Antibiotic Resistant and Biofilm-Associated Escherichia coli Isolates from Diarrheic and Healthy Dogs
Abstract
1. Introduction
2. Materials and Methods
2.1. Canine Samples, Isolation and Identification of E. coli
2.2. Phylogenetic Groups
2.3. Antimicrobial Sensitivity
2.4. Detection of Resistance Genes
2.5. Detection of Biofilm Formation
3. Results and Discussion
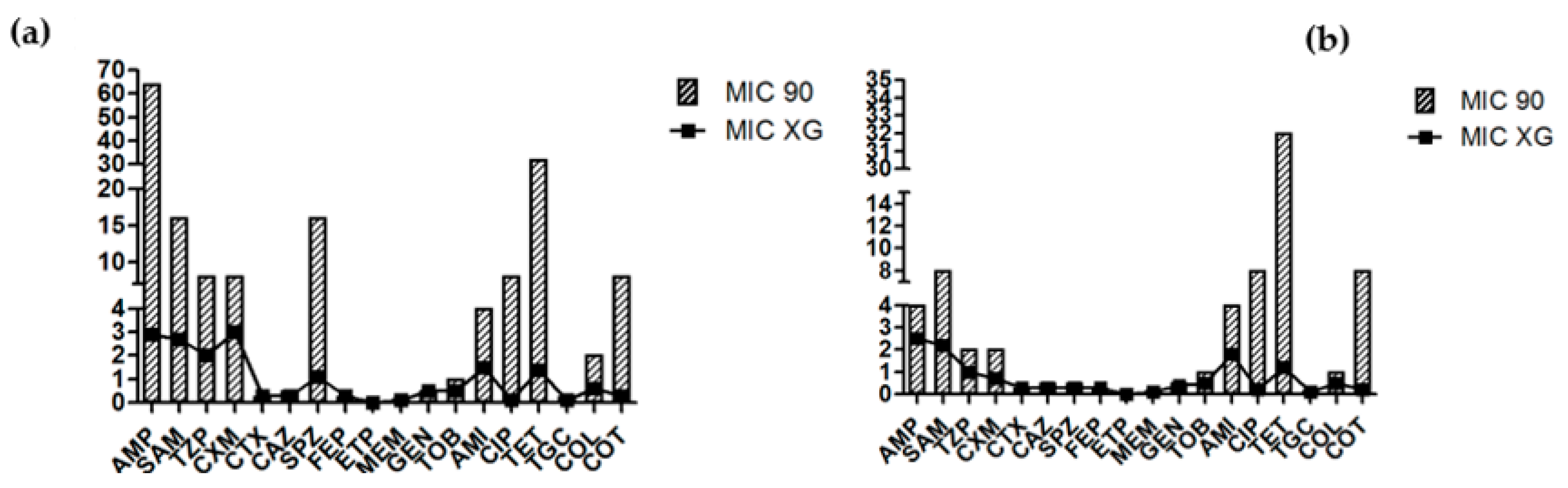
3.1. Antimicrobial Sensitivity
3.2. Interpretative Reading of the Antibiogram and Detection of Resistance Genes
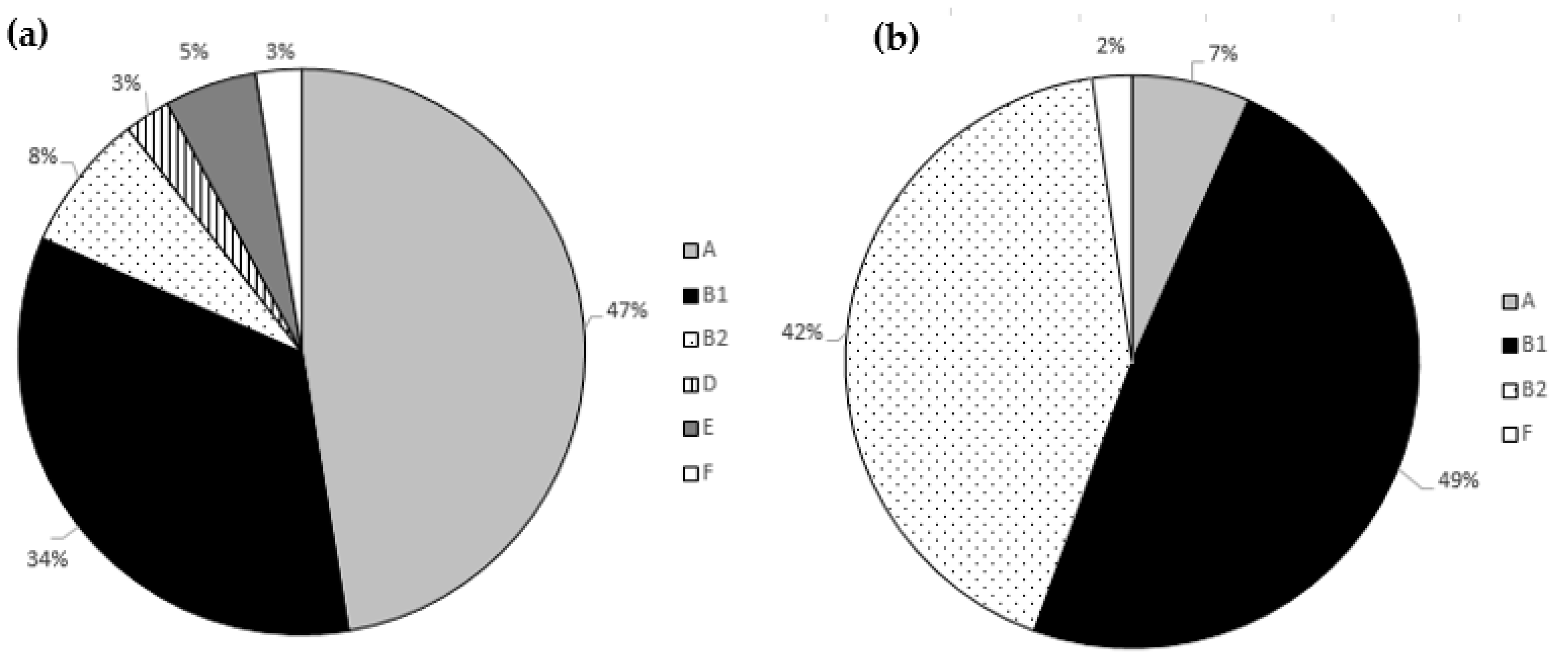
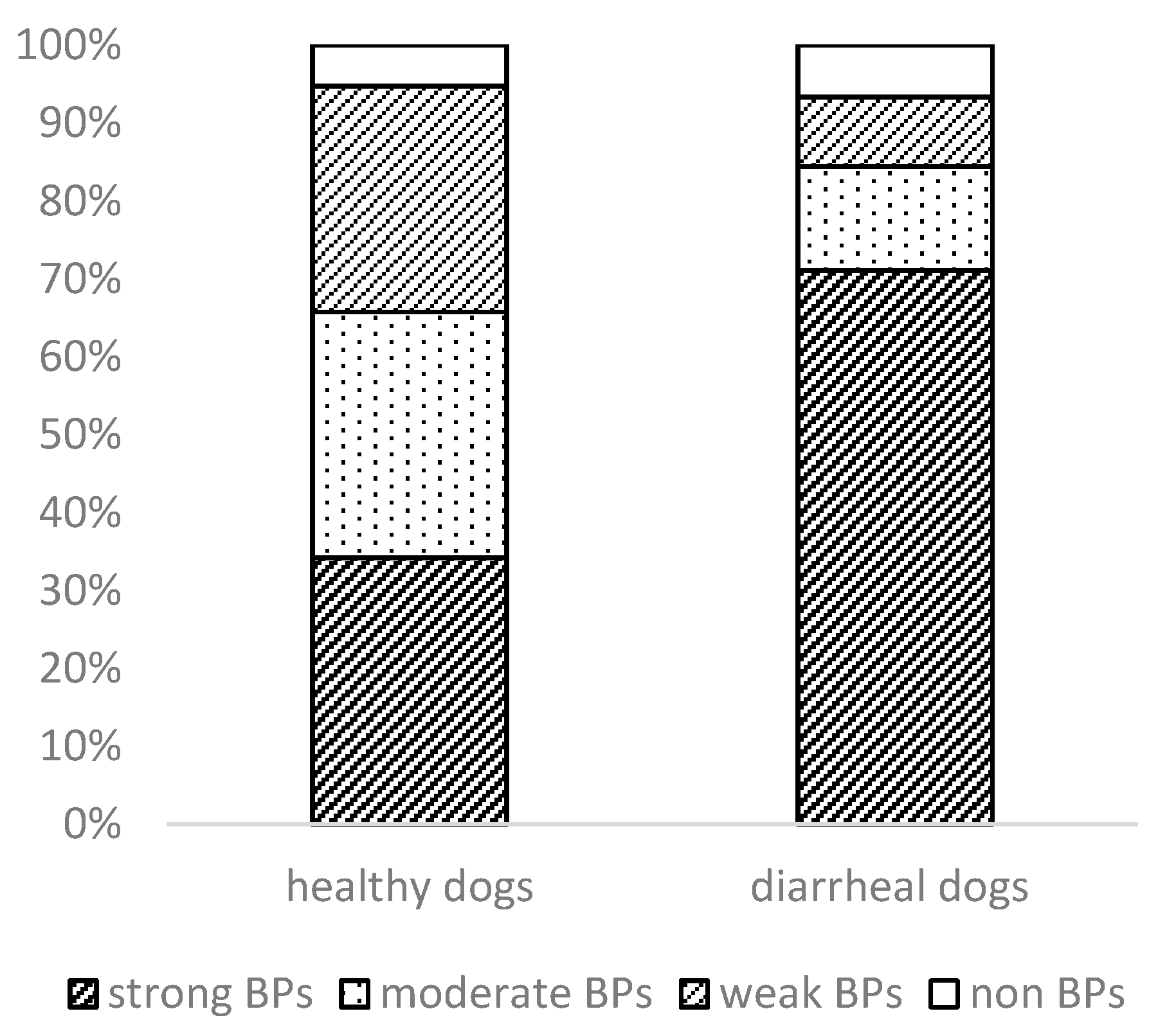
3.3. Phylogenetic Analysis and Biofilm Formation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L.T. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef]
- Puño-Sarmiento, J.; Medeiros, L.; Chiconi, C.; Martins, F.; Pelayo, J.; Rocha, S.; Blanco, J.; Blanco, M.; Zanutto, M.; Kobayashi, R.; et al. Detection of Diarrheagenic Escherichia coli Strains Isolated from Dogs and Cats in Brazil. Vet. Microbiol. 2013, 166, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Majowicz, S.E.; Scallan, E.; Jones-Bitton, A.; Sargeant, J.M.; Stapleton, J.; Angulo, F.J.; Yeung, D.H.; Kirk, M.D. Global Incidence of Human Shiga Toxin–Producing Escherichia coli Infections and Deaths: A Systematic Review and Knowledge Synthesis. Foodborne Pathog. Dis. 2014, 11, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus Biofilm: An Emerging Battleground in Microbial Communities. Antimicrob. Resist. Infect. Control 2019, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.P.; Staerk, K. Antimicrobial Resistance and Antimicrobial Use Animal Monitoring Policies in Europe: Where Are We? J. Public Health Policy 2017, 38, 185–202. [Google Scholar] [CrossRef][Green Version]
