Lipoteichoic Acid from Staphylococcus aureus Activates the Complement System via C3 Induction and CD55 Inhibition
Abstract
1. Introduction
2. Materials and Methods
2.1. LTA Preparation
2.2. Cell Culture
2.3. Real-Time PCR
2.4. Western Blot Analysis
2.5. Immunofluorescence
2.6. Bactericidal Assay
2.7. C9 Deposition Assay
2.8. Mouse Study
2.9. Statistical Analysis
3. Results
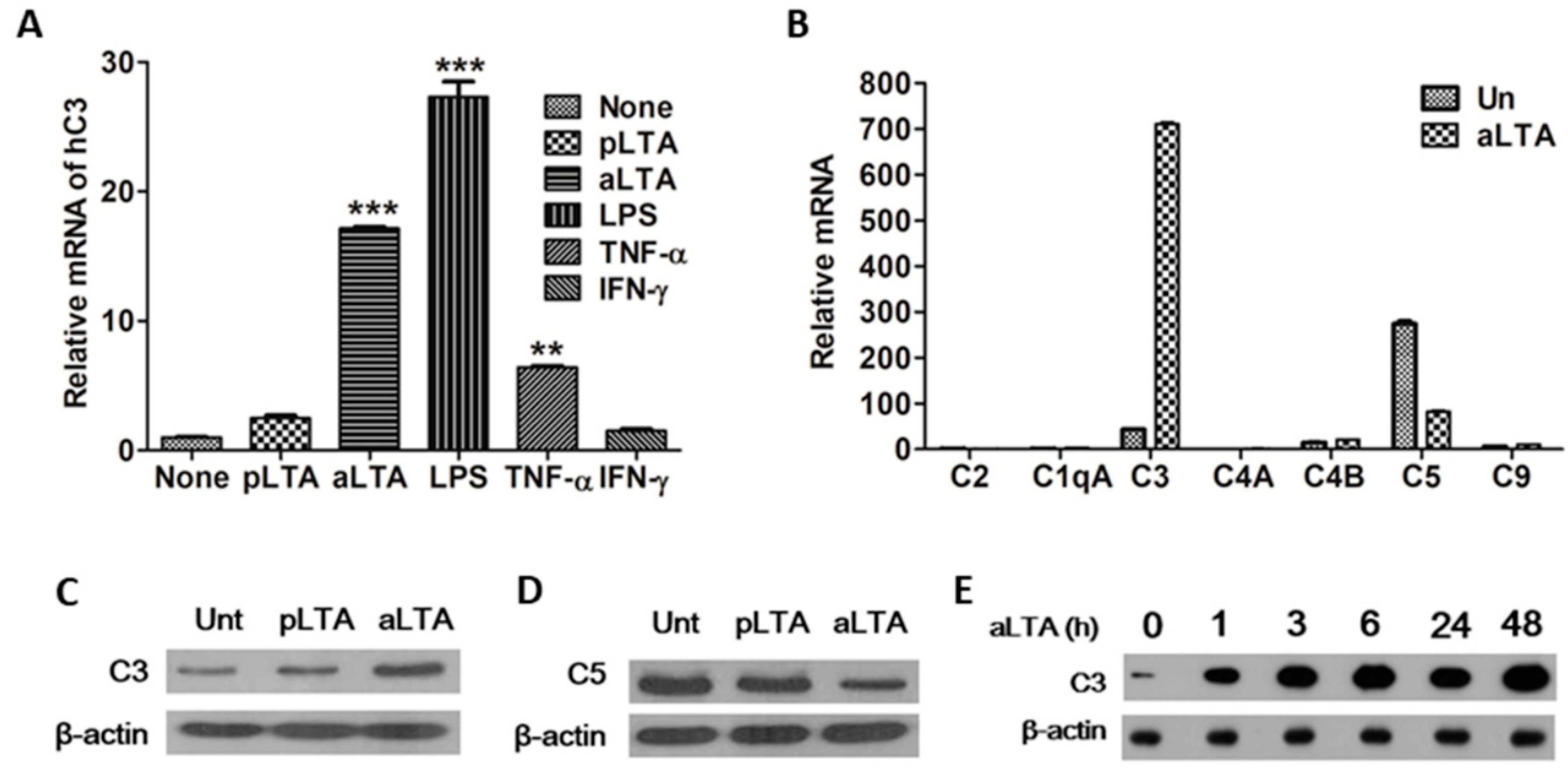
3.1. C3 Expression Is Significantly Increased by the aLTA Treatment
3.2. Toll-Like Recptor (TLR) 2 Is Involved in aLTA-Mediated C3 Expression
3.3. IRAK2 Plays an Important Role in aLTA-TLR2-Mediated C3 Expression
3.4. NF-κB Is a Central Mediator for aLTA-Mediated C3 Production
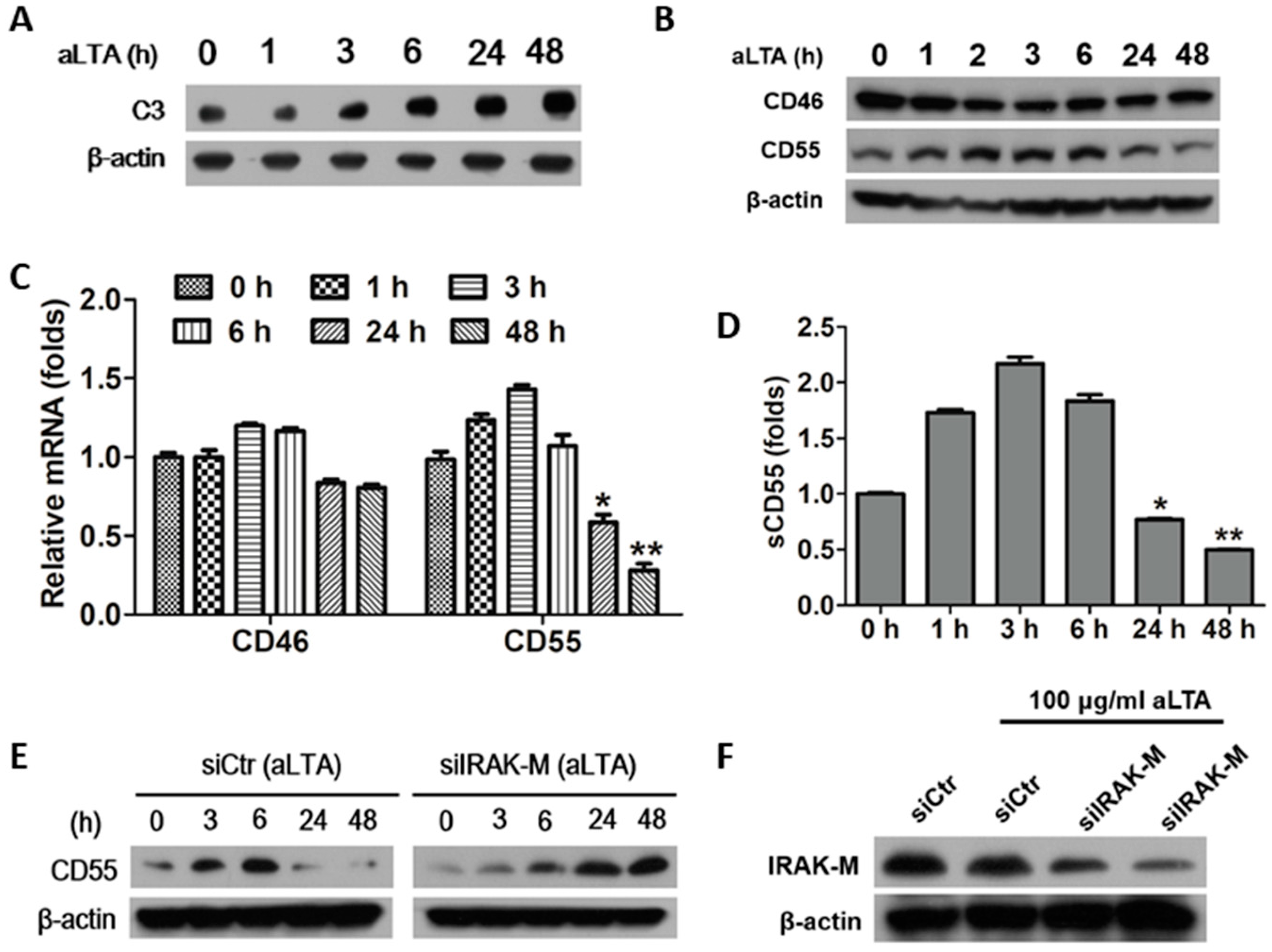
3.5. The aLTA Down-Regulated CD55 Production in HepG2 Cells through the Induction of IRAK-M
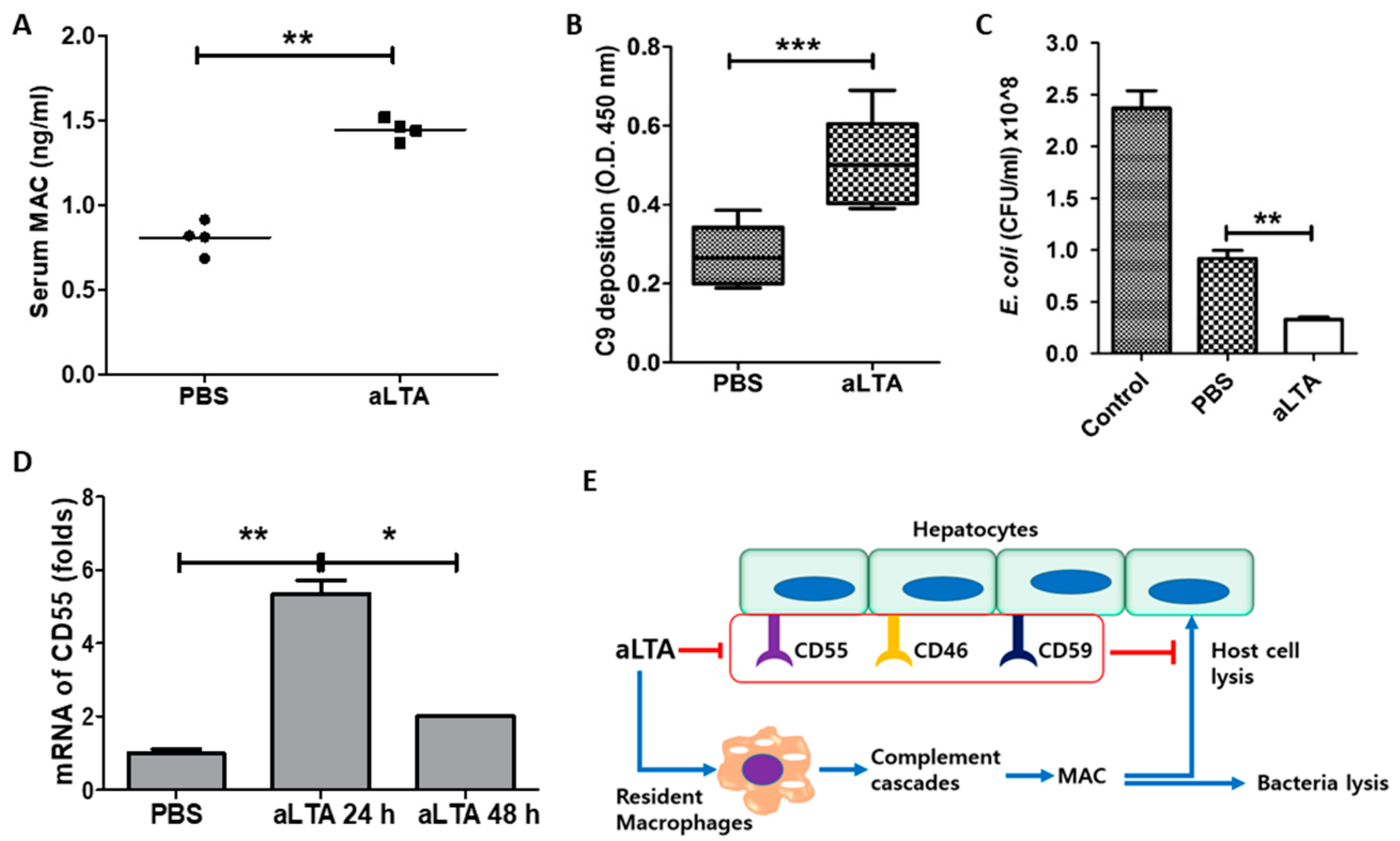
3.6. The aLTA Treatment Increased C3 Activation in Mice
3.7. Activation of MAC by aLTA Induces Liver Damage
3.8. Repeated Treatment with aLTA Induces Organ Injury as Well as MAC Activation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Janeway, C.A., Jr.; Travers, P.; Walport, M.; Shlomchik, M.J. Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001. Available online: https://www.ncbi.nlm.nih.gov/books/NBK10775/ (accessed on 24 May 2021).
- Na, M.; Jarneborn, A.; Ali, A.; Welin, A.; Magnusson, M.; Stokowska, A.; Pekna, M.; Jin, T. Deficiency of the Complement component 3 but not factor B aggravates Staphylococcus aureus septic arthritis in mice. Infect. Immun. 