Fermentation Strategies to Improve Soil Bio-Inoculant Production and Quality
Abstract
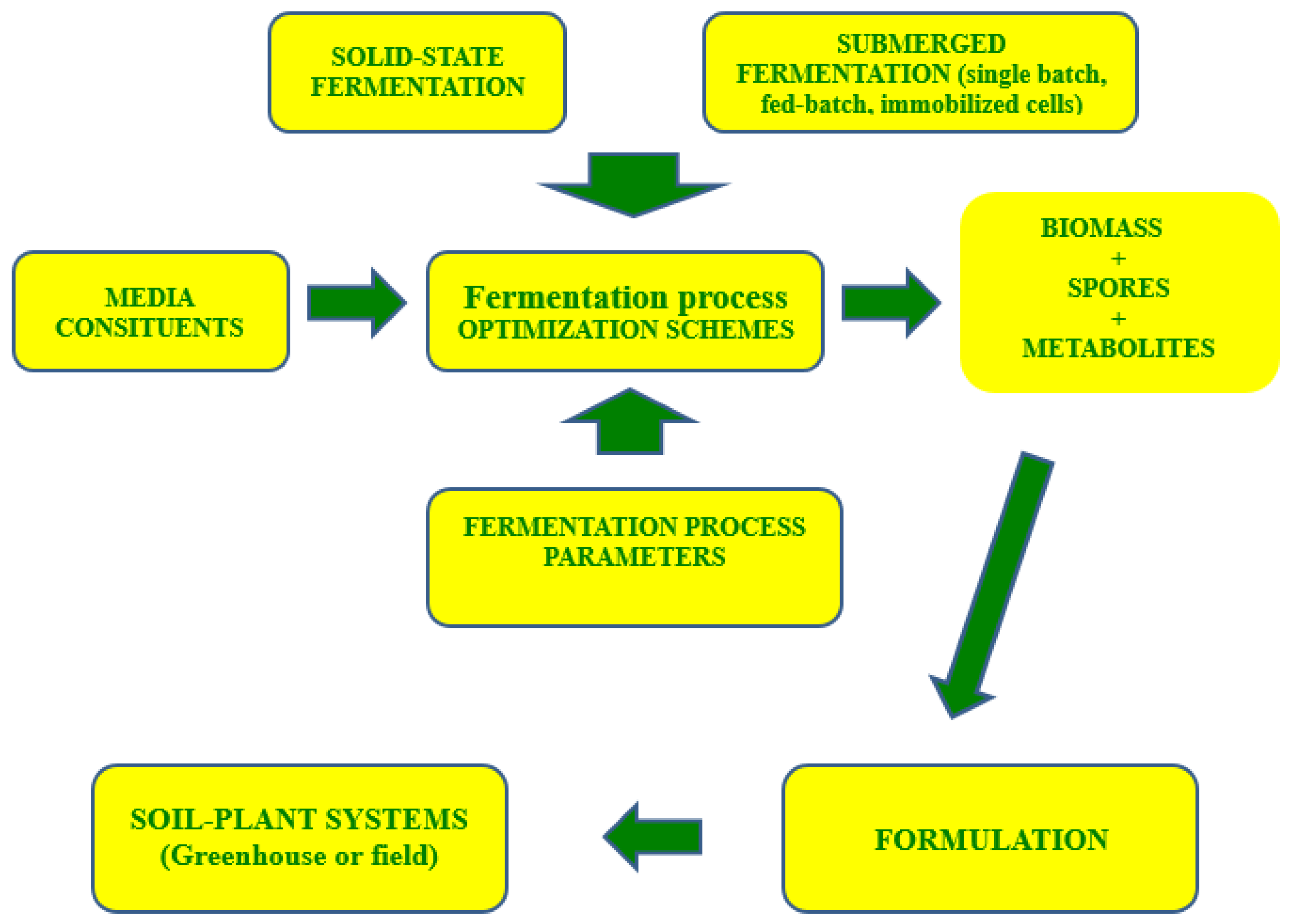
1. Introduction
2. Submerged Liquid Fermentation
2.1. Single Batch Operations
2.2. Fed-Batch Operations
3. Immobilized-Cell-Based Fermentation Processes
4. Solid-State Fermentations
5. Key Fermentation Parameters
6. Selection of the Fermentation Mode for Industrial Production
7. The special Case of Mycorrhiza
8. Perspective Developments and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Malusà, E.; Vassilev, N. A contribution to set a legal framework for biofertilisers. Appl. Microbiol. Biotechnol. 2014, 98, 6599–6607. [Google Scholar] [CrossRef] [PubMed]
- Meena, V.S.; Meena, S.K.; Verma, J.P.; Kumar, A.; Aeron, A.; Mishra, P.K.; Bisht, J.K.; Pattanayak, A.; Naveed, M.; Dotaniya, M.L. Plant beneficial rhizospheric microorganism (PBRM) strategies to improve nutrients use efficiency: A review. Ecol. Eng. 2017, 107, 8–32. [Google Scholar] [CrossRef]
- Singh, D.P.; Prabha, R.; Gupta, V.K. Microbial inoculants for sustainable crop management. In Microbial Interventions in Agriculture and Environment. Vol. 2 Rhizosphere, Microbiome and Agro-Ecology; Springer Nature: Singapore, 2019; pp. 1–35. [Google Scholar]
- Owen, D.; Williams, A.P.; Griffith, G.W.; Withers, P.J.A. Use of commercial bio-inoculants to increase agricultural production through improved phosphorus acquisition. Appl. Soil Ecol. 2015, 86, 41–54. [Google Scholar] [CrossRef]
- Figueiredo, M.V.B.; Bonifacio, A.; Rodrigues, A.C.; de Araujo, F.F.; Stamford, N.P. Beneficial microorganisms: Current challenges to increase crop performance. In Bioformulations for Sustainable Agriculture; Arora, N.K., Mehnaz, S., Balestrini, R., Eds.; Springer: New Delhi, India, 2016; pp. 53–70. [Google Scholar]
- Pathak, D.V.; Kumar, M. Microbial Inoculants as Biofertilizers and Biopesticides. In Microbial Inoculants in Sustainable Agricultural Productivity; Singh, D.P., Ed.; Springer: New Delhi, India, 2016; pp. 197–209. [Google Scholar] [CrossRef]
- Pii, Y.; Mimmo, T.; Tomasi, N.; Terzano, R.; Cesco, S.; Crecchio, C. Microbial interactions in the rhizosphere: Beneficial influences of plant growth- promoting rhizobacteria on nutrient acquisition process. A review. Biol. Fertil. Soils 2015, 51, 403–415. [Google Scholar] [CrossRef]
- Shilev, S.; Naydenov, M.; Sancho Prieto, M.; Vassilev, N.; Sancho, D.E. PGPR as Inoculants in Management of Lands Contaminated with Trace Elements. In Bacteria in Agrobiology; Maheshwari Dinesh, K., Ed.; Springer: New York, NY, USA; Berlin, Germany, 2012; pp. 259–278. [Google Scholar]
- Vassilev, N.; Vassileva, M.; Nikolaeva, I. Simultaneous P-solubilizing and biocontrol activity of microorganisms: Potentials and future trends. Appl. Microbiol. Biotechnol. 2006, 71, 137–144. [Google Scholar] [CrossRef]
- Vassileva, M.; Flor-Peregrin, E.; Malusá, E.; Vassilev, N. Towards better understanding of the interactions and efficient application of plant beneficial prebiotics, probiotics, postbiotics and synbiotics. Front. Plant Sci. 2020, 11, 1068. [Google Scholar] [CrossRef]
- Vassileva, M.; Serrano, M.; Bravo, V.; Jurado, E.; Nikolaeva, I.; Martos, V.; Vassilev, N. Multifunctional properties of phosphate- solubilizing microorganisms grown on agro-industrial wastes in fermentation and soil conditions. Appl. Microbiol. Biotechnol. 2010, 85, 1287–1299. [Google Scholar] [CrossRef]
- Shilev, S.; Azaizeh, H.; Vassilev, N.; Georgiev, D.; Babrikova, I. Interactions in soil-microbe-plant system: Adaptation to stressed agriculture. In Microbial Interventions in Agriculture and Environment; Singh, D., Gupta, V., Prabha, R., Eds.; Springer: Singapore, 2019; pp. 131–171. [Google Scholar] [CrossRef]
- Barret, M.; Tan, H.; Egan, F.; Morrissey, J.P.; Reen, J.; O’Gara, F. Exploiting new systems-based strategies to elucidate plant–bacterial interactions in the rhizosphere. In Molecular Microbial Ecology of the Rhizosphere; de Bruijn, F.J., Ed.; Wiley Blackwell: Hoboken, NJ, USA, 2013; Volume 1, pp. 57–68. [Google Scholar]
- Adesemoye, A.O.; Torbert, H.A.; Kloepper, J.W. Enhanced plant nutrient use efficiency with PGPR and AMF in an integrated nutrient management system. Can. J. Microbiol. 2008, 54, 876–886. [Google Scholar] [CrossRef]
- Kowalska, J.; Tyburski, J.; Matysiak, K.; Tylkowski, B.; Malusá, E. Field Exploitation of Multiple Functions of Beneficial Microorganisms for Plant Nutrition and Protection: Real Possibility or Just a Hope? Front. Microbiol. 2020, 11, 1904. [Google Scholar] [CrossRef]
