Ecological Role of Volatile Organic Compounds Emitted by Pantoea agglomerans as Interspecies and Interkingdom Signals
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Plants
2.2. Evaluation of Compatibility between Pantoea agglomerans MVC 21 and Pseudomonas putida MVC17
2.3. Assessment of the Interaction Effect on Plant Growth-Promoting Activities of Pantoea agglomerans MVC 21 and Pseudomonas putida MVC 17
2.4. Evaluation of the Effect of VOCs Emitted by Pantoea agglomerans MVC 21 and Pseudomonas putida MVC 17 on Tomato Plant Growth
2.5. Metabolite Profiling of VOCs Emitted by Pantoea agglomerans MVC 21
2.5.1. Preparation of Samples for Headspace Analysis
2.5.2. Headspace GC-MS Analysis
2.5.3. Preparation of Volatile Organic Compound Solutions for Functional Assays
2.6. Evaluation of the Effect of Pure VOCs on Tomato Plant Growth and Plant Growth-Promoting Traits of Pseudomonas putida MVC 17
2.7. Effect of VOCs Released by Pantoea agglomerans MVC 21 on the Interaction between Pseudomonas putida MVC 17 and Tomato Seedlings
2.8. Evaluation of the Effect of Dimethyl Disulfide and Volatile Organic Compounds Emitted by Pantoea agglomerans MVC 21 on the Production of Indole-3-Acetic Acid in Pseudomonas putida MVC 17
2.9. Statistical Analysis
3. Results
3.1. Compatibility between Pantoea agglomerans MVC 21 and Pseudomonas putida MVC 17
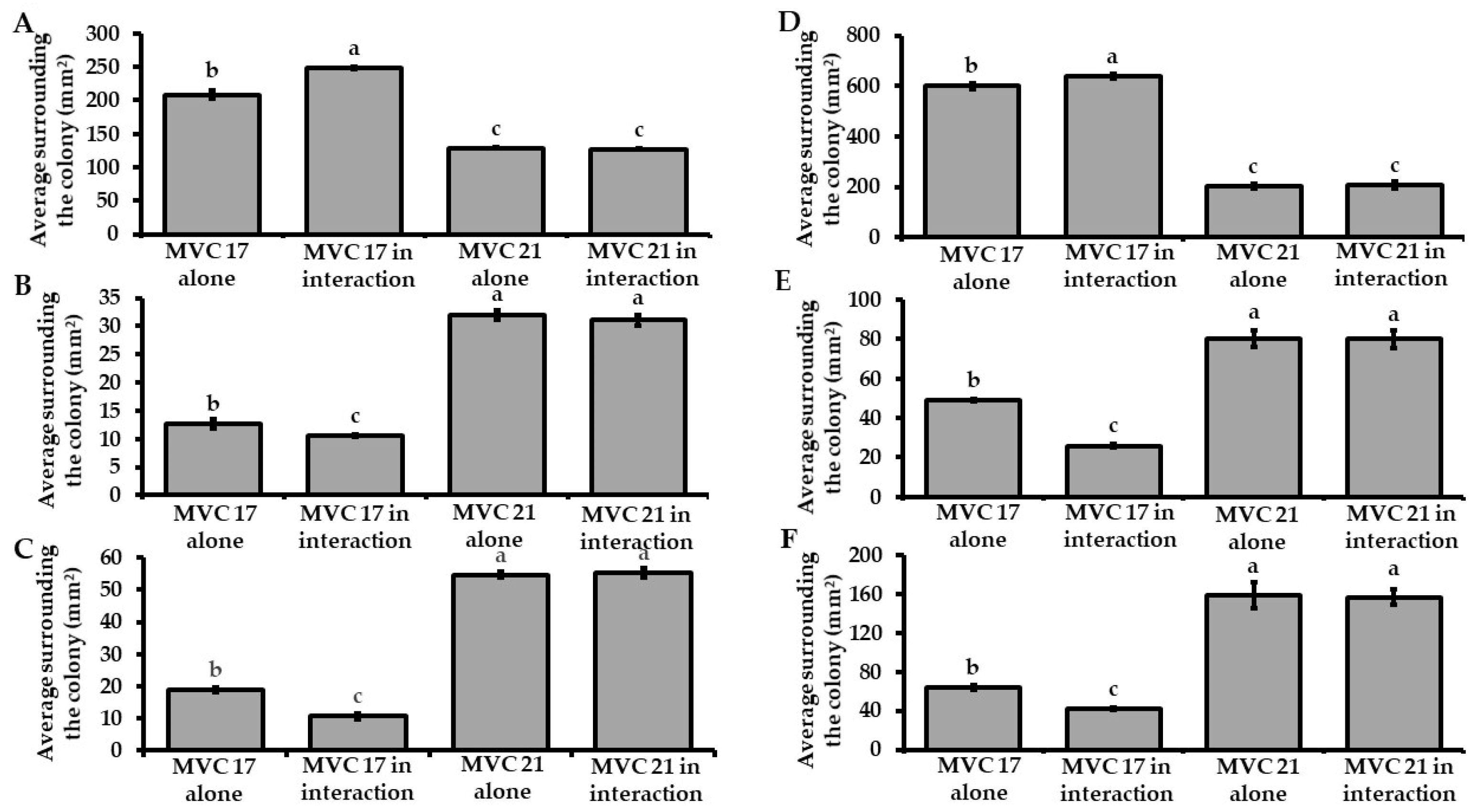
3.2. Interaction with Pantoea agglomerans MVC 21 Modulates the Plant Growth-Promoting Activities of Pseudomonas putida MVC 17
3.3. Pantoea agglomerans MVC 21 Releases VOCs with a Positive Impact on Tomato Seedling Growth
3.4. Headspace Analysis of VOCs Using GC-MS Only Few VOCs Were Produced at Levels Above the Limit of Detection of the Applied GC-MS Method
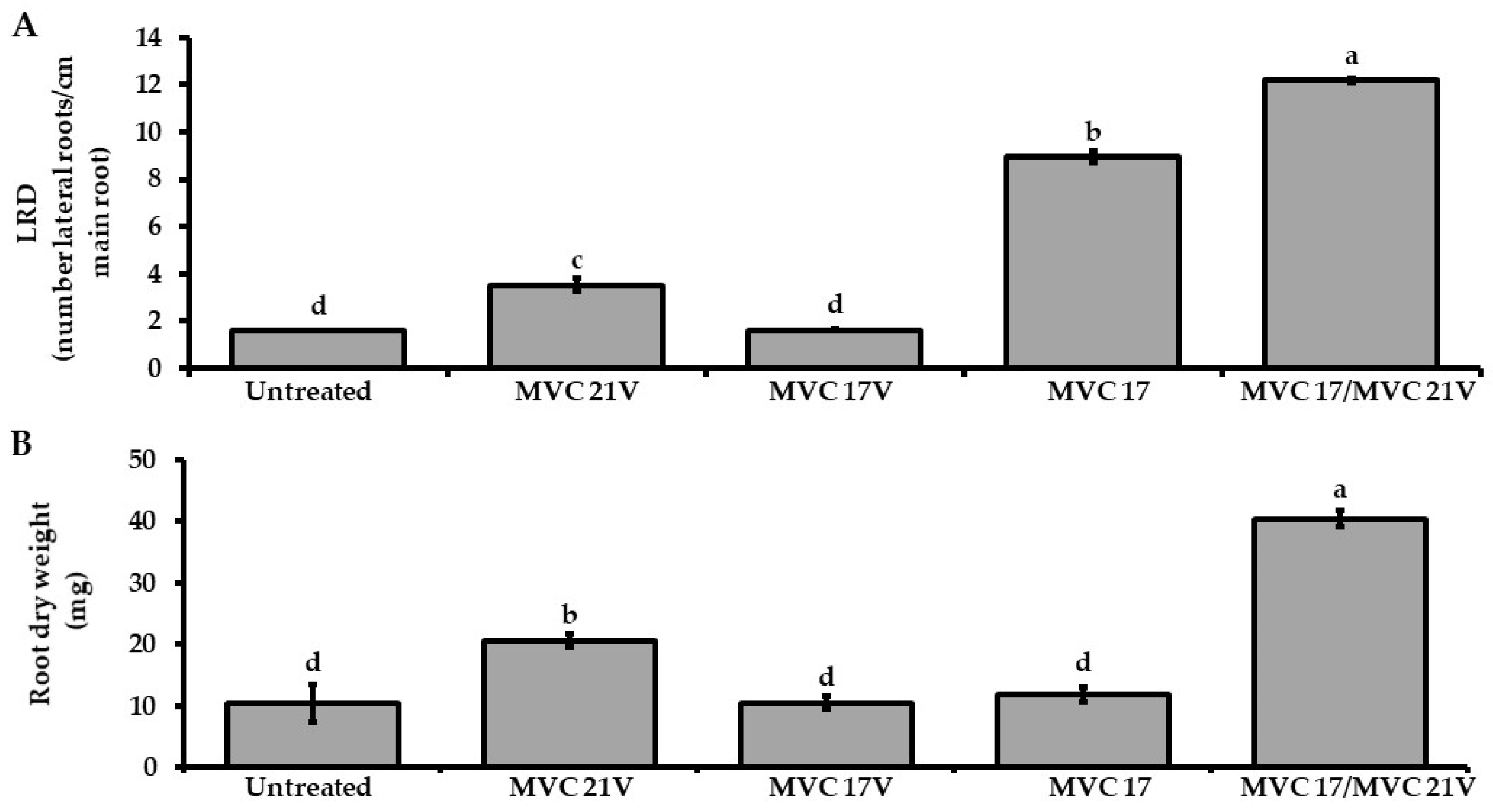
3.5. Dimethyl Disulfide Shows a Positive Effect on Pseudomonas putida MVC 17 Plant Growth-Promoting Activities and on Tomato Plant Growth
3.6. Dimethyl Disulfide and VOCs Emitted by Pantoea agglomerans MVC 21 Affect the Interaction between Pseudomonas putida MVC17 and Tomato Seedlings
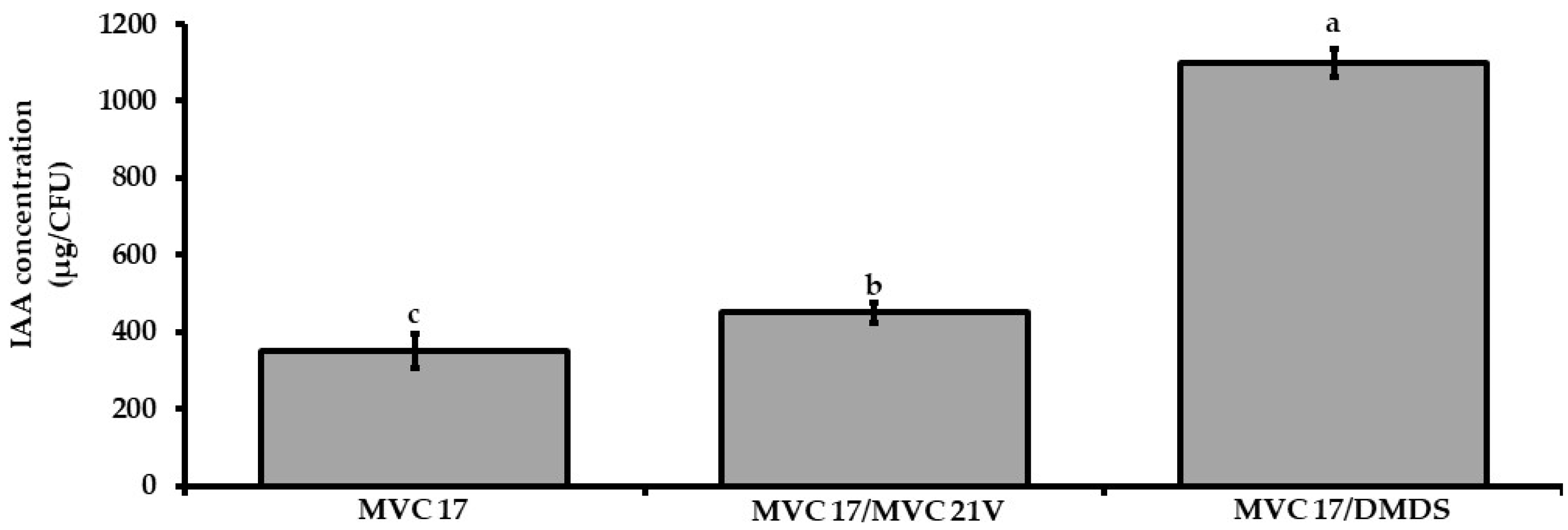
3.7. Dimethyl Disulfide and VOCs Emitted by Pantoea agglomerans MVC 21 Show a Positive Effect on the Production of Indole-3-Acetic Acid by Pseudomonas putida MVC 21
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bakker, P.A.H.M.; Berendsen, R.L.; Doornbos, R.F.; Wintermans, P.C.A.; Pieterse, C.M.J. The rhizosphere revisited: Root microbiomics. Front. Plant Sci. 2013, 4, 1–7. [Google Scholar] [CrossRef]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663R. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Paulitz, T.C.; Steinberg, C.; Alabouvette, C.; Moënne-Loccoz, Y. The rhizosphere: A playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 2009, 321, 341–361. [Google Scholar] [CrossRef]