- Collignon, P.; McEwen, S. One Health—Its Importance in Helping to Better Control Antimicrobial Resistance. Trop. Med. Infect. Dis. 2019, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Guardabassi, L.; Schwarz, S.; Lloyd, D.H. Pet Animals as Reservoirs of Antimicrobial-Resistant BacteriaReview. J. Antimicrob. Chemother. 2004, 54, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Penakalapati, G.; Swarthout, J.; Delahoy, M.J.; McAliley, L.; Wodnik, B.; Levy, K.; Freeman, M.C. Exposure to Animal Feces and Human Health: A Systematic Review and Proposed Research Priorities. Environ. Sci. Technol. 2017, 51, 11537–11552. [Google Scholar] [CrossRef]
- Swedres-Svarm 2019. Sales of Antibiotics and Occurrence of Resistance in Sweden. Solna/Uppsala ISSN1650-6332. Available online: www.sva.se/swedres-svarm/ (accessed on 19 March 2021).
- The European Union Summary Report on Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Humans, Animals and Food in 2014. EFSA J. 2016, 14. [CrossRef]
- Bessède, E.; Angla-Gre, M.; Delagarde, Y.; Hieng, S.S.; Ménard, A.; Mégraud, F. Matrix-Assisted Laser-Desorption/Ionization Biotyper: Experience in the Routine of a University Hospital. Clin. Microbiol. Infect. 2011, 17, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Clermont, O.; Christenson, J.K.; Denamur, E.; Gordon, D.M. The Clermont Escherichia coli Phylo-Typing Method Revisited: Improvement of Specificity and Detection of New Phylo-Groups. Environ. Microbiol. Rep. 2013, 5, 58–65. [Google Scholar] [CrossRef]
- Mazel, D.; Dychinco, B.; Webb, V.A.; Davies, J. Antibiotic Resistance in the ECOR Collection: Integrons and Identification of a Novel Aad Gene. Antimicrob. Agents Chemother. 2000, 44, 1568–1574. [Google Scholar] [CrossRef]
- Weill, F.-X.; Demartin, M.; Fabre, L.; Grimont, P.A.D. Extended-Spectrum-β-Lactamase (TEM-52)-Producing Strains of Salmonella enterica of Various Serotypes Isolated in France. J. Clin. Microbiol. 2004, 42, 3359–3362. [Google Scholar] [CrossRef] [PubMed]
- Navia, M.M.; Ruiz, J.; Sanchez-Cespedes, J.; Vila, J. Detection of Dihydrofolate Reductase Genes by PCR and RFLP. Diagn. Microbiol. Infect. Dis. 2003, 46, 295–298. [Google Scholar] [CrossRef]