- Vassilev, N.; Vassileva, M.; Martos, V.; Garcia Del Moral, L.F.; Kowalska, J.; Tylkowski, B.; Malusá, E. Formulation of microbial inoculants by encapsulation in natural polysaccharides: Focus on beneficial properties of carrier additives and derivates. Front. Plant Sci. 2020, 11, 270. [Google Scholar] [CrossRef]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R.; Hernandez, J.-P. Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013). Plant. Soil 2014, 378, 1–33. [Google Scholar] [CrossRef]
- Vassilev, N.; Vassileva, M.; Lopez, A.; Martos, V.; Reyes, A.; Maksimovic, I.; Eichler-Löbermann, B.; Malusá, E. Unexploited potential of some biotechnological techniques for biofertilizer production and formulation. Appl. Microbiol. Biotechnol. 2015, 99, 4983–4996. [Google Scholar] [CrossRef] [PubMed]
- Canfora, L.; Costa, C.; Pallottino, F.; Mocali, S. Trends in Soil Microbial Inoculants Research: A Science Mapping Approach to Unravel Strengths and Weaknesses of their Application. Agriculture 2021, 11, 158. [Google Scholar] [CrossRef]
- Willey, J.M.; Sherwood, L.M.; Wolverton, C.J. Applied and Industrial Microbiology. In Prescott, Harley, and Klein’s Microbiology, 7th ed.; McGraw-Hill: New York, NY, USA, 2008; pp. 1049–1088. [Google Scholar]
- Vassilev, N.; Eichler-Löbermann, B.; Flor-Peregrin, E.; Martos, V.; Reyes, A.; Vassileva, M. Production of a potential liquid plant biostimulant by immobilized Piriformospora indica in repeated-batch fermentation process. AMB Express 2017, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Mutturi, S.; Sahai, V.; Sharma, S.; Bisaria, V.S. Strategies for high-density cultivation of bio-inoculants in submerged culture with special reference to Pseudomonads. In Microbial Inoculants in Sustainable Agricultural Productivity; Singh, D.P., Singh, H.B., Prabha, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; Volume 1, pp. 181–196. [Google Scholar]
- Subramaniam, R.; Thirumal, V.; Chistoserdov, A.; Bajpai, R.; Bader, J.; Popovic, M. High-density cultivation in the production of microbial products. Chem. Biochem. Eng. Q. 2018, 32, 451–464. [Google Scholar] [CrossRef]
- Vassilev, N.; Mendes, G.; Costa, M.; Vassileva, M. Biotechnological tools for enhancing microbial solubilization of insoluble inorganic phosphates. Geomicrobiol. J. 2014, 31, 751–763. [Google Scholar] [CrossRef]
- Mukhtar, H.; Bashir, H.; Nawaz, A.; Haq, I. Optimization of growth conditions for Azotobacter species and their use as biofertilizer. J. Bacteriol. Mycol. 2018, 6, 274–278. [Google Scholar] [CrossRef]
- Ben Rebah, F.; Prevost, D.; Yezza, A.; Tyagi, R.D. Agro-indusrial waste materials and wastewater sludge for rhizobial inoculant production: A review. Biores Technol. 2007, 98, 3535–3546. [Google Scholar] [CrossRef]
- Estrella, M.J.; Pieckenstein, F.L.; Marina, M.; Diaz, L.E.; Ruiz, O.A. Cheese whey: An alternative growth and protective médium for Rhizobium loti cells. J. Ind. Microbiol. Biotechnol. 2004, 31, 122–126. [Google Scholar] [CrossRef]
- Singh, A.K.; Gauri Bhatt, R.P.; Pant, S. Optimization and comparative study of the sugar waste for the growth of Rhizobium cells along with traditional laboratory media. Res. J. Microbiol. 2011, 6, 715–723. [Google Scholar]
- Yezza, A.; Tyagi, R.D.; Valero, J.R.; Surampalli, R.Y. Production of Bacillus thuriengiensis-based biopesticides in batch and fed batch cultures using wastewater sludge as a raw material. J. Chem. Technol. Biotechnol. 2005, 80, 502–510. [Google Scholar] [CrossRef]
- Behle, R.W.; Jackson, M.A. Effect of Fermentation Media on the Production, Efficacy, and Storage Stability of Metarhizium brunneum Microsclerotia Formulated as a Prototype Granule. J. Econ. Entom. 2014, 107, 582–590. [Google Scholar] [CrossRef]
- Mendes, G.; Vassilev, N.; Araújo, V.H.; da Silva, I.R.; Júnior, J.I.; Costa, M. Inhibition of fungal phosphate solubilization by fluoride released from rock phosphate. Appl. Environ. Microbiol. 2013, 79, 4906–4913. [Google Scholar] [CrossRef]
- Vassilev, N.; Nikolaeva, I.; Vassileva, M. Indole-3-acetic acid production by gel-entrapped Bacillus thuringiensis in the presence of rock phosphate ore. Chem. Eng. Commun. 2007, 194, 441–445. [Google Scholar] [CrossRef]
- Bai, Z.; Jin, B.; Li, Y.; Chen, J.; Li, Z. Utilization of winery wastes for Trichoderma viride biocontrol agent production by solid state fermentation. J. Environ. Sci. 2008, 20, 353–358. [Google Scholar] [CrossRef]
- Zhang, J.D.; Yang, Q. Optimization of solid-state fermentation conditions for Trichoderma harzianum using an orthogonal test. Genet. Mol. Res. 2015, 14, 1771–1781. [Google Scholar] [CrossRef]
- Jaronski, S.T.; Mascarin, G.M. Mass Production of Fungal Entomopathogens. In Microbial Control of Insect and Mite Pests; Lawrence, A.L., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 141–155. [Google Scholar]
