias in a Randomized Trial|Cochrane Training. Available online: https://training.cochrane.org/handbook/current/chapter-08 (accessed on 25 April 2021).
- Oxman, A.D. Grading quality of evidence and strength of recommendations. Br. Med. J. 2004, 328, 1490–1494. [Google Scholar]
- Chapter 15: Interpreting Results and Drawing Conclusions|Cochrane Training. Available online: https://training.cochrane.org/handbook/current/chapter-15 (accessed on 28 April 2021).
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maldonado, J.; Cañabate, F.; Sempere, L.; Vela, F.; Sánchez, A.R.; Narbona, E.; López-Huertas, E.; Geerlings, A.; Valero, A.D.; Olivares, M.; et al. Human Milk Probiotic Lactobacillus fermentum CECT5716 Reduces the Incidence of Gastrointestinal and Upper Respiratory Tract Infections in Infants. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, J.; Gil-Campos, M.; Maldonado-Lobón, J.A.; Benavides, M.R.; Flores-Rojas, K.; Jaldo, R.; Jiménez Del Barco, I.; Bolívar, V.; Valero, A.D.; Prados, E.; et al. Evaluation of the safety, tolerance and efficacy of 1-year consumption of infant formula supplemented with Lactobacillus fermentum CECT5716 Lc40 or Bifidobacterium breve CECT7263: A randomized controlled trial. BMC Pediatr. 2019, 19, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, A.; Villa, C.R.; Comelli, E.M. Probiotics in early life: A preventative and treatment approach. Food Funct. 2016, 7, 1752–1768. [Google Scholar] [CrossRef]
- Lai, H.H.; Chiu, C.H.; Kong, M.S.; Chang, C.J.; Chen, C.C. Probiotic Lactobacillus casei: Effective for managing childhood diarrhea by altering gut microbiota and attenuating fecal inflammatory markers. Nutrients 2019, 11, 1150. [Google Scholar] [CrossRef] [Green Version]
- Mahon, J.; Lifschitz, C.; Ludwig, T.; Thapar, N.; Glanville, J.; Miqdady, M.; Saps, M.; Quak, S.H.; Lenoir Wijnkoop, I.; Edwards, M.; et al. The costs of functional gastrointestinal disorders and related signs and symptoms in infants: A systematic literature review and cost calculation for England. BMJ Open 2017, 7, e015594. [Google Scholar] [CrossRef]
- Fischer Walker, C.L.; Rudan, I.; Liu, L.; Nair, H.; Theodoratou, E.; Bhutta, Z.A.; O’Brien, K.L.; Campbell, H.; Black, R.E. Global burden of childhood pneumonia and diarrhoea. Lancet 2013, 381, 1405–1416. [Google Scholar] [CrossRef]
- Zimmerman, C.M.; Bresee, J.S.; Parashar, U.D.; Riggs, T.L.; Holman, R.C.; Glass, R.I. Cost of diarrhea-associated hospitalizations and outpatient visits in an insured population of young children in the United States. Pediatr. Infect. Dis. J. 2001, 20, 14–19. [Google Scholar] [CrossRef]
- Farthing, M.J.G. Novel targets for the pharmacotherapy of diarrhoea: A view for the millennium. J. Gastroenterol. Hepatol. 2000, 15, G38–G45. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Maia, A.P.; Matijasevich, A.; Wang, Y.P. Epidemiology of functional gastrointestinal disorders in infants and toddlers: A systematic review. World J. Gastroenterol. 2016, 22, 6547–6558. [Google Scholar] [CrossRef]
- Lyons, K.E.; Ryan, C.A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. Breast Milk, a Source of Beneficial Microbes and Associated Benefits for Infant Health. Nutrients 2020, 12, 1039. [Google Scholar] [CrossRef]
- Jara, S.; Sánchez, M.; Vera, R.; Cofré, J.; Castro, E. The inhibitory activity of Lactobacillus spp. isolated from breast milk on gastrointestinal pathogenic bacteria of nosocomial origin. Anaerobe 2011, 17, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef] [Green Version]
- Schultz, M.; Göttl, C.; Young, R.J.; Iwen, P.; Vanderhoof, J.A. Administration of Oral Probiotic Bacteria to Pregnant Women Causes Temporary Infantile Colonization. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 293–297. [Google Scholar] [CrossRef]
- Martín, R.; Jiménez, E.; Heilig, H.; Fernández, L.; Marín, M.L.; Zoetendal, E.G.; Rodríguez, J.M. Isolation of bifidobacteria from breast milk and assessment of the bifidobacterial population by PCR-denaturing gradient gel electrophoresis and quantitative real-time PCR. Appl. Environ. Microbiol. 2009, 75, 965–969. [Google Scholar] [CrossRef] [Green Version]
- Guarino, A.; Lo Vecchio, A.; Canani, R.B. Probiotics as prevention and treatment for diarrhea. Curr. Opin. Gastroenterol. 2009, 25, 18–23. [Google Scholar] [CrossRef]