- Guillaume, G.; Verbrugge, D.; Chasseur-Libotte, M.-L.; Moens, W.; Collard, J.-M. PCR Typing of Tetracycline Resistance Determinants (Tet A–E) in Salmonella enterica Serotype Hadar and in the Microbial Community of Activated Sludges from Hospital and Urban Wastewater Treatment Facilities in Belgium. FEMS Microbiol. Ecol. 2000, 32, 77–85. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, X.; Zhang, W.; Pan, W.; Yin, J.; Pan, Z.; Gao, S.; Jiao, X. Prevalence of Qnr, Aac(6′)-Ib-Cr, QepA, and OqxAB in Escherichia coli Isolates from Humans, Animals, and the Environment. Antimicrob. Agents Chemother. 2012, 56, 3423–3427. [Google Scholar] [CrossRef]
- Yamane, K.; Wachino, J.; Suzuki, S.; Arakawa, Y. Plasmid-Mediated QepA Gene among Escherichia coli Clinical Isolates from Japan. Antimicrob. Agents Chemother. 2008, 52, 1564–1566. [Google Scholar] [CrossRef]
- Robicsek, A.; Strahilevitz, J.; Sahm, D.F.; Jacoby, G.A.; Hooper, D.C. Qnr Prevalence in Ceftazidime-Resistant Enterobacteriaceae Isolates from the United States. Antimicrob. Agents Chemother. 2006, 50, 2872–2874. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Wang, Y.; Walsh, T.R.; Yi, L.-X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of Plasmid-Mediated Colistin Resistance Mechanism MCR-1 in Animals and Human Beings in China: A Microbiological and Molecular Biological Study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Xavier, B.B.; Lammens, C.; Ruhal, R.; Kumar-Singh, S.; Butaye, P.; Goossens, H.; Malhotra-Kumar, S. Identification of a Novel Plasmid-Mediated Colistin-Resistance Gene, Mcr-2, in Escherichia coli, Belgium, June 2016. Eurosurveillance 2016, 21, 30280. [Google Scholar] [CrossRef]
- Kerrn, M.B.; Klemmensen, T.; Frimodt-Møller, N.; Espersen, F. Susceptibility of Danish Escherichia coli Strains Isolated from Urinary Tract Infections and Bacteraemia, and Distribution of Sul Genes Conferring Sulphonamide Resistance. J. Antimicrob. Chemother. 2002, 50, 513–516. [Google Scholar] [CrossRef]