- Vassilev, N.; Baca, M.T.; Vassileva, M.; Franco, I.; Azcon, R. Rock phosphate solubilization by Aspergillus niger grown on sugar beet wastes. Appl. Microbiol. Biotechnol. 1995, 44, 546–549. [Google Scholar] [CrossRef]
- Vassileva, M.; Vassilev, N.; Azcon, R. Rock phosphate solubilization by Aspergillus niger on olive cake-based médium and its further application in a soil-plant system. W. J. Microbiol. Biotechnol. 1997, 14, 281–284. [Google Scholar] [CrossRef]
- Vassilev, N.; Medina, A.; Azcon, R.; Vassileva, M. Microbial solubilization of rock phosphate on media containing agro-industrial wastes and effect of the resulting products on plant growth and P uptake. Plant. Soil 2006, 287, 77–84. [Google Scholar] [CrossRef]
- Vassilev, N.; Malusà, E.; Reyes, A.; Lopez, A.; Martos, V.; Maksimovic, I.; Vassileva, M. Potential application of glycerol in the production of plant beneficial microorganisms. J. Ind. Microbiol. Biotechnol. 2017, 44, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Vassilev, N.; Medina, A.; Mendes, G.; Galvez, A.; Martos, V.; Vassileva, M. Solubilization of animal bonechar by a filamentous fungus employed in solid state fermentation. Ecol. Eng. 2013, 58, 165–169. [Google Scholar]
- Mendes, G.; Zafra, D.; Vassilev, N.; Silva, I.R.; Ribeiro, J.I., Jr.; Costa, M.D. Biochar Enhances Aspergillus niger Rock Phosphate Solubilization by Increasing Organic Acid Production and Alleviating Fluoride Toxicity. Appl. Environ. Microbiol. 2014, 80, 3081–3085. [Google Scholar] [CrossRef]
- Sanchez, S.; Demain, A. Metabolite regulation of fermentation processes. Enz. Microb. Technol. 2002, 31, 895–906. [Google Scholar] [CrossRef]
- Aleksieva, P.; Micheva-Viteva, S. Regulation of extracellular acid phosphatase biosynthesis by phosphates in proteinase producing fungus Humicola lutea. Enzym. Microb. Technol. 2000, 27, 570–575. [Google Scholar] [CrossRef]
- Vassilev, N.; Requena, A.; Nieto, L.; Nikolaeva, I.; Vassileva, M. Production of manganese peroxidase by Phanerochaete chrysosporium grown on medium containing agro-wastes/rock phosphate and biocontrol properties of the final product. Ind. Crops Prod. 2009, 30, 28–32. [Google Scholar] [CrossRef]
- Vassilev, N.; Nikolaeva, I.; Vassileva, M. An improved technique for preparation of gel-entrapped AM fungal spores. Minerva Biotechnol. 2007, 19, 51–55. [Google Scholar]
- Lozano-Tovar, M.D.; Garrido-Jurado, I.; Quesada-Moraga, E.; Raya-Ortega, M.C.; Trapero-Casas, A. Metarhizium brunneum and Beauveria bassiana release secondary metabolites with antagonistic activity against Verticillium dahliae and Phytophthora megasperma olive pathogens. Crop. Prot. 2017, 100, 186–195. [Google Scholar] [CrossRef]
- Mendes, G.; Galvez, A.; Vassileva, M.; Vassilev, N. Fermentation liquid containing microbially solubilized P significantly improved plant growth and P uptake in both soil and soilless experiments. Appl. Soil Ecol. 2017, 117, 208–211. [Google Scholar] [CrossRef]
- Lu, K.H.; Jin, Q.; Lin, Y.B.; Lu, W.W.; Li, S.S.; Zhou, C.H.; Jin, J.R.; Jiang, Q.Y.; Ling, L.C.; Xiao, M. Cell-free Fermentation Broth of Bacillus velezensis Strain S3-1 Improves Pak Choi Nutritional Quality and Changes the Bacterial Community Structure of the Rhizosphere Soil. Front. Microbiol. 2020, 11, 2043. [Google Scholar] [CrossRef]
- Kumar, V.; Rajauria, G.; Sahai, V.; Bisaria, V.S. Culture filtrate of root endophytic fungus Piriformospora indica promotes the growth and lignan production of Linum album hairy root cultures. Process. Biochem. 2012, 47, 901–907. [Google Scholar] [CrossRef]
- Compant, S.; Brader, G.; Muzammil, S.; Sessitsch, A.; Lebrihi, A.; Mathieu, F. Use of beneficial bacteria and their secondary metabolites to control grapevine pathogen diseases. BioControl 2013, 58, 435–455. [Google Scholar] [CrossRef]
- Nasr-Eldin, M.; Messiha, N.; Othman, B.; Megaheb, A.; Elhalag, K. Induction of potato systemic resistance against the potato virus Y (PVYNTH), using crude filtrates of Streptomyces spp. under greenhouse conditions. Egypt. J. Biol. Pest. Control. 2019, 29–62. [Google Scholar] [CrossRef]
- Inglis, G.D.; Johnson, D.L.; Cheng, K.-J.; Goettel, M.S. Use of pathogen combinations to overcome the constraints of temperature on entomopathogenic hyphomycetes against grasshoppers. Biol. Control. 1997, 8, 143–152. [Google Scholar] [CrossRef]
- Canfora, L.; Abu-Samra, N.; Tartanus, M.; Łabanowska, B.H.; Benedetti, A.; Pinzari, F.; Malusà, E. Co-inoculum of Beauveria brongniartii and B. bassiana shows in vitro different metabolic behaviour in comparison to single inoculums. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.Y.; Xue, L.; YuanLong, L.C.; DongYing, J.; Kai, Y. Characterization of co-culturing microorganisms for simultaneous degradation of β-Cypermethrin and 3-phenoxybenzoic acid. Fres Environ. Bull. 2018, 27, 4249–4257. [Google Scholar]