- Leach, J.E.; Triplett, L.R.; Argueso, C.T.; Trivedi, P. Communication in the Phytobiome. Cell 2017, 169, 587–596. [Google Scholar] [CrossRef]
- Venturi, V.; Keel, C. Signalling in the Rhizosphere. Trends Plant Sci. 2016, 21, 187–198. [Google Scholar] [CrossRef]
- Waters, C.M.; Bassler, B.L. Quorum sensing: Cell-to-cell communication in bacteria. Annu Rev Cell Dev. Biol. 2005, 21, 319–346. [Google Scholar] [CrossRef] [PubMed]
- Fuqua, W.C.; Winans, S.C. A LuxR-LuxI type regulatory system activates Agrobacterium Ti plasmid conjugal transfer in the presence of a plant tumor metabolite. J. Bacteriol. 1994, 176, 2796–2806. [Google Scholar] [CrossRef] [PubMed]
- Maddula, V.S.; Zhang, Z.; Pierson, E.A.; Pierson, L.S., III. Quorum sensing and phenazines are involved in biofilm formation by Pseudomonas chlororaphis (aureofaciens) strain 30-84. Microb. Ecol. 2006, 52, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Pierson, E.A.; Wood, D.W.; Cannon, J.A.; Blachere, F.M.; Pierson, L.S., III. Interpopulation signaling via N-acyl-homoserine lactone among bacteria in the wheat rhizosphere. Mol. Plant Microbe Interact. 1998, 11, 1078–1084. [Google Scholar] [CrossRef]
- Evans, K.C.; Benomar, S.; Camuy-Vélez, L.A.; Nasseri, E.B.; Wang, X.; Neuenswander, B.; Chandler, J.R. Quorum-sensing control of antibiotic resistance stabilizes cooperation in Chromobacterium violaceum. ISME J. 2018, 12, 1263–1272. [Google Scholar] [CrossRef]
- Lloyd, D.G.; Whitworth, D.E. The myxobacterium Myxococcus xanthus can sense and respond to the quorum signals secreted by potential prey organisms. Front. Microbiol. 2017, 8, 439. [Google Scholar] [CrossRef]
- Venturi, V.; Fuqua, C. Chemical signaling between plants and plant-pathogenic bacteria. Annu Rev Phytopathol 2013, 51, 17–37. [Google Scholar] [CrossRef]
- Fuqua, C.; Parsek, M.R.; Greenberg, E.P. Regulation of gene expression by cell-to-cell communication: Acyl-homoserine lactone quorum sensing. Annu. Rev. Genet. 2001, 35, 439–468. [Google Scholar] [CrossRef]
- Abisado, R.G.; Benomar, S.; Klaus, J.R.; Dandekar, A.A.; Chandler, J.R. Bacterial quorum sensing and microbial community interactions. mBio 2018, 9, e02331-17. [Google Scholar] [CrossRef]