- Guerra, B.; Junker, E.; Helmuth, R. Incidence of the Recently Described Sulfonamide Resistance Gene Sul3 among German Salmonella enterica Strains Isolated from Livestock and Food. Antimicrob. Agents Chemother. 2004, 48, 2712–2715. [Google Scholar] [CrossRef][Green Version]
- Lescat, M.; Clermont, O.; Woerther, P.L.; Glodt, J.; Dion, S.; Skurnik, D.; Djossou, F.; Dupont, C.; Perroz, G.; Picard, B.; et al. Commensal Escherichia coli Strains in Guiana Reveal a High Genetic Diversity with Host-Dependant Population Structure. Environ. Microbiol. Rep. 2013, 5, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Yates, C.; Brown, D.; Edwards, G.; Amyes, S. Detection of TEM-52 in Salmonella enterica Serovar Enteritidis Isolated in Scotland. J. Antimicrob. Chemother. 2004, 53, 407–408. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Villalobos, H.; Malaviolle, V.; Frankard, J.; de Mendonça, R.; Nonhoff, C.; Struelens, M.J. In Vitro Activity of Temocillin against Extended Spectrum Beta-Lactamase-Producing Escherichia coli. J. Antimicrob. Chemother. 2006, 57, 771–774. [Google Scholar] [CrossRef]
- Pérez-Pérez, P.J.; Hanson, N.D. Detection of Plasmid-Mediated AmpC beta-Lactamase Genes in Clinical Isolates by Using Multiplex PCR. J. Clin. Microbiol. 2002, 40, 2153–2162. [Google Scholar] [CrossRef] [PubMed]
- Gattringer, R.; Niks, M.; Ostertág, R.; Schwarz, K.; Medvedovic, H.; Graninger, W.; Georgopoulos, A. Evaluation of MIDITECH Automated Colorimetric MIC Reading for Antimicrobial Susceptibility Testing. J. Antimicrob. Chemother. 2002, 49, 651–659. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 10.0. 2020. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Resistance_mechanisms/EUCAST_detection_of_resistance_mechanisms_170711.pdf (accessed on 10 October 2020).