- BioVoicenews. IIT Innovation Series: Boosting Biofertilizer Productivity. 2016. Available online: https://www.biovoicenews.com/iit-innovation-series-boosting-biofertilizer-productivity (accessed on 20 March 2021).
- Quiroga, G.; Diaz, A.; Gomez, M. Improving biomass production for two biofertilizers strains of Azotobacter chroococcum at laboratory level. J. Biotechnol. Biomater. 2015, 5, 6. [Google Scholar] [CrossRef]
- Damir, O.; Mladen, P.; Bozidar, S.; Srnan, N. Cultivation of the bacterium Azotobacter chroococcum for preparation of biofertilizers. Afr. J. Biotechnol. 2011, 10, 3104–3111. [Google Scholar] [CrossRef][Green Version]
- Sarma, M.V.R.K.; Gautam, A.; Kumar, L.; Saharan, K.; Kapoor, A.; Shrivastava, N.; Sahai, V.; Bisaria, V.S. Bioprocess strategies for mass multiplication of and metabolite synthesis by plant growth promoting pseudomonads for agronomical applications. Proc. Biochem. 2013, 48, 1418–1424. [Google Scholar] [CrossRef]
- Klaic, R.; Plotegher, F.; Ribeiro, C.; Zangirolami, T.C.; Farinas, C.S. A fed-batch strategy integrated with mechanical activation improves the solubilization of phosphate rock by Aspergillus niger. ACS Sustain. Chem. Eng. 2018, 6, 11326–11334. [Google Scholar] [CrossRef]
- Chen, S.; Hong, J.Y.; Wu, W.T. Fed batch culture of Bacillus thuringiensis based on motile intensity. J. Ind. Microbiol. Biotechnol. 2003, 30, 677–681. [Google Scholar] [CrossRef]
- Kuppusamy, M.; Balaraman, K. Fed batch fermentation studies with Bacillus thuringiensis H-14 synthesizing delta endotoxin. Indian J. Exp. Biol. 1991, 29, 1031–1034. [Google Scholar]
- Kang, B.C.; Lee, S.Y.; Chang, H.N. Enhanced spore production of Bacillus thuringiensis by fed batch culture. Biotechnol. Lett. 1992, 14, 721–726. [Google Scholar] [CrossRef]
- Karel, S.F.; Libicki, S.B.; Robertson, C.R. The immobilization of whole cells: Engineering principles. Chem. Eng. Sci. 1985, 40, 1321–1354. [Google Scholar] [CrossRef]
- Vassilev, N.; Vassileva, M.; Fenice, M.; Federici, F. Immobilized cell technology applied in solubilization of insoluble inorganic (rock) phosphates and P plant acquisition. Biores Technol. 2001, 79, 263–271. [Google Scholar] [CrossRef]
- Sahu, P.K.; Brahmaprakash, G.P. Formulations of Biofertilizers—Approaches and Advances. In Microbial Inoculants in Sustainable Agricultural Productivity; Singh, D.P., Ed.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 179–198. [Google Scholar] [CrossRef]
- Abbott, B.J. Immobilized cells. Annu. Rep. Ferment. Process. 1978, 2, 91–123. [Google Scholar]
- Fukui, S.; Tanaka, A. Immobilized microbial cells. Ann. Rev. Microbiol. 1982, 36, 145–172. [Google Scholar] [CrossRef]
- Chibata, I.; Tosa, T. Immobilized cells: Historical background. Appl. Biochem. Bioeng. 1983, 4, 1–9. [Google Scholar]
- Vassilev, N.; Vassileva, M. Production of organic acids by immobilized filamentous fungi. Mycol. Res. 1992, 96, 563–570. [Google Scholar] [CrossRef]
- Bashan, Y. Alginate Beads as Synthetic Inoculant Carriers for Slow Release of Bacteria That Affect Plant Growth. Appl. Environ. Microbiol. 1986, 51, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Vassilev, N.; Toro, M.; Vassileva, M.; Azcon, R.; Barea, J.M. Rock phosphate solubilization by encapsulated Enterobacter sp. in fermentation and soil conditions. Biores Technol. 1997, 61, 29–33. [Google Scholar] [CrossRef]
- Malusá, E.; Sas-Paszt, L.; Ciesielska, J. Technologies for beneficial microorganisms inocula used as biofertilizers. Sci. World J. 2012, 491206. [Google Scholar] [CrossRef]
- Vassilev, N.; Fenice, M.; Federici, F. Rock phosphate solubilization with gluconic acid produced by imobilized Penicillium variabile P16. Biotechnol. Technol. 1996, 10, 585–588. [Google Scholar] [CrossRef]
- Kautola, H.; Vassilev, N.; Linko, Y.Y. Itaconic acid production by immobilized Aspergillus terreus. Biotechnol. Lett. 1989, 11, 313–318. [Google Scholar] [CrossRef]
- Vassilev, N.; Medina, A.; Eichler-Löbermann, B.; Flor-Peregrín, E.; Vassileva, M. Animal Bone Char Solubilization with Itaconic Acid Produced by Free and Immobilized Aspergillus terreus Grown on Glycerol-Based Medium. Appl. Biochem. Biotechnol. 2012, 68, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Cereti, C.F.; Rossini, F.; Federici, F.; Quarantino, D.; Vassilev, N.; Fenice, M. Reuse of microbially-treated olive mill wastewater as fertilizer for wheat (Triticum durum Desf.). Biores. Technol. 2004, 91, 135–140. [Google Scholar] [CrossRef]
- Vassileva, M.; Peregrin, E.; Martos, V.; Vassilev, N. Biotechnological strategies aimed at sustainable mineral plant nutrition and food safety. J. Int. Sci. Publ. Ecol. Saf. 2012, 6, 330–340. [Google Scholar]