- Nawaz, M.S.; Arshad, A.; Rajput, L.; Fatima, K.; Ullah, S.; Ahmad, M.; Imran, A. Growth-stimulatory effect of quorum sensing signal molecule n-acyl-homoserine lactone-producing multi-trait Aeromonas spp. on wheat genotypes under salt stress. Front Microbiol. 2020, 11, 553621. [Google Scholar] [CrossRef]
- Schenk, S.T.; Stein, E.; Kogel, K.H.; Schikora, A. Arabidopsis growth and defense are modulated by bacterial quorum sensing molecules. Plant Signal Behav. 2012, 7, 178–181. [Google Scholar] [CrossRef]
- Schikora, A.; Schenk, S.T.; Stein, E.; Molitor, A.; Zuccaro, A.; Kogel, K.H. N-acyl-homoserine lactone confers resistance toward biotrophic and hemibiotrophic pathogens via altered activation of AtMPK6. Plant Physiol. 2011, 157, 1407–1418. [Google Scholar] [CrossRef]
- Schulz-Bohm, K.; Martín-Sánchez, L.; Garbeva, P. Microbial volatiles: Small molecules with an important role in intra- and inter-kingdom interactions. Front Microbiol. 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Schulz, S.; Dickschat, J.S. Bacterial volatiles: The smell of small organisms. Nat. Prod. Rep. 2007, 24, 814–842. [Google Scholar] [CrossRef]
- Audrain, B.; Farag, M.A.; Ryu, C.M.; Ghigo, J.M. Role of bacterial volatile compounds in bacterial biology. FEMS Microbiol. Rev. 2015, 39, 222–233. [Google Scholar] [CrossRef]
- Zhang, H.; Kim, M.S.; Krishnamachari, V.; Payton, P.; Sun, Y.; Grimson, M.; Farag, M.A.; Ryu, C.-M.; Allen, R.; Melo, I.S.; et al. Rhizobacterial volatile emissions regulate auxin homeostasis and cell expansion in Arabidopsis. Planta 2007, 226, 839–851. [Google Scholar] [CrossRef]
- Aziz, M.; Nadipalli, R.K.; Xie, X.; Sun, Y.; Surowiec, K.; Zhang, J.L.; Paré, P.W. Augmenting sulfur metabolism and herbivore defense in Arabidopsis by bacterial volatile signaling. Front. Plant Sci. 2016, 27, 1–14. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Y.; Xie, X.; Kim, M.S.; Dowd, S.E.; Paré, P.W. A soil bacterium regulates plant acquisition of iron via deficiency-inducible mechanisms. Plant J. 2006, 58, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Farag, M.A.; Park, H.B.; Kloepper, J.W.; Lee, S.H.; Ryu, C.M. Induced Resistance by a Long-Chain Bacterial Volatile: Elicitation of Plant Systemic Defense by a C13 Volatile Produced by Paenibacillus polymyxa. PLoS ONE 2012, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kim, M.S.; Sun, Y.; Dowd, S.E.; Shi, H.; Paré, P.W. Soil bacteria confer plant salt tolerance by tissue-specific regulation of the sodium transporter HKT1. Mol. Plant Microbe Interact. 2008, 21, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xie, X.; Kim, M.S.; Kornyeyev, D.A.; Holaday, S.; Paré, P.W. Soil bacteria augment Arabidopsis photosynthesis by decreasing glucose sensing and abscisic acid levels in planta. Plant J. 2008, 56, 264–273. [Google Scholar] [CrossRef]