- Stepanović, S.; Vuković, D.; Hola, V.; Bonaventura, G.D.; Djukić, S.; Ćirković, I.; Ruzicka, F. Quantification of Biofilm in Microtiter Plates: Overview of Testing Conditions and Practical Recommendations for Assessment of Biofilm Production by Staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef]
- Wedley, A.L.; Maddox, T.W.; Westgarth, C.; Coyne, K.P.; Pinchbeck, G.L.; Williams, N.J.; Dawson, S. Prevalence of Antimicrobial-Resistant Escherichia coli in Dogs in a Cross-Sectional, Community-Based Study. Vet. Rec. 2011, 168, 354. [Google Scholar] [CrossRef]
- German, A.J.; Halladay, L.J.; Noble, P.-J.M. First-Choice Therapy for Dogs Presenting with Diarrhea in Clinical Practice. Vet. Rec. 2010, 167, 810–814. [Google Scholar] [CrossRef]
- Werner, M.; Suchodolski, J.S.; Straubinger, R.K.; Wolf, G.; Steiner, J.M.; Lidbury, J.A.; Neuerer, F.; Hartmann, K.; Unterer, S. Effect of Amoxicillin-Clavulanic Acid on Clinical Scores, Intestinal Microbiome, and Amoxicillin-Resistant Escherichia coli in Dogs with Uncomplicated Acute Diarrhea. J. Vet. Intern. Med. 2020, 34, 1166–1176. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, H.E.; Jernberg, C.; Andersson, A.F.; Sjölund-Karlsson, M.; Jansson, J.K.; Engstrand, L. Short-Term Antibiotic Treatment Has Differing Long-Term Impacts on the Human Throat and Gut Microbiome. PLoS ONE 2010, 5, e9836. [Google Scholar] [CrossRef] [PubMed]
- Jernberg, C.; Löfmark, S.; Edlund, C.; Jansson, J.K. Long-Term Impacts of Antibiotic Exposure on the Human Intestinal Microbiota. Microbiology 2010, 156, 3216–3223. [Google Scholar] [CrossRef]
- Rzewuska, M.; Czopowicz, M.; Kizerwetter-Świda, M.; Chrobak, D.; Błaszczak, B.; Binek, M. Multidrug Resistance in Escherichia coli Strains Isolated from Infections in Dogs and Cats in Poland (2007–2013). Available online: https://www.hindawi.com/journals/tswj/2015/408205/ (accessed on 9 October 2020). [CrossRef]
- Escher, M.; Vanni, M.; Intorre, L.; Caprioli, A.; Tognetti, R.; Scavia, G. Use of Antimicrobials in Companion Animal Practice: A Retrospective Study in a Veterinary Teaching Hospital in Italy. J. Antimicrob. Chemother. 2011, 66, 920–927. [Google Scholar] [CrossRef]
- Thomson, K.H.; Rantala, M.H.J.; Viita-Aho, T.K.; Vainio, O.M.; Kaartinen, L.A. Condition-Based Use of Antimicrobials in Cats in Finland: Results from Two Surveys. J. Feline Med. Surg. 2009, 11, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Odensvik, K.; Grave, K.; Greko, C. Antibacterial Drugs Prescribed for Dogs and Cats in Sweden and Norway 1990–1998. Acta Vet. Scand. 2001, 42, 189. [Google Scholar] [CrossRef] [PubMed]
- Kvaale, M.K.; Grave, K.; Kristoffersen, A.B.; Norström, M. The Prescription Rate of Antibacterial Agents in Dogs in Norway—Geographical Patterns and Trends during the Period 2004–2008. J. Vet. Pharmacol. Ther. 2013, 36, 285–291. [Google Scholar] [CrossRef]
- Mateus, A.; Brodbelt, D.C.; Barber, N.; Stärk, K.D.C. Antimicrobial Usage in Dogs and Cats in First Opinion Veterinary Practices in the UK. J. Small Anim. Pract. 2011, 52, 515–521. [Google Scholar] [CrossRef]
- Marques, C.; Gama, L.T.; Belas, A.; Bergström, K.; Beurlet, S.; Briend-Marchal, A.; Broens, E.M.; Costa, M.; Criel, D.; Damborg, P.; et al. European Multicenter Study on Antimicrobial Resistance in Bacteria Isolated from Companion Animal Urinary Tract Infections. BMC Vet. Res. 2016, 12, 213. [Google Scholar] [CrossRef]
- Joosten, P.; Ceccarelli, D.; Odent, E.; Sarrazin, S.; Graveland, H.; Van Gompel, L.; Battisti, A.; Caprioli, A.; Franco, A.; Wagenaar, J.A.; et al. Antimicrobial Usage and Resistance in Companion Animals: A Cross-Sectional Study in Three European Countries. Antibiotics 2020, 9, 87. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Doi, Y.; Huang, X.; Li, H.-Y.; Zhong, L.-L.; Zeng, K.-J.; Zhang, Y.-F.; Patil, S.; Tian, G.-B. Possible Transmission of Mcr-1 –Harboring Escherichia coli between Companion Animals and Human. Emerg. Infect. Dis. 2016, 22, 1679–1681. [Google Scholar] [CrossRef]
- Guenther, S.; Falgenhauer, L.; Semmler, T.; Imirzalioglu, C.; Chakraborty, T.; Roesler, U.; Roschanski, N. Environmental Emission of Multiresistant Escherichia coli Carrying the Colistin Resistance Gene Mcr-1 from German Swine Farms. J. Antimicrob. Chemother. 2017, dkw585. [Google Scholar] [CrossRef]
- Comms, V. Colistin Resistance Detected in Shelter Dogs Imported from Russia. Available online: https://www.helsinki.fi/en/news/health/colistin-resistance-detected-in-shelter-dogs-imported-from-russia (accessed on 14 October 2020).