- Ahuja, A.; D’Souza, S.F. Bioprocess for solubilization of rock phosphate on starch based medium by Paecilomyces marquandii immobilized on polyurethane foam. Appl. Biochem. Biotechnol. 2009, 152, 1. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.Q.; Peng, H.; Zeng, S.Y.; Ji, R.Y.; Shi, K.; Huang, H.; Ji, X.J. Microbial production of plant hormones: Opportunities and challenges. Bioengineering 2017, 8, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.K.R.; Lonsane, B.K. Immobilized growing cells of Gibberella fujikuroi P-3 for production of gibberellic acid and pigment in batch and semi-continuous cultures. Appl. Microbiol. Biotechnol. 1988, 28, 537–542. [Google Scholar] [CrossRef]
- Nava-Saucedo, J.E.; Barbotin, J.N.; Thomas, D. Continuous production of gibberellic acid in a fixed-bed reactor by immobilized mycelia of Gibberella fujikuroi in calcium alginate beads. Appl. Microbiol. Biotechnol. 1989, 30, 226–233. [Google Scholar]
- Meleigy, S.A.; Khalaf, M.A. Biosynthesis of gibberellic acid from milk permeate in repeated batch operation by a mutant Fusarium moniliforme, cells immobilized on loofa sponge. Biores. Technol. 2009, 100, 374–379. [Google Scholar] [CrossRef]
- Eleazar, M.E.; Dendooven, L.; Magana, I.P.; Parra, R.; De la Torre, M. Optimization of gibberellic acid production by immobilized Gibberella fujikuroi, mycelium in fluidized bioreactors. J. Biotechnol. 2000, 76, 147–155. [Google Scholar]
- Camara, M.C.; Vandenberghe, L.P.S.; Rodrigues, C.; de Oliveira, J.; Fauld, C.; Bertrand, E.; Soccol, C.R. Current advances in gibberellic acid (GA3) production, patented technologies and potential applications. Planta 2018, 248, 1049–1062. [Google Scholar] [CrossRef]
- Muge, G.; Selma, A.T. Extractive fermentation of gibberellic acid with free and immobilized Gibberella fujikuroi. Prep. Biochem. Biotechnol. 2014, 44, 80–89. [Google Scholar]
- Teale, W.D.; Paponov, I.A.; Palme, K. Auxin in action: Signalling, transport and the control of plant growth and development. Nat. Rev. Mol. Cell Biol. 2006, 7, 847–859. [Google Scholar] [CrossRef]
- Celloto, V.R.; Oliveira, A.J.B.; Goncalves, J.E.; Watanabe, C.S.F.; Matioloi, G.; Goncalves, R.A.C. Biosynthesis of indole-3-acetic acid by new Klebsiella oxytoca free and immobilized cells on inorganic matrices. Sci. World J. 2012, 495970. [Google Scholar]
- Copping, L.G.; Duke, S.O. Natural products that have been used commercially as crop protection agents. Pest. Manag. Sci. 2007, 63, 524–554. [Google Scholar] [CrossRef]
- Tanaka, K.; Cho, S.H.; Lee, H.; Pham, A.Q.; Batek, J.M.; Cui, S.; Qiu, J.; Khan, S.M.; Joshi, T.; Zhang, Z.J.; et al. Effect of lipo-chitooligosaccharide on early growth of C4 grass seedlings. J. Exp. Bot. 2015, 66, 5727–5738. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A. Solid-state fermentation. Biochem. Eng. J. 2003, 13, 81–84. [Google Scholar] [CrossRef]
- Pandey, A.; Soccol, C.R.; Larroche, C. Current Developments in Solid State Fermentation; Asiatech Publishers Inc.: New Delhi, India, 2007; p. 517. [Google Scholar]
- Vassilev, N.; Mendes, G. Solid-state fermentation and plant-beneficial microorganisms. In Current Developments in Biotechnology and Bioengineering: Current Advances in Solid-State Fermentation; Pandey, A., Larroche, C., Soccol, C.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 402–416. [Google Scholar]
- Vassilev, N.; Fenice, M.; Jurado, E.; Reyes, A.; Nikolaeva, I.; Vassileva, M. Antagonistic Effect of Microbially-Treated Mixture of Agro-Industrial Wastes and Inorganic Insoluble Phosphate to Fusarium Wilt Disease. In Fusarium: Epidemiology, Environmental Sources and Prevention; Nova Publishers: Hauppauge, NY, USA, 2012; pp. 253–264. [Google Scholar]
- Medina, A.; Vassilev, N.; Alguacil, M.; Roldan, A.; Azcon, R. Increased plant growth, nutrient uptake and soil enzymatic activities in a decertified Mediterranean soil amended with treated residues and inoculated with native AM fungi and plant-growth-promoting yeast. Soil Sci. 2004, 169, 260–270. [Google Scholar] [CrossRef]
- Medina, A.; Vassilev, N.; Barea, J.M.; Azcon, R. Application of Aspergillus niger- treated agrowaste residue and Glomus mosseae for improving growth and nutrition of Trifolium repens in a Cd-contaminated soil. J. Biotechnol. 2005, 116, 369–378. [Google Scholar] [CrossRef]
- Vassileva, M.; Medina, A.; Reyes, A.; Martos, V.; Vassilev, N. Remediation of Heavy Metal Contaminated Soils by Phosphate-Bearing Biotechnological Products. In Bioremediation: Biotechnology, Engineering and Environmental Management; Mason, A., Ed.; Nova Publishers: Hauppauge, NY, USA, 2012; pp. 465–474. [Google Scholar]