- Tyc, O.; Putra, R.; Gols, R.; Harvey, J.A.; Garbeva, P. The ecological role of bacterial seed endophytes associated with wild cabbage in the United Kingdom. Microbiol. Open 2020, 9, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Wood, T.K.; Lee, J. Roles of indole as an interspecies and interkingdom signaling molecule. Trends Microbiol. 2015, 23, 707–718. [Google Scholar] [CrossRef]
- Wang, D.; Ding, X.; Rather, P.N. Indole can act as an extracellular signal in Escherichia coli. J. Bacteriol. 2001, 183, 4210–4216. [Google Scholar] [CrossRef]
- Garbeva, P.; Hordijk, C.; Gerards, S.; de Boer, W. Volatile-mediated interactions between phylogenetically different soil bacteria. Front. Microbiol. 2014, 5, 1–9. [Google Scholar] [CrossRef]
- Raio, A.; Brilli, F.; Baraldi, R.; Neri, L.; Puopolo, G. Impact of spontaneous mutations on physiological traits and biocontrol activity of Pseudomonas chlororaphis M71. Microbiol. Res. 2020, 239, 126517. [Google Scholar] [CrossRef]
- Tyc, O.; van den Berg, M.; Gerards, S.; Van Veen, J.A.; Raaijmakers, J.M.; de Boer, W.; Garbeva, P. Impact of interspecific interactions on antimicrobial activity among soil bacteria. Front. Microbiol. 2014, 5, 1–10. [Google Scholar] [CrossRef]
- Vasseur-Coronado, M.; Dupré du Boulois, H.; Pertot, I.; Puopolo, G. Selection of plant growth promoting rhizobacteria sharing suitable features to be commercially developed as biostimulant products. Microbiol. Res. 2021, 245, 126672. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant 1962, 15, 474–497. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Cordovez, V.; Schop, S.; Hordijk, K.; Dupré de Boulois, H.; Coppens, F.; Hanssen, I.; Raaijmakers, J.M.; Carrión, V. Priming of plant growth promotion by volatiles of root-associated Microbacterium spp. Appl. Environ. Microbiol. 2018, 84, e01865-18. [Google Scholar] [CrossRef]
- Lazazzara, V.; Perazzolli, M.; Pertot, I.; Biasioli, F.; Puopolo, G.; Cappellin, L. Growth media affect the volatilome and antimicrobial activity against Phytophthora infestans in four Lysobacter type strains. Microbiol. Res. 2017, 201, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Vlassi, A.; Nesler, A.; Perazzolli, M.; Lazazzara, V.; Büschl, C.; Parich, A.; Puopolo, G.; Schuhmacher, R. Volatile organic compounds from Lysobacter capsici AZ78 as potential candidates for biological control of soilborne plant pathogens. Front. Microbiol. 2020, 1, 1748. [Google Scholar] [CrossRef]