- Ortega-Paredes, D.; Haro, M.; Leoro-Garzón, P.; Barba, P.; Loaiza, K.; Mora, F.; Fors, M.; Vinueza-Burgos, C.; Fernández-Moreira, E. Multidrug-Resistant Escherichia coli Isolated from Canine Faeces in a Public Park in Quito, Ecuador. J. Glob. Antimicrob. Resist. 2019, 18, 263–268. [Google Scholar] [CrossRef]
- Grossman, T.H. Tetracycline Antibiotics and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025387. [Google Scholar] [CrossRef] [PubMed]
- Chopra, I.; Roberts, M. Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef]
- Costa, D.; Poeta, P.; Sáenz, Y.; Coelho, A.C.; Matos, M.; Vinué, L.; Rodrigues, J.; Torres, C. Prevalence of Antimicrobial Resistance and Resistance Genes in Faecal Escherichia coli Isolates Recovered from Healthy Pets. Vet. Microbiol. 2008, 127, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Torkan, S.; Bahadoranian, M.; Khamesipour, F.; Anyanwu, M. Detection of Virulence and Antimicrobial Resistance Genes in Escherichia coli Isolates from Diarrhoiec Dogs in Iran. Arch. Med. Vet. 2016, 48, 181–190. [Google Scholar] [CrossRef][Green Version]
- Yousefi, A.; Torkan, S. Uropathogenic Escherichia coli in the Urine Samples of Iranian Dogs: Antimicrobial Resistance Pattern and Distribution of Antibiotic Resistance Genes. BioMed Res. Int. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Martinez-Martinez, L. Interaction of Plasmid and Host Quinolone Resistance. J. Antimicrob. Chemother. 2003, 51, 1037–1039. [Google Scholar] [CrossRef][Green Version]
- Yu, T.; Jiang, X.; Fu, K.; Liu, B.; Xu, D.; Ji, S.; Zhou, L. Detection of Extended-Spectrum β-Lactamase and Plasmid-Mediated Quinolone Resistance Determinants in Escherichia coli Isolates from Retail Meat in China: ESBL and PMQR Genes in Escherichia coli. J. Food Sci. 2015, 80, M1039–M1043. [Google Scholar] [CrossRef]
- Ishida, Y.; Ahmed, A.M.; Mahfouz, N.B.; Kimura, T.; El-Khodery, S.A.; Moawad, A.A.; Shimamoto, T. Molecular Analysis of Antimicrobial Resistance in Gram-Negative Bacteria Isolated from Fish Farms in Egypt. J. Vet. Med. Sci. 2010, 72, 727–734. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, X.; Zhu, D.; Ye, X.; Wang, M. Decreased Quinolone Susceptibility in High Percentage of Enterobacter Cloacae Clinical Isolates Caused Only by Qnr Determinants. Diagn. Microbiol. Infect. Dis. 2010, 67, 110–113. [Google Scholar] [CrossRef]
- Aslantas, Ö.; Yilmaz, E.S. Prevalence and Molecular Characterization of Extended-Spectrum β-Lactamase (ESBL) and Plasmidic AmpC β-Lactamase (PAmpC) Producing Escherichia coli in Dogs. J. Vet. Med. Sci. 2017, 79, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- LeCuyer, T.E.; Byrne, B.A.; Daniels, J.B.; Diaz-Campos, D.V.; Hammac, G.K.; Miller, C.B.; Besser, T.E.; Davis, M.A. Population Structure and Antimicrobial Resistance of Canine Uropathogenic Escherichia coli. J. Clin. Microbiol. 2018, 56, e00788-18. [Google Scholar] [CrossRef]
- Carvalho, A.C.; Barbosa, A.V.; Arais, L.R.; Ribeiro, P.F.; Carneiro, V.C.; Cerqueira, A.M.F. Resistance Patterns, ESBL Genes, and Genetic Relatedness of Escherichia coli from Dogs and Owners. Braz. J. Microbiol. 2016, 47, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Tenaillon, O.; Skurnik, D.; Picard, B.; Denamur, E. The Population Genetics of Commensal Escherichia coli. Nat. Rev. Microbiol. 2010, 8, 207–217. [Google Scholar] [CrossRef]