- Vassilev, N.; Eichler-Löbermann, B.; Vassileva, M. Stress tolerant P-soubilizing microorganisms. Appl. Microbiol. Biotechnol. 2012, 95, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Mendes, G.; Silva, N.M.R.M.; Anastacio, T.C.; Vassilev, N. Optimization of Aspergillus niger phosphate solubilization in solid state fermentation and use of the resulting product as a P fertilizer. Microb. Biotechnol. 2015, 8, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Vassilev, N.; Franco, I.; Vassileva, M.; Azcon, R. Improved plant growth with rock phosphate solubilized by Aspergillus niger grown on sugar beet waste. Biores. Technol. 1996, 55, 237–241. [Google Scholar] [CrossRef]
- Ooijkaas, L.P.; Wilkinson, E.C.; Tramper, J.; Buitelaar, R. Medium optimization for spore production of Coniothyrium minitans using statistically-based experimental designs. Biotechnol. Bioeng. 1999, 64, 92–100. [Google Scholar] [CrossRef]
- Weber, F.J.; Tramper, J.; Rinzema, A. A simplified material and energy balance approach for process development and scale-up of Coniothyrium minitans conidia production by solid-state cultivation in a packed-bed reactor. Biotechnol. Bioeng. 1999, 65, 447–458. [Google Scholar] [CrossRef]
- McQuilken, M.P.; Budge, S.P.; Whipps, J.M. Production, survival and evaluation of liquid culture-produced inocula of Coniothyrium minitans against Sclerotinia sclerotiorum. Biocontrol Sci. Technol. 1997, 7, 23–36. [Google Scholar] [CrossRef]
- Pascual, S.; De Cal, A.; Magan, N.; Melgarejo, P. Surface hydrophobicity, viability and efficacy in biological control of Penicillium oxalicum spores produced in aerial and submerged culture. J. Applied Microbiol. 2000, 89, 847–853. [Google Scholar] [CrossRef]
- De la Cruz Quiroz, R.; Roussos, S.; Aguilar, C.N. Production of a biological control agent: Effect of a drying process of solid-state fermentation on viability of Trichoderma spores. Int. J. Green Technol. 2018, 4, 1–6. [Google Scholar]
- Vassilev, N.; Vassileva, M. Biotechnological solubilization of rock phosphate on media containing agro-industrial wastes. Appl. Microbiol. Biotechnol. 2003, 61, 435–440. [Google Scholar] [CrossRef]
- Boonyuen, N.; Manoch, L.; Luangsaard, J.J.; Piasai, O.; Chamswarng, C.; Chuaseeharonnachai, C.; Ueapattanakit, J.; Arnthong, J.; Sriindrasutdhi, V. Decomposition of sugarcane bagasse with lignocellulose-derived thermotolerant and thermoresistant Penicillia and Aspergilli. Int. Biodeter. Biodegr. 2014, 92, 86–100. [Google Scholar] [CrossRef]
- Vassileva, M.; Eichler-Löbermann, B.; Vassilev, N. Compatibility of P-solubilizing Aspergillus niger with bioeffectors. Int. Multidiscip. Sci. Geo Conf. Surv. Geol. Min. Ecol. Manag. SGEM 2017, 17, 601–608. [Google Scholar]
- Germec, M.; Turhan, I. Effect of pH control and aeration on inulinase production from sugarbeet molasses in a bench-scale biorreactor. Biomass Conv. Bioref. 2021. [Google Scholar] [CrossRef]
- Mattey, M. The production of organic acids. Crit. Rev. Biotechnol. 1992, 12, 87–132. [Google Scholar] [CrossRef] [PubMed]
- Netik, A.; Torres, N.V.; Riol, J.-M.; Kubicek, C.P. Uptake and export of citric acid by Aspergillus niger is reciprocally regulated by manganese ions. BBA Biomembr. 1997, 1326, 287–294. [Google Scholar] [CrossRef][Green Version]
- Juhász, T.; Szengyel, Z.; Szijártó, N.; Réczey, K. Effect of pH on cellulase production of Trichoderma reesei RUT C30. Appl. Biochem. Biotechnol. 2004, 113–116, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Haei, M.; Rousk, J.; Ilstedt, U.; Öquist, M.; Baath, E.; Laudon, H. Effects of soil frost on growth, composition and respiration of the soil microbial decomposer community. Soil Biol. Biochem. 2011, 43, 2069–2077. [Google Scholar] [CrossRef]
- Lobo, C.B.; Juárez Tomás, M.S.; Viruel, E.; Ferrero, M.A.; Lucca, M.E. Development of low-cost formulations of plant growth-promoting bacteria to be used as inoculants in beneficial agricultural technologies. Microbiol. Res. 2019, 219, 12–25. [Google Scholar] [CrossRef]
- Hernández, A.; Larsson, C.U.; Sawicki, R.; van Niel, E.W.J.; Roos, S.; Håkansson, S. Impact of the fermentation parameters pH and temperature on stress resilience of Lactobacillus reuteri DSM 17938. AMB Expr. 2019, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Kautola, H.; Vassilev, N.; Linko, Y.Y. Continuous Itaconic Acid Production by Immobilized Biocatalysts. J. Biotechnol. 1990, 116, 369–378. [Google Scholar] [CrossRef]
- Bodizs, L.; Titica, M.; Faria, N.; Srinivasan, B.; Dochain, D.; Bonvin, D. Oxygen control for an industrial pilot-scale fed-batch filamentous fungal fermentation. J. Proc. Control. 2007, 17, 595–606. [Google Scholar] [CrossRef][Green Version]