- Hiller, K.; Hangebrauk, J.; Jäger, C.; Spura, J.; Schreiber, K.; Schomburg, D. Metabolite detector: Comprehensive analysis tool for targeted and nontargeted GC/MS based metabolome analysis. Anal Chem. 2009, 81, 3429–3439. [Google Scholar] [CrossRef] [PubMed]
- Blaženović, I.; Kind, T.; Ji, J.; Fiehn, O. Software tools and approaches for compound identification of LC-MS/MS data in metabolomics. Metabolites 2018, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Etalo, D.W.; Jager, V.; de Gerards, S.; Zweers, H.; de Boer, W.; Garbeva, P. Microbial small talk: Volatiles in fungal-bacterial interactions. Front. Microbiol. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Meldau, D.G.; Meldau, S.; Hoang, L.H.; Underberg, S.; Wünsche, H.; Baldwin, I.T. DMDS produced by the naturally associated bacterium Bacillus sp B55 promotes Nicotiana attenuata growth by enhancing sulfur nutrition. Plant Cell 2013, 25, 2731–2747. [Google Scholar] [CrossRef] [PubMed]
- Tyc, O.; Song, C.; Dickschat, J.S.; Vos, M.; Garbeva, P. The ecological role of volatile and soluble secondary metabolites produced by soil bacteria. Trends Microbiol. 2017, 25, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Malboobi, M.A.; Behbahani, M.; Madani, H.; Owlia, P.; Deljou, A.; Yakhchali, B.; Moradi, M.; Hassanabadi, H. Performance evaluation of potent phosphate solubilizing bacteria in potato rhizosphere. World J. Microbiol. Biotechnol. 2009, 25, 1479–1484. [Google Scholar] [CrossRef]
- Marchi, G.; Sisto, A.; Cimmino, A.; Andolfi, A.; Cipriani, M.G.; Evidente, A.; Surico, G. Interaction between Pseudomonas savastanoi pv. savastanoi and Pantoea agglomerans in olive knots. Plant Pathol. 2006, 55, 614–624. [Google Scholar] [CrossRef]
- Hosni, T.; Moretti, C.; Devescovi, G.; Suarez-Moreno, Z.R.; M’ Barek, F.; Guarnaccia, C.; Pongor, S.; Onofri, A.; Buonaurio, R.; Venturi, V. Sharing of quorum-sensing signals and role of interspecies communities in a bacterial plant disease. ISME J. 2011, 5, 1857–1870. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Lee, S.; Ryu, C.M. Interspecific bacterial sensing through airborne signals modulates locomotion and drug resistance. Nat. Commun. 2013, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Molina-Santiago, C.; Daddaoua, A.; Fillet, S.; Duque, E.; Ramos, J.L. Interspecies signalling: Pseudomonas putida efflux pump TtgGHI is activated by indole to increase antibiotic resistance. Environm. Microbiol. 2014, 16, 1267–1281. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, J.; Wang, L.; Liu, S.; Bai, Z.; Zhuang, X.; Zhuang, G. Environmental adaptability and quorum sensing: Iron uptake regulation during biofilm formation by Paracoccus denitrificans. Appl. Environ. Microb. 2018, 84, 1–15. [Google Scholar] [CrossRef]