- Russo, T.A.; Johnson, J.R. Proposal for a New Inclusive Designation for Extraintestinal Pathogenic Isolates of Escherichia coli: ExPEC. J. Infect. Dis. 2000, 181, 1753–1754. [Google Scholar] [CrossRef] [PubMed]
- Hutton, T.A.; Innes, G.K.; Harel, J.; Garneau, P.; Cucchiara, A.; Schifferli, D.M.; Rankin, S.C. Phylogroup and Virulence Gene Association with Clinical Characteristics of Escherichia coli Urinary Tract Infections from Dogs and Cats. J. Vet. Diagn. Investig. 2018, 30, 64–70. [Google Scholar] [CrossRef]
- Vega-Manriquez, X.D.; Ubiarco-López, A.; Verdugo-Rodríguez, A.; Hernández-Chiñas, U.; Navarro-Ocaña, A.; Ahumada-Cota, R.E.; Ramírez-Badillo, D.; Hernández-Díaz de León, N.; Eslava, C.A. Pet Dogs Potential Transmitters of Pathogenic Escherichia coli with Resistance to Antimicrobials. Arch. Microbiol. 2020, 202, 1173–1179. [Google Scholar] [CrossRef]
- Valat, C.; Drapeau, A.; Beurlet, S.; Bachy, V.; Boulouis, H.-J.; Pin, R.; Cazeau, G.; Madec, J.-Y.; Haenni, M. Pathogenic Escherichia coli in Dogs Reveals the Predominance of ST372 and the Human-Associated ST73 Extra-Intestinal Lineages. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Vijay, D.; Dhaka, P.; Vergis, J.; Negi, M.; Mohan, V.; Kumar, M.; Doijad, S.; Poharkar, K.; Kumar, A.; Malik, S.S.; et al. Characterization and Biofilm Forming Ability of Diarrhoeagenic Enteroaggregative Escherichia coli Isolates Recovered from Human Infants and Young Animals. Comp. Immunol. Microbiol. Infect. Dis. 2015, 38, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, K.; Nishi, J.; Imuta, N.; Fujiyama, R.; Kamenosono, A.; Manago, K.; Kawano, Y. Characterization of Typical and Atypical Enteroaggregative Escherichia coli in Kagoshima, Japan: Biofilm Formation and Acid Resistance. Microbiol. Immunol. 2010, 54, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Garcia, F.; Gutierrez-Jimenez, J.; Garcia-Tovar, C.; Castro, L.A.; Salazar-Gonzalez, H.; Cordova, V. Pic, an Autotransporter Protein Secreted by Different Pathogens in the Enterobacteriaceae Family, Is a Potent Mucus Secretagogue. Infect. Immun. 2010, 78, 4101–4109. [Google Scholar] [CrossRef] [PubMed]
| Gene | Primer Sequences (5′–3′) | Annealing (°C) | Size Product (bp) | Reference |
|---|---|---|---|---|
| int1 | F:GGGTCAAGGATCTGGATTTCG R:ACATGCGTGTAAATCATCGTCG | 62 | 483 | [13] |
| tn3 | F:CACGAATGAGGGCCGACAGGA R:ACCCACTCGTGCACCCAACTG | 58 | 500 | [14] |
| dfrA | F:GTGAAACTATCACTAATGG R:TTAACCCTTTTGCCAGATTT | 55 | 474 | [15] |
| dfrB | F:GATCGCCTGCGCAAGAAATC R:AAGCGCAGCCACAGGATAAAT | 60 | 141 | [15] |
| tetA | F:GGCCTCAATTTCCTGACG R:AAGCAGGATGTAGCCTGTGC | 55 | 372 | [16] |
| tetB | F:GAGACGCAATCGAATTCGG R:TTTAGTGGCTATTCTTCCTGCC | 55 | 228 | [16] |
| oqxA | F:GACAGCGTCGCACAGAATG R:GGAGACGAGGTTGGTATGGA | 62 | 339 | [17] |
| oqxB | F:CGAAGAAAGACCTCCCTACCC R:CGCCGCCAATGAGATACA | 62 | 240 | [17] |
| qepA | F:GCAGGTCCAGCAGCGGGTAG R:CTTCCTGCCCGAGTATCGTG | 60 | 199 | [18] |
| qnrS | F:ACGACATTCGTCAACTGCAA R:TAAATTGGCACCCTGTAGGC | 53 | 417 | [19] |
| qnrA | F:ATTTCTCACGCCAGGATTTG R:GATCGGCAAAGGTTAGGTCA | 53 | 516 | [19] |