- Martín, R.; Olivares, M.; Marín, M.L.; Fernández, L.; Xaus, J.; Rodríguez, J.M. Probiotic potential of 3 Lactobacilli strains isolated from breast milk. J. Hum. Lact. 2005, 21, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Kunz, C.; Rudloff, S. Potential anti-inflammatory and anti-infectious effects of human milk oligosaccharides. Adv. Exp. Med. Biol. 2008, 606, 455–465. [Google Scholar]
- Taipale, T.; Pienihkkinen, K.; Isolauri, E.; Larsen, C.; Brockmann, E.; Alanen, P.; Jokela, J.; Söderling, E. Bifidobacterium animalis subsp. lactis BB-12 in reducing the risk of infections in infancy. Br. J. Nutr. 2011, 105, 409–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taipale, T.J.; Pienihäkkinen, K.; Isolauri, E.; Jokela, J.T.; Söderling, E.M. Bifidobacterium animalis subsp. lactis BB-12 in reducing the risk of infections in early childhood. Pediatr. Res. 2016, 79, 65–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bocquet, A.; Lachambre, E.; Kempf, C.; Beck, L. Effect of Infant and Follow-on Formulas Containing B lactis and Galacto- and Fructo-oligosaccharides on Infection in Healthy Term Infants. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 180–187. [Google Scholar] [CrossRef]
- Grenov, B.; Namusoke, H.; Lanyero, B.; Nabukeera-Barungi, N.; Ritz, C.; Mølgaard, C.; Friis, H.; Michaelsen, K.F. Effect of Probiotics on Diarrhea in Children With Severe Acute Malnutrition. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 396–403. [Google Scholar] [CrossRef]
- Rautava, S.; Salminen, S.; Isolauri, E. Specific probiotics in reducing the risk of acute infections in infancy—A randomised, double-blind, placebo-controlled study. Br. J. Nutr. 2009, 101, 1722. [Google Scholar] [CrossRef]
- Laursen, R.P.; Larnkjær, A.; Ritz, C.; Hauger, H.; Michaelsen, K.F.; Mølgaard, C. Probiotics and Child Care Absence Due to Infections: A Randomized Controlled Trial. Pediatrics 2017, 140, e20170735. [Google Scholar] [CrossRef] [Green Version]
- Olek, A.; Woynarowski, M.; Ahrén, I.L.; Kierkuś, J.; Socha, P.; Larsson, N.; Önning, G. Efficacy and Safety of Lactobacillus plantarum DSM 9843 (LP299V) in the Prevention of Antibiotic-Associated Gastrointestinal Symptoms in Children—Randomized, Double-Blind, Placebo-Controlled Study. J. Pediatr. 2017, 186, 82–86. [Google Scholar] [CrossRef]
- Baglatzi, L.; Gavrili, S.; Stamouli, K.; Zachaki, S.; Favre, L.; Pecquet, S.; Benyacoub, J.; Costalos, C. Effect of Infant Formula Containing a Low Dose of the Probiotic Bifidobacterium lactis CNCM I-3446 on Immune and Gut Functions in C-Section Delivered Babies: A Pilot Study. Clin. Med. Insights. Pediatr. 2016, 10, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radke, M.; Picaud, J.-C.; Loui, A.; Cambonie, G.; Faas, D.; Lafeber, H.N.; de Groot, N.; Pecquet, S.S.; Steenhout, P.G.; Hascoet, J.-M. Starter formula enriched in prebiotics and probiotics ensures normal growth of infants and promotes gut health: A randomized clinical trial. Pediatr. Res. 2016, 81, 622–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weizman, Z.; Asli, G.; Alsheikh, A. Effect of a probiotic infant formula on infections in child care centers: Comparison of two probiotic agents. Pediatrics 2005, 115, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Szajewska, H.; Wanke, M.; Patro, B. Meta-analysis: The effects of Lactobacillus rhamnosus GG supplementation for the prevention of healthcare-associated diarrhoea in children. Aliment. Pharmacol. Ther. 2011, 34, 1079–1087. [Google Scholar] [CrossRef]
- Szajewska, H.; Canani, R.B.; Guarino, A.; Hojsak, I.; Indrio, F.; Kolacek, S.; Orel, R.; Shamir, R.; Vandenplas, Y.; van Goudoever, J.B.; et al. Probiotics for the Prevention of Antibiotic-Associated Diarrhea in Children. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 495–506. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Cho, D.Y.; Lee, S.H.; Han, K.S.; Yang, S.W.; Kim, J.H.; Lee, S.H.; Kim, S.M.; Kim, K.N. A randomized clinical trial of synbiotics in irritable bowel syndrome: Dose-dependent effects on gastrointestinal symptoms and fatigue. Korean J. Fam. Med. 2019, 40, 2–8. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Guo, C.; Mu, D.; Feng, B.; Zuo, X.; Li, Y. Effects of probiotic type, dose and treatment duration on irritable bowel syndrome diagnosed by Rome III criteria: A meta-analysis. BMC Gastroenterol. 