2016, 84, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jacobsson, K.; Ström, K.; Lindberg, M.; Frykberg, L. Staphylococcus aureus expresses a cell surface protein that binds both IgG and beta2-glycoprotein I. Microbiology 1999, 145 Pt 1, 177–183. [Google Scholar] [CrossRef]
- Burman, J.D.; Leung, E.; Atkins, K.L.; O’Seaghdha, M.N.; Lango, L.; Bernadó, P.; Bagby, S.; Svergun, D.I.; Foster, T.J.; Isenman, D.E.; et al. Interaction of human complement with Sbi, a staphylococcal immunoglobulin-binding protein: Indications of a novel mechanism of complement evasion by Staphylococcus aureus. J. Biol. Chem. 2008, 283, 17579–17593. [Google Scholar] [CrossRef]
- Lee, L.Y.; Höök, M.; Haviland, D.; Wetsel, R.A.; Yonter, E.O.; Syribeys, P.; Vernachio, J.; Brown, E.L. Inhibition of complement activation by a secreted Staphylococcus aureus protein. J. Infect. Dis. 2004, 190, 571–579. [Google Scholar] [CrossRef]
- Wilkinson, B.J.; Kim, Y.; Peterson, P.K.; Quie, P.G.; Michael, A.F. Activation of complement by cell surface components of Staphylococcus aureus. Infect. Immun. 1978, 20, 388–392. [Google Scholar] [CrossRef]
- Loos, M.; Clas, F.; Fischer, W. Interaction of purified lipoteichoic acid with the classical complement pathway. Infect. Immun. 1986, 53, 595–599. [Google Scholar] [CrossRef]
- Zeng, Z.; Surewaard, B.G.; Wong, C.H.; Geoghegan, J.A.; Jenne, C.N.; Kubes, P. CRIg Functions as a Macrophage Pattern Recognition Receptor to Directly Bind and Capture Blood-Borne Gram-Positive Bacteria. Cell Host Microbe 2016, 20, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Kim, N.R.; Gim, M.G.; Lee, J.M.; Lee, S.Y.; Ko, M.Y.; Kim, J.Y.; Han, S.H.; Chung, D.K. Lipoteichoic acid isolated from Lactobacillus plantarum inhibits lipopolysaccharide-induced TNF-alpha production in THP-1 cells and endotoxin shock in mice. J. Immunol. 2008, 180, 2553–2561. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kobayashi, K.; Hernandez, L.D.; Galán, J.E.; Janeway, C.A., Jr.; Medzhitov, R.; Flavell, R.A. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell 2002, 110, 191–202. [Google Scholar] [CrossRef]
- Sim, R.B.; Day, A.J.; Moffatt, B.E.; Fontaine, M. Complement factor I and its cofactors in control of the complement system convertase enzymes. Methods Enzymol. 1993, 223, 13–35. [Google Scholar] [PubMed]
- Schmidt, J.; Klempp, C.; Büchler, M.W.; Märten, A. Release of iC3b from apoptotic tumor cells induces tolerance by binding to immature dendritic cells in vitro and in vivo. Cancer Immunol. Immunother. 2006, 55, 31–38. [Google Scholar] [CrossRef]
- Qin, X.; Gao, B. The complement system in liver diseases. Cell Mol. Immunol. 2006, 3, 333–340. [Google Scholar] [PubMed]
- Thorgersen, E.B.; Barratt-Due, A.; Haugaa, H.; Harboe, M.; Pischke, S.E.; Nilsson, P.H.; Mollnes, T.E. The Role of Complement in Liver Injury, Regeneration, and Transplantation. Hepatology 2019, 70, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Karmen, A.; Wroblewski, F.; Ladue, J.S. Transaminase activity in human blood. J. Clin. Investig. 1955, 34, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Hall, P.; Cash, J. What is the real function of the liver ‘function’ tests? Ulster Med. J. 2012, 81, 30–36. [Google Scholar] [PubMed]