- Tang, W.; Pan, A.; Lu, H.; Xia, J.; Zhuang, Y.; Zhang, S.; Chu, J.; Noorman, H. Improvement of glucoamylase production using axial impellers with low power consumption and homogeneous mass transfer. Biochem. Eng. J. 2015, 99, 167–176. [Google Scholar] [CrossRef]
- Hamrouni, R.; Molinet, J.; Mitropoulou, G.; Kourkoutas, Y.; Dupuy, N.; Masmoudi, A.; Roussos, S. From flasks to single used bioreactor: Scale-up of solid state fermentation process for metabolites and conidia production by Trichoderma asperellum. J. Environ. Manag. 2019, 252, 109496. [Google Scholar] [CrossRef]
- Costa, J.A.V.; Treichel, H.; Kumar, V.; Pandey, A. Advances in Solid-State Fermentation. In Current Developments in Biotechnology and Bioengineering: Current Advances in Solid-State Fermentation; Pandey, A., Larroche, C., Soccol, C.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–17. [Google Scholar]
- Magnuson, J.K.; Lasure, L.L. Organic Acid Production by Filamentous Fungi. In Advances in Fungal Biotechnology for Industry, Agriculture, and Medicine; Tkacz, J.S., Lange, L., Eds.; Springer: Boston, MA, USA, 2004. [Google Scholar] [CrossRef]
- Papagianni, M. Fungal morphology and metabolite production in submerged mycelial processes. Biotechnol. Adv. 2004, 22, 189–259. [Google Scholar] [CrossRef]
- Tang, Y.J.; Zhong, J.J. Role of oxygen supply in submerged fermentation of Ganoderma lucidum for production of Ganoderma polysaccharide and ganoderic acid. Enz. Microb. Technol. 2003, 32, 478–484. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Liu, J.H.; Zhou, Y.M.; Zhang, Y.Y.; Liu, Y.; Gong, T.Y.; Wang, J. A new two-phase kinetic model of sporulation of Clonostachys rosea in a new solid-state fermentation reactor. Proc. Biochem. 2013, 48, 1119–1125. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK, 2008. [Google Scholar]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; De Pascale, S.; Bonini, P.; Colla, G. Arbuscular mycorrhizal fungi as a biostimulants in horticultural crops. Sci. Horticul. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Giovannetti, M.; Avio, L.; Sbrana, C. Improvement of nutraceutical value of food by plant symbionts. In Natural Products, Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Merillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 2641–2662. [Google Scholar]
- Barea, J.M.; Pozo, M.J.; Azcon, R.; Azcon-Anguilar, C. Microbial cooperation in the rhizosphere. J. Exp. Bot 2005, 56, 1761–1778. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, R.; Adholeya, A. Biotechnological Advancements in Industrial Production of Arbuscular Mycorrhizal Fungi: Achievements, Challenges, and Future Prospects. In Developments in Fungal Biology and Applied Mycology; Satyanarayana, T., Deshmukh, S.K., Johri, B.N., Eds.; Springer: Singapore, 2017; pp. 413–431. [Google Scholar]
- Vassilev, N.; Nikolaeva, I.; Vassileva, M. Polymer-based preparation of soil inoculants: Applications to arbuscular mycorrhizal fungi. Rev. Environ. Sci. Bio Technol. 2005, 4, 235–243. [Google Scholar] [CrossRef]
- Mugnier, J.; Mosse, B. Vesicular–arbuscular mycorrhizal infection in transformed root inducing T-DNA roots grown axenically. Phytopathology 1987, 77, 1045–1050. [Google Scholar] [CrossRef]
- Becard, G.; Piche, Y. New aspects on the acquisition of biotrophic status by a vesicular–arbuscular mycorrhizal fungus, Gigaspora margarita. N. Phytol. 1989, 112, 77–83. [Google Scholar] [CrossRef]
- Hung, L.-L.; O’Keefe, D.; Sylvia, D. Use of hydrogel as a sticking agent and carrier for vesicular–arbuscular mycorrhizal fungi. Mycol. Res. 1991, 95, 427–429. [Google Scholar] [CrossRef]
- Jarstfer, A.G.; Sylvia, D. Inoculum production and inoculation strategies for vesicular–arbuscular mycorrhizal fungi. In Soil Microbial Ecology: Applications in Agriculture and Environmental Management; Metting, B., Ed.; Marcel Dekker: New York, NY, USA, 1995; pp. 349–377. [Google Scholar]
- Verma, A.; Aldholeya, A. Cost-economics of existing methodologies for inoculum production of vesicular–arbuscular mycorrhizal fungi. In Concepts in Mycorrhizal Research; Mukerji, K.G., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996; pp. 179–194. [Google Scholar]
- St-Arnaud, M.C.; Hamel, C.; Vimard, B.; Caron, M.; Fortin, J.A. Enhanced hyphal growth and spore production of the arbuscular mycorrhizal fungus Glomus intraradices in an in vitro system in the absence of host roots. Mycol. Res. 1996, 100, 328–332. [Google Scholar] [CrossRef]
- Douds, D.D., Jr. Increased spore production by Glomus intraradices in the split-plate monoxenic culture system by repeated harvest, gel replacement, and resupply of glucose to the mycorrhiza. Mycorrhiza 2002, 12, 163–167. [Google Scholar] [CrossRef] [PubMed]