- Fong, K.P.; Gao, L.; Demuth, D.R. luxS and arcB control aerobic growth of Actinobacillus actinomycetemcomitans under iron limitation. Infect Immun. 2003, 71, 298–308. [Google Scholar] [CrossRef]
- James, C.E.; Hasegawa, Y.; Park, Y.; Yeung, V.; Tribble, G.D.; Kuboniwa, M.; Demuth, D.R.; Lamont, R.J. LuxS involvement in the regulation of genes coding for hemin and iron acquisition systems in Porphyromonas gingivalis. Infect. Immun. 2006, 74, 3834–3844. [Google Scholar] [CrossRef] [PubMed]
- Bredenbruch, F.; Geffers, R.; Nimtz, M.; Buer, J.; Häussler, S. The Pseudomonas aeruginosa quinolone signal (PQS) has an iron-chelating activity. Environ. Microbiol. 2006, 8, 1318–1329. [Google Scholar] [CrossRef]
- Van Mooy, B.A.S.; Hmelo, L.R.; Sofen, L.E.; Campagna, S.R.; May, A.L.; Dyhrman, S.T.; Heithoff, A.; Webb, E.A.; Momper, L.; Mincer, T.J. Quorum sensing control of phosphorus acquisition in Trichodesmium consortia. ISME J. 2006, 6, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Luziatelli, F.; Ficca, A.G.; Cardarelli, M.; Melini, F.; Cavalieri, A.; Ruzzi, M. Genome sequencing of Pantoea agglomerans C1 provides Insights into molecular and genetics mechanisms of plant growth-promotion and tolerance to heavy metals. Microorganisms 2020, 8, 153. [Google Scholar] [CrossRef]
- Malboobi, M.A.; Owlia, P.; Behbahani, M.; Sarokhani, E.; Moradi, S.; Yakhchali, B.; Deljou, A.; Heravi, K.M. Solubilization of organic and inorganic phosphates by three highly efficient soil bacterial isolates. World J. Microbiol. Biotechnol. 2009, 25, 1471–1477. [Google Scholar] [CrossRef]
- Omer, Z.S.; Björkman, P.O.; Nicander, B.; Tillberg, E.; Gerhardson, B. 5′-Deoxyisopentenyladenosine and other cytokinins in culture filtrates of the bacterium Pantoea agglomerans. Physiol. Plant. 2004, 121, 439–447. [Google Scholar] [CrossRef]
- Quecine, M.C.; Araújo, W.L.; Rossetto, P.B.; Ferreira, A.; Tsui, S.; Lacava, P.T.; Mondin, M.; Azevedo, J.L.; Pizzirani-Kleiner, A.A. Sugarcane growth promotion by the endophytic bacterium Pantoea agglomerans 33.1. Appl. Environ. Microbiol. 2012, 78, 7511–7518. [Google Scholar] [CrossRef]
- Viruel, E.; Lucca, M.E.; Siñeriz, F. Plant growth promotion traits of phosphobacteria isolated from Puna, Argentina. Arch. Microbiol. 2011, 193, 489–496. [Google Scholar] [CrossRef]