- Farkas, I.; Baranyi, L.; Ishikawa, Y.; Okada, N.; Bohata, C.; Budai, D.; Fukuda, A.; Imai, M.; Okada, H. CD59 blocks not only the insertion of C9 into MAC but inhibits ion channel formation by homologous C5b-8 as well as C5b-9. J. Physiol. 2002, 539, 537–545. [Google Scholar] [CrossRef]
- Anwar, M.; Basith, S.; Choi, S. Negative regulatory approaches to the attenuation of Toll-like receptor signaling. Exp. Mol. Med. 2013, 45, e11. [Google Scholar] [CrossRef]
- Huggins, T.; Haught, J.C.; Xie, S.; Tansky, C.S.; Klukowska, M.; Miner, M.C.; White, D.J. Quantitation of endotoxin and lipoteichoic acid virulence using toll receptor reporter gene. Am. J. Dent. 2016, 29, 321–327. [Google Scholar]
- Kurokawa, K.; Lee, H.; Roh, K.B.; Asanuma, M.; Kim, S.; Nakayama, H.; Shiratsuchi, A.; Choi, Y.; Takeuchi, O.; Kang, H.J.; et al. The triacylated ATP binding cluster transporter substrate-binding lipoprotein of Staphylococcus aureus functions as a native ligand for Toll-like receptor 2. J. Biol. Chem. 2009, 284, 8406–8411. [Google Scholar] [CrossRef]
- Hashimoto, M.; Tawaratsumida, K.; Kariya, H.; Aoyama, K.; Tamura, T.; Suda, Y. Lipoprotein is a predominant Toll-like receptor 2 ligand in Staphylococcus aureus cell wall components. Int. Immunol. 2006, 18, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Tawaratsumida, K.; Kariya, H.; Kiyohara, A.; Suda, Y.; Krikae, F.; Kirikae, T.; Götz, F. Not lipoteichoic acid but lipoproteins appear to be the dominant immunobiologically active compounds in Staphylococcus aureus. J. Immunol. 2006, 177, 3162–3169. [Google Scholar] [CrossRef]