- El-Tarabily, K.A.; Sivasithamparam, K. Potential of yeasts as biocontrol agents of soil-borne fungal plant pathogens and as plant growth promoters. Mycoscience 2006, 47, 25–35. [Google Scholar] [CrossRef]
- Mukherjee, A.; Verma, J.P.; Gaurav, A.K.; Chouhan, G.K.; Patel, J.S.; Hesham, A.E. Yeast a potential bio-agent: Future for plant growth and postharvest disease management for sustainable agriculture. Appl. Microbiol. Biotechnol. 2020, 104, 1497–1510. [Google Scholar] [CrossRef] [PubMed]
- Limpens, E.; van Zeijl, A.; Geurts, R. Lipochitooligosaccharides modulate plant host immunity to enable endosymbiosis. Annu. Rev. Phytopathol. 2015, 53, 311–334. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.A.; Janarthanam, L. Polymicrobial Formulations for Enhancing Plant Productivity. U.S. Patent US8822190B2, 9 February 2014. [Google Scholar]
- Allaga, H.; Bóka, B.; Poór, P.; Nagy, V.D.; Szűcs, A.; Stankovics, I.; Takó, M.; Manczinger, L.; Vágvölgyi, C.; Kredics, L.; et al. A composite bioinoculant based on the combined application of beneficial bacteria and fungi. Agronomy 2020, 10, 220. [Google Scholar] [CrossRef]
- Bitterlich, M.; Rouphael, Y.; Graefe, J.; Franken, P. Arbuscular Mycorrhizas: A Promising Component of Plant Production Systems Provided Favorable Conditions for Their Growth. Front. Plant Sci. 2018, 9, 1329. [Google Scholar] [CrossRef]
- Dar, R.A.; Parmar, M.; Dar, E.A.; Sani, R.K.; Phutela, U.G. Biomethanation of agricultural residues: Potential, limitations and possible solutions. Renew. Sustain. Energy Rev. 2021, 135, 110217. [Google Scholar] [CrossRef]
- Mahjoub, B.; Domscheit, E. Chances and challenges of an organic waste–based bioeconomy. Curr. Opin. Green Sustain. Chem. 2020, 25, 100388. [Google Scholar] [CrossRef]
- Anonymous. Biopesticides Market Size, Share and Industry Analysis by Product Type, Source, Mode of Application, Crops and Regional Forecast 2018–2025; Fortune Business Insights: Maharashtra, India, September 2020. [Google Scholar]
- Anonymous. Biofertilizers Market by Product, Microorganism Type and Application, Crop Type: Global Opportunity Analysis and Industry Forecast, 2019–2026; Fortune Business Insights: Maharashtra, India, September 2019. [Google Scholar]
- Regulation EU 2019/1009. Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 laying down rules on the making available on the market of EU fertilising products and amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and repealing Regulation (EC) No 2003/2003. Off. J. Eur. Union 2019, 62, 1–114. [Google Scholar]

| Fermentation Mode | Utilization Mode | Materials Used | References |
|---|---|---|---|
| Liquid submerged | Substrate source | Glycerol | [21] |
| Agro-industrial wastes | [26] | ||
| Cheese whey | [27] | ||
| Malt sprouts | [28] | ||
| Beet and cane molasses | [24] and ref. therein | ||
| Sewage sludge | [29] | ||
| Substrate additive | Cotton-seed | [30] | |
| Soy flours | [30] | ||
| Biochar | [31] | ||
| Solid State | Substrate source | Corn steep liquor | [32] |
| Grape marc and wine lees | [33] | ||
| Straw | [34] | ||
| Wheat bran | [34] | ||
| Cereal grains (rice, barley, rye, wheat, sorghum, corn) | [35] | ||
| Olive mill solid wastes | |||
| Sugar cane bagasse | [36,37,38] | ||
| Sugar beet wastes | [36] | ||
| Vinasse | [31] | ||
| [24] and ref. therein | |||
| Substrate additive | Glycerol | [39,40] | |
| Biochar | [41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vassileva, M.; Malusà, E.; Sas-Paszt, L.; Trzcinski, P.; Galvez, A.; Flor-Peregrin, E.; Shilev, S.; Canfora, L.; Mocali, S.; Vassilev, N. Fermentation Strategies to Improve Soil Bio-Inoculant Production and Quality. Microorganisms 2021, 9, 1254. https://doi.org/10.3390/microorganisms9061254
Vassileva M, Malusà E, Sas-Paszt L, Trzcinski P, Galvez A, Flor-Peregrin E, Shilev S, Canfora L, Mocali S, Vassilev N. Fermentation Strategies to Improve Soil Bio-Inoculant Production and Quality. Microorganisms. 2021; 9(6):1254. https://doi.org/10.3390/microorganisms9061254
Chicago/Turabian StyleVassileva, Maria, Eligio Malusà, Lidia Sas-Paszt, Pawel Trzcinski, Antonia Galvez, Elena Flor-Peregrin, Stefan Shilev, Loredana Canfora, Stefano Mocali, and Nikolay Vassilev. 2021. "Fermentation Strategies to Improve Soil Bio-Inoculant Production and Quality" Microorganisms 9, no. 6: 1254. https://doi.org/10.3390/microorganisms9061254
APA StyleVassileva, M., Malusà, E., Sas-Paszt, L., Trzcinski, P., Galvez, A., Flor-Peregrin, E., Shilev, S., Canfora, L., Mocali, S., & Vassilev, N. (2021). Fermentation Strategies to Improve Soil Bio-Inoculant Production and Quality. Microorganisms, 9(6), 1254. https://doi.org/10.3390/microorganisms9061254