- Shariati, V.; Malboobi, M.A.; Tabrizi, Z.; Tavakol, E.; Owilia, P.; Safari, M. Comprehensive genomic analysis of a plant growth-promoting rhizobacterium Pantoea agglomerans strain P5. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Tyc, O.; Zweers, H.; de Boer, W.; Garbeva, P. Volatiles in inter-specific bacterial interactions. Front. Microbiol. 2015, 6, 1–15. [Google Scholar] [CrossRef]
- Popova, A.A.; Koksharova, O.A.; Lipasova, V.A.; Zaitseva, J.V.; Katkova-Zhukotskaya, O.A.; Eremina, S.I.; Mironov, A.S.; Chernin, L.S.; Khmel, I.A. Inhibitory and toxic effects of volatiles emitted by strains of Pseudomonas and Serratia on growth and survival of selected microorganisms, Caenorhabditis elegans and Drosophila melanogaster. Biomed. Res. Int. 2014, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Dandurishvili, N.; Toklikishvili, N.; Ovadis, M.; Eliashvili, P.; Giorgobiani, N.; Keshelava, R.; Tediashvili, M.; Vainstein, A.; Khmel, I.; Szegedi, E.; et al. Broad-range antagonistic rhizobacteria Pseudomonas fluorescens and Serratia plymuthica suppress Agrobacterium crown gall tumours on tomato plants. J. Appl. Microbiol. 2011, 110, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Asari, S.; Matzén, S.; Petersen, M.A.; Bejai, S.; Meijer, J. Multiple effects of Bacillus amyloliquefaciens volatile compounds: Plant growth promotion and growth inhibition of phytopathogens. FEMS Microbiol. Ecol. 2016, 92, 1–11. [Google Scholar] [CrossRef]
- Huang, C.J.; Tsay, J.F.; Chang, S.; Yang, H.P.; Wu, W.S.; Chen, C.Y. DMDS is an induced systemic resistance elicitor produced by Bacillus cereus C1L. Pest Manag. Sci. 2012, 68, 1306–1310. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Solís, D.; Zetter-Salmón, E.; Contreras-Pérez, M.; Rocha-Granados, M.D.C.; Macías-Rodríguez, L.; Santoyo, G. Pseudomonas stutzeri E25 and Stenotrophomonas maltophilia CR14 endophytes produce antifungal volatile organic compounds and exhibit additive plant growth-promoting effects. Biocatal. Agric. Biotechnol. 2018, 13, 46–52. [Google Scholar] [CrossRef]
- Groenhagen, U.; Baumgartner, R.; Bailly, A.; Gardiner, A.; Eberl, L.; Schulz, S.; Weisskopf, L. Production of Bioactive Volatiles by Different Burkholderia ambifaria Strains. J. Chem. Ecol. 2013, 39, 892–906. [Google Scholar] [CrossRef] [PubMed]
- Bailly, A.; Groenhagen, U.; Schulz, S.; Geisler, M.; Eberl, L.; Weisskopf, L. The inter kingdom volatile signal indole promotes root development by interfering with auxin signalling. Plant J. 2014, 80, 758–771. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Kim, K.; Cho, M.; Lee, K.J. Volatile DMDS affects root system architecture of Arabidopsis via modulation of canonical auxin signaling pathways. Environ. Sustain. 2019, 2, 211–216. [Google Scholar] [CrossRef]