- Jin, M.S.; Kim, S.E.; Heo, J.Y.; Lee, M.E.; Kim, H.M.; Paik, S.G.; Lee, H.; Lee, J.O. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 2007, 130, 1071–1082. [Google Scholar] [CrossRef]
- Kang, J.Y.; Nan, X.; Jin, M.S.; Youn, S.J.; Ryu, Y.H.; Mah, S.; Han, S.H.; Lee, H.; Paik, S.G.; Lee, J.O. Recognition of lipopeptide patterns by Toll-like receptor 2-Toll-like receptor 6 heterodimer. Immunity 2009, 31, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Wardenburg, J.B.; Williams, W.A.; Missiakas, D. Host defenses against Staphylococcus aureus infection require recognition of bacterial lipoproteins. Proc. Natl. Acad. Sci. USA 2006, 103, 13831–13836. [Google Scholar] [CrossRef] [PubMed]
- Stoll, H.; Dengjel, J.; Nerz, C.; Götz, F. Staphylococcus aureus deficient in lipidation of prelipoproteins is attenuated in growth and immune activation. Infect. Immun. 2005, 73, 2411–2423. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.S.; Kim, S.K.; Baik, J.E.; Ko, E.B.; Ahn, K.B.; Yun, C.H.; Han, S.H. Staphylococcal LTA antagonizes the B cell-mitogenic potential of LPS. Sci. Rep. 2018, 8, 1496. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, M.; Jiang, N.; Zhang, A. Sodium Phenylbutyrate Ameliorates Inflammatory Response Induced by Staphylococcus aureus Lipoteichoic Acid via Suppressing TLR2/NF-κB/NLRP3 Pathways in MAC-T Cells. Molecules 2018, 23, 3056. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Guo, S.; Yang, J.; Liu, J.; Shaukat, A.; Zhao, G.; Wu, H.; Deng, G. Matrine alleviates Staphylococcus aureus lipoteichoic acid-induced endometritis via suppression of TLR2-mediated NF-κB activation. Int. Immunopharmacol. 2019, 70, 201–207. [Google Scholar] [CrossRef]
- Kim, H.; Meyer, K.; Di Bisceglie, A.M.; Ray, R. Hepatitis C virus suppresses C9 complement synthesis and impairs membrane attack complex function. J. Virol. 2013, 87, 5858–5867. [Google Scholar] [CrossRef] [PubMed][Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, B.J.; Kim, H.; Jang, K.O.; Kim, S.; Chung, D.K. Lipoteichoic Acid from Staphylococcus aureus Activates the Complement System via C3 Induction and CD55 Inhibition. Microorganisms 2021, 9, 1135. https://doi.org/10.3390/microorganisms9061135
Jung BJ, Kim H, Jang KO, Kim S, Chung DK. Lipoteichoic Acid from Staphylococcus aureus Activates the Complement System via C3 Induction and CD55 Inhibition. Microorganisms. 2021; 9(6):1135. https://doi.org/10.3390/microorganisms9061135
Chicago/Turabian StyleJung, Bong Jun, Hangeun Kim, Kyoung Ok Jang, Seongjae Kim, and Dae Kyun Chung. 2021. "Lipoteichoic Acid from Staphylococcus aureus Activates the Complement System via C3 Induction and CD55 Inhibition" Microorganisms 9, no. 6: 1135. https://doi.org/10.3390/microorganisms9061135
APA StyleJung, B. J., Kim, H., Jang, K. O., Kim, S., & Chung, D. K. (2021). Lipoteichoic Acid from Staphylococcus aureus Activates the Complement System via C3 Induction and CD55 Inhibition. Microorganisms, 9(6), 1135. https://doi.org/10.3390/microorganisms9061135