| qnrB | F:GATCGTGAAAGCCAGAAAGG R:ACGATGCCTGGTAGTTGTCC | 53 | 469 | [19] |
| aac(6′)-Ib-cr | F:GATCTCATATCGTCGAGTGGTGG R:GAACCATGTACACGGCTGGAC | 58 | 435 | [19] |
| mcr-1 | F:CGGTCAGTCCGTTTGTTC R:CTTGGTCGGTCTGTAGGG | 58 | 309 | [20] |
| mcr-2 | F: TGTTGCTTGTGCCGATTGGA R:AGATGGTATTGTTGGTTGCTG | 58 | 567 | [21] |
| sul1 | F:CGGCGTGGGCTACCTGAACG R:GCCGATCGCGTGAAGTTCCG | 69 | 433 | [22] |
| sul2 | F:GCGCTCAAGGCAGATGGCATT R:GCGTTTGATACCGGCACCCGT | 69 | 293 | [22] |
| sul3 | F: GAGCAAGATTTTTGGAATCG R:CATCTGCAGCTAACCTAGGGCTTTGA | 51 | 990 | [23] |
| arpA | F:AACGCTATTCGCCAGCTTGC R:TCTCCCCATACCGTACGCTA | 59 | 400 | [12] |
| chuA | F:ATGGTACCGGACGAACCAAC R:TGCCGCCAGTACCAAAGACA | 59 | 288 | [12] |
| yjaA | F:CAAACGTGAAGTGTCAGGAG R: AATGCGTTCCTCAACCTGTG | 59 | 211 | [12] |
| TspE4.C2 | F: CACTATTCGTAAGGTCATCC R: AGTTTATCGCTGCGGGTCGC | 59 | 152 | [12] |
| arpAgpE | F:GATTCCATCTTGTCAAAATATGCC R:GAAAAGAAAAAGAATTCCCAAGAG | 57 | 301 | [24] |
| trpAgpC | F:AGTTTTATGCCCAGTGCGAG R:TCTGCGCCGGTCACGCCC | 59 | 219 | [24] |
| blaTEM-1 | F:ATGAGTATTCAACATTTCCG R:CCAATGCTTAATCAGTGAGG | 55 | 858 | [25] |
| blaSHV | F:ATGCGTTATATTCGCCTGTG R:TTAGCGTTGCCAGTGCTCGATG | 58 | 301 | [26] |
| cit | F: TGGCCAGAACTGACAGGCAAA R: TTTCTCCTGAACGTGGCTGGC | 64 | 462 | [27] |
| Phylogroups of Healthy Dogs | Phenotypic Antimicrobial Resistance Profile | Number of Isolates |
|---|---|---|
| A | Without AMR profile | n = 8 |
| A | TET | n = 2 |
| A | AMP, COT | n = 1 |
| A | AMP, TET, COT | n = 2 |
| A | AMP, SAM, TET | n = 1 |
| A | AMP, CIP, TET, COT | n = 2 |
| A | AMP, CIP, TET, COL, COT | n = 2 |
| B1 | Without AMR profile | n = 9 |
| B1 | AMP, TET, COT | n = 2 |
| B1 | AMP, CIP, TET, COT | n = 2 |
| B2 | Without AMR profile | n = 3 |
| D | Without AMR profile | n = 1 |
| E | Without AMR profile | n = 2 |
| F | Without AMR profile | n = 1 |
| Phylogroups of sick dogs | Phenotypic antimicrobial resistance profile | Number of isolates |
| A | Without AMR profile | n = 3 |
| B1 | Without AMR profile | n = 20 |
| B1 | COL | n = 2 |
| B2 | CIP | n = 4 |
| B2 | TET | n = 3 |
| B2 | CIP, TET | n = 7 |
| B2 | TET, COT | n = 2 |
| B2 | CIP, COT | n = 1 |
| B2 | SAM, TET, COT | n = 2 |
| F | Without AMR profile | n = 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karahutová, L.; Mandelík, R.; Bujňáková, D. Antibiotic Resistant and Biofilm-Associated Escherichia coli Isolates from Diarrheic and Healthy Dogs. Microorganisms 2021, 9, 1334. https://doi.org/10.3390/microorganisms9061334
Karahutová L, Mandelík R, Bujňáková D. Antibiotic Resistant and Biofilm-Associated Escherichia coli Isolates from Diarrheic and Healthy Dogs. Microorganisms. 2021; 9(6):1334. https://doi.org/10.3390/microorganisms9061334
Chicago/Turabian StyleKarahutová, Lívia, René Mandelík, and Dobroslava Bujňáková. 2021. "Antibiotic Resistant and Biofilm-Associated Escherichia coli Isolates from Diarrheic and Healthy Dogs" Microorganisms 9, no. 6: 1334. https://doi.org/10.3390/microorganisms9061334
APA StyleKarahutová, L., Mandelík, R., & Bujňáková, D. (2021). Antibiotic Resistant and Biofilm-Associated Escherichia coli Isolates from Diarrheic and Healthy Dogs. Microorganisms, 9(6), 1334. https://doi.org/10.3390/microorganisms9061334