| Metabolite 1 | RI 2 | Sim Score 3 | Level of Identification 4 | HS Vials 5 |
|---|---|---|---|---|
| Dimethyl disulfide | 735 | 0.98 | 1 | 10/10 |
| 3-Methyl-1-butanol | 752 | 0.99 | 1 | 10/10 |
| 2-Phenylethyl-alcohol | 1115 | 0.97 | 1 | 10/10 |
| 2-Tridecanone | 1498 | 0.89 | 1 | 5/10 |
| 2-Nonanone | 1093 | 0.87 | 1 | 2/10 |
| 1-Tetradecanol | 1671 | 0.91 | 1 | 2/10 |
| 2-Undecanone | 1295 | 0.87 | 1 | 1/10 |
| DMDS Concentration * | Siderophore Release (mm2) ** | Phosphate Solubilisation (mm2) | Potassium Solubilisation (mm2) |
|---|---|---|---|
| 0 | 592.10 ± 2.69 c | 27.35 ± 0.79 a | 76.37 ± 0.25 a |
| 0.02 | 610.16 ± 1.25 c | 24.77 ± 0.56 a | 74.34 ± 0.56 a |
| 0.2 | 612.33 ± 2.33 c | 20.39 ± 0.36 b | 66.18 ± 0.36 b |
| 2 | 675.53 ± 1.09 b | 17.21 ± 0.44 b | 65.81 ± 0.44 b |
| 20 | 707.01 ± 2.45 a | 16.37 ± 0.74 b | 63.30 ± 0.74 b |
| DMDS Concentration * | Lateral Root Density *** | Rood Dry Weight (mg) | Shoot Dry Weight (mg) |
| 0 | 1.46 ± 0.05 c | 12.32 ± 0.55 d | 31.14 ± 0.92 d |
| 0.02 | 1.82 ± 0.03 b | 26.43 ± 1.33 c | 53.31 ± 2.33 c |
| 0.2 | 1.94 ± 0.06 b | 37.87 ± 0.21 c | 79.25 ± 1.25 c |
| 2 | 2.31 ± 0.12 a | 47.31 ± 1.23 b | 84.25 ± 0.99 b |
| 20 | 2.40 ± 0.16 a | 57.65 ± 2.36 a | 102.33 ± 1.22 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasseur-Coronado, M.; Vlassi, A.; Boulois, H.D.d.; Schuhmacher, R.; Parich, A.; Pertot, I.; Puopolo, G. Ecological Role of Volatile Organic Compounds Emitted by Pantoea agglomerans as Interspecies and Interkingdom Signals. Microorganisms 2021, 9, 1186. https://doi.org/10.3390/microorganisms9061186
Vasseur-Coronado M, Vlassi A, Boulois HDd, Schuhmacher R, Parich A, Pertot I, Puopolo G. Ecological Role of Volatile Organic Compounds Emitted by Pantoea agglomerans as Interspecies and Interkingdom Signals. Microorganisms. 2021; 9(6):1186. https://doi.org/10.3390/microorganisms9061186
Chicago/Turabian StyleVasseur-Coronado, Maria, Anthi Vlassi, Hervé Dupré du Boulois, Rainer Schuhmacher, Alexandra Parich, Ilaria Pertot, and Gerardo Puopolo. 2021. "Ecological Role of Volatile Organic Compounds Emitted by Pantoea agglomerans as Interspecies and Interkingdom Signals" Microorganisms 9, no. 6: 1186. https://doi.org/10.3390/microorganisms9061186
APA StyleVasseur-Coronado, M., Vlassi, A., Boulois, H. D. d., Schuhmacher, R., Parich, A., Pertot, I., & Puopolo, G. (2021). Ecological Role of Volatile Organic Compounds Emitted by Pantoea agglomerans as Interspecies and Interkingdom Signals. Microorganisms, 9(6), 1186. https://doi.org/10.3390/microorganisms9061186