2016, 16, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koletzko, B.; Szajewska, H.; Ashwell, M.; Shamir, R.; Aggett, P.; Baerlocher, K.; Noakes, P.; Braegger, C.; Calder, P.; Campoy Folgoso, C.; et al. Documentation of functional and clinical effects of infant nutrition: Setting the scene for COMMENT. Ann. Nutr. Metab. 2012, 60, 222–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guarino, A.; Ashkenazi, S.; Gendrel, D.; Lo Vecchio, A.; Shamir, R.; Szajewska, H. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases Evidence-Based Guidelines for the Management of Acute Gastroenteritis in Children in Europe. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 132–152. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Lobón, J.A.; Gil-Campos, M.; Maldonado, J.; López-Huertas, E.; Flores-Rojas, K.; Valero, A.D.; Rodríguez-Benítez, M.V.; Bañuelos, O.; Lara-Villoslada, F.; Fonollá, J.; et al. Long-term safety of early consumption of Lactobacillus fermentum CECT5716: A 3-year follow-up of a randomized controlled trial. Pharmacol. Res. 2015, 95–96, 12–19. [Google Scholar] [CrossRef] [PubMed]

| Authors (Year) | Study Design | Setting | N | Sample | Intervention | Time of Intervention | Findings Summary |
|---|---|---|---|---|---|---|---|
| Gil-Campos et al. (2012) | RCT, double blind, controlled, multi-center | Hospital Virgen de las Nieves (Granada, Spain), Hospital Reina Sofía (Córdoba, Spain), Hospital Carlos Haya (Málaga, Spain) | Randomized: 137 (IG:66, CG:71) Analyzed at the end of the study: 121 (IG:61, CG:60) | Healthy 1-month-old infants with exclusively formula-fed | Intervention: Standard powdered infant formula a with GOS supplemented or not with a probiotic strain. Follow up: 4 visits Experimental product and dose L. fermentum CECT5716 (8.4 × 108 cfu/day) b | From 1 to 6 months old | IG: ↓ incidence of GI (IR: 0.082 ± 0.037) vs. CG (IR: 0.283 ± 0.068) |
| Maldonado et al. (2012) | RCT, double blind, controlled, multi-center | Hospital San Cecilio (Granada, Spain), Hospital Virgen de las Nieves (Granada, Spain), Hospital de Poniente (El Ejido, Almeria, Spain) | Randomized: 215 (IG:117, CG:98) Analyzed at the end of the study: 188 (IG:97, CG:91) | Healthy 6-month-old infants, exclusively formula-fed | Intervention: Standard powdered infant formula a with GOS supplemented or not with a probiotic strain. Follow up: 3 visits Experimental product and dose: : L. fermentum CECT5716 (2 × 108 cfu/day) | From 6 to 12 months old | IG: ↓ incidence of GI (IR: 0.196 ± 0.51) vs. CG (IR: 0.363 ± 0.53) |
| Maldonado et al. (2019) | RCT, double blind, controlled, multi-center | Hospital Virgen de las Nieves (Granada, Spain), Hospital Reina Sofía (Córdoba, Spain), Pediatric Clinic Roquetas de Mar (Almeria, Spain), Pediatric Clinic Cristo de la Salud (Granada, Spain), 7 Pediatric Services from the Andalusian Public Health System (Spain) | Randomized: 160 (IG1:83, CG:77) Analyzed per-protocol: 126 (IG1:65, CG:61) | Healthy 1-month-old infants, exclusively formula-fed | Intervention: Standard powdered infant formula a supplemented or not with a probiotic strain. Follow up: 6 visits Experimental product and dose: : L. fermentum CECT5716 (1 × 109–8 × 108 cfu/day) c | From 1 to 12 months old | IG: ↓ incidence of GI (IR: 0.385 ± 0.077) vs. CG (IR: 0.689 ± 0.106) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastor-Villaescusa, B.; Blanco-Rojo, R.; Olivares, M. Evaluation of the Effect of Limosilactobacillus fermentum CECT5716 on Gastrointestinal Infections in Infants: A Systematic Review and Meta-Analysis. Microorganisms 2021, 9, 1412. https://doi.org/10.3390/microorganisms9071412
Pastor-Villaescusa B, Blanco-Rojo R, Olivares M. Evaluation of the Effect of Limosilactobacillus fermentum CECT5716 on Gastrointestinal Infections in Infants: A Systematic Review and Meta-Analysis. Microorganisms. 2021; 9(7):1412. https://doi.org/10.3390/microorganisms9071412
Chicago/Turabian StylePastor-Villaescusa, Belén, Ruth Blanco-Rojo, and Mónica Olivares. 2021. "Evaluation of the Effect of Limosilactobacillus fermentum CECT5716 on Gastrointestinal Infections in Infants: A Systematic Review and Meta-Analysis" Microorganisms 9, no. 7: 1412. https://doi.org/10.3390/microorganisms9071412
APA StylePastor-Villaescusa, B., Blanco-Rojo, R., & Olivares, M. (2021). Evaluation of the Effect of Limosilactobacillus fermentum CECT5716 on Gastrointestinal Infections in Infants: A Systematic Review and Meta-Analysis. Microorganisms, 9(7), 1412. https://doi.org/10.3390/microorganisms9071412







