Survey of Pathogen-Lowering and Immuno-Modulatory Effects Upon Treatment of Campylobacter coli-Infected Secondary Abiotic IL-10−/− Mice with the Probiotic Formulation Aviguard®
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Secondary Abiotic IL-10−/− Mice
2.3. Campylobacter Coli Infection
2.4. Treatment of Mice With the Commercial Exclusion Product Aviguard®
2.5. Gastrointestinal C. coli Loads
2.6. Cultural Intestinal Microbiota Analysis
2.7. Culture-Independent Intestinal Microbiota Analysis
2.8. Clinical Conditions
2.9. Sampling Procedures
2.10. Histopathology
2.11. In Situ Immunohistochemistry
2.12. Proinflammatory Cytokines
2.13. Statistical Analyses
3. Results
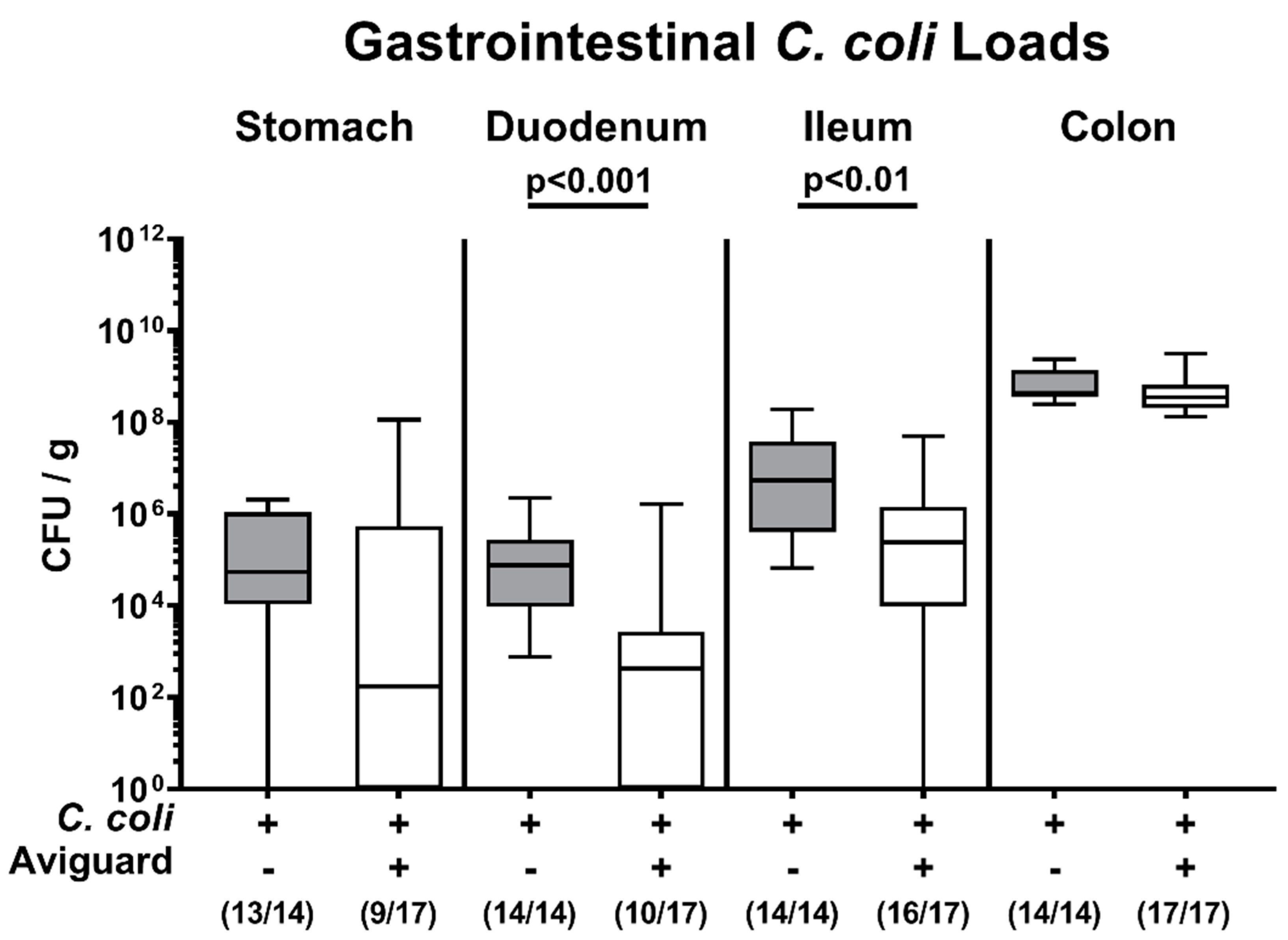
3.1. Gastrointestinal Pathogen Loads Following Oral Aviguard® Treatment of C. coli Infected Secondary Abiotic IL-10−/− Mice
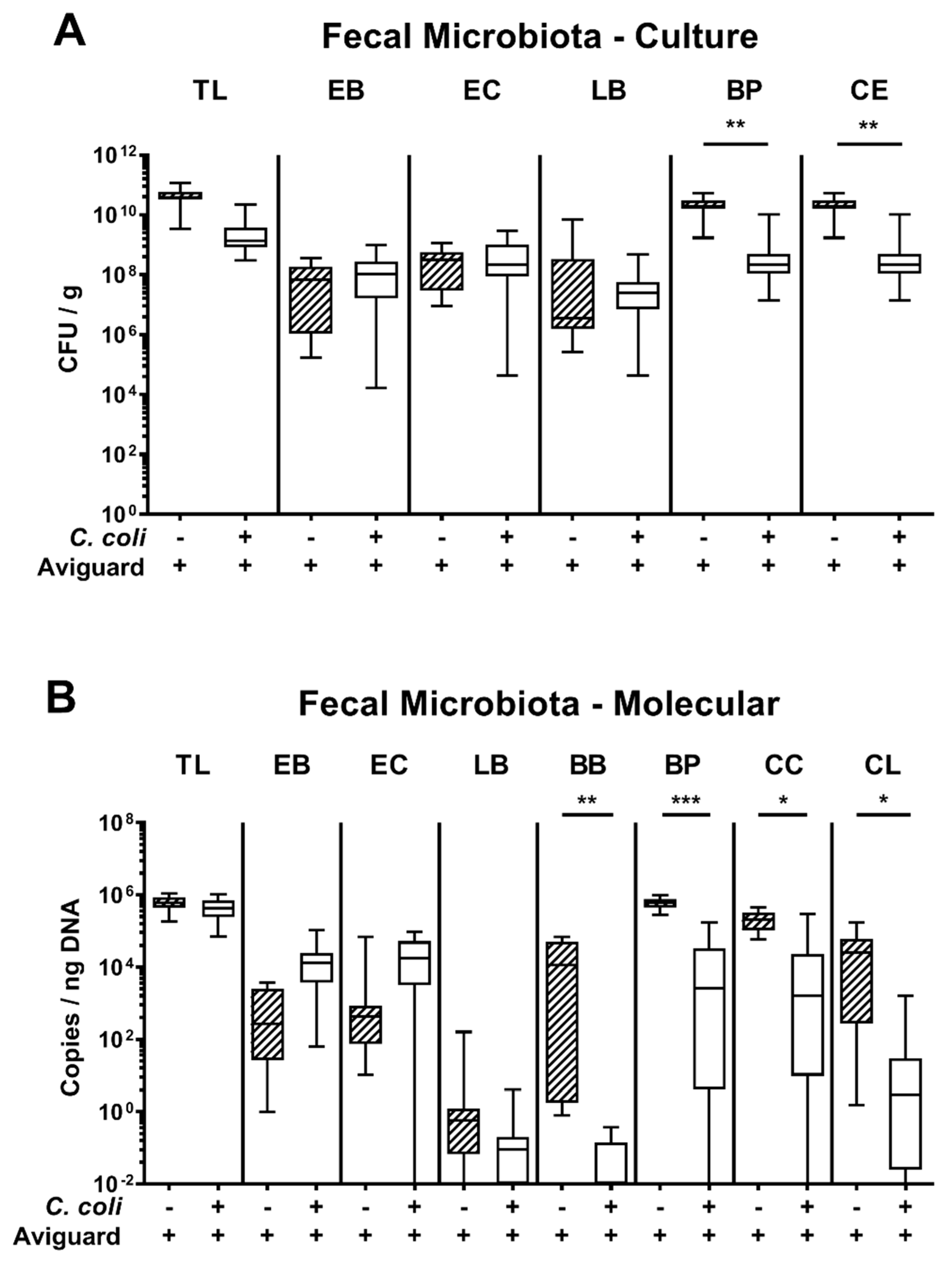
3.2. Fecal Microbiota Composition Following Oral Aviguard® Treatment of C. coli-Infected Secondary Abiotic IL-10−/− Mice
3.3. Clinical and Histopathological Sequelae Upon Oral Aviguard® Treatment of C. coli-Infected Secondary Abiotic IL-10−/− Mice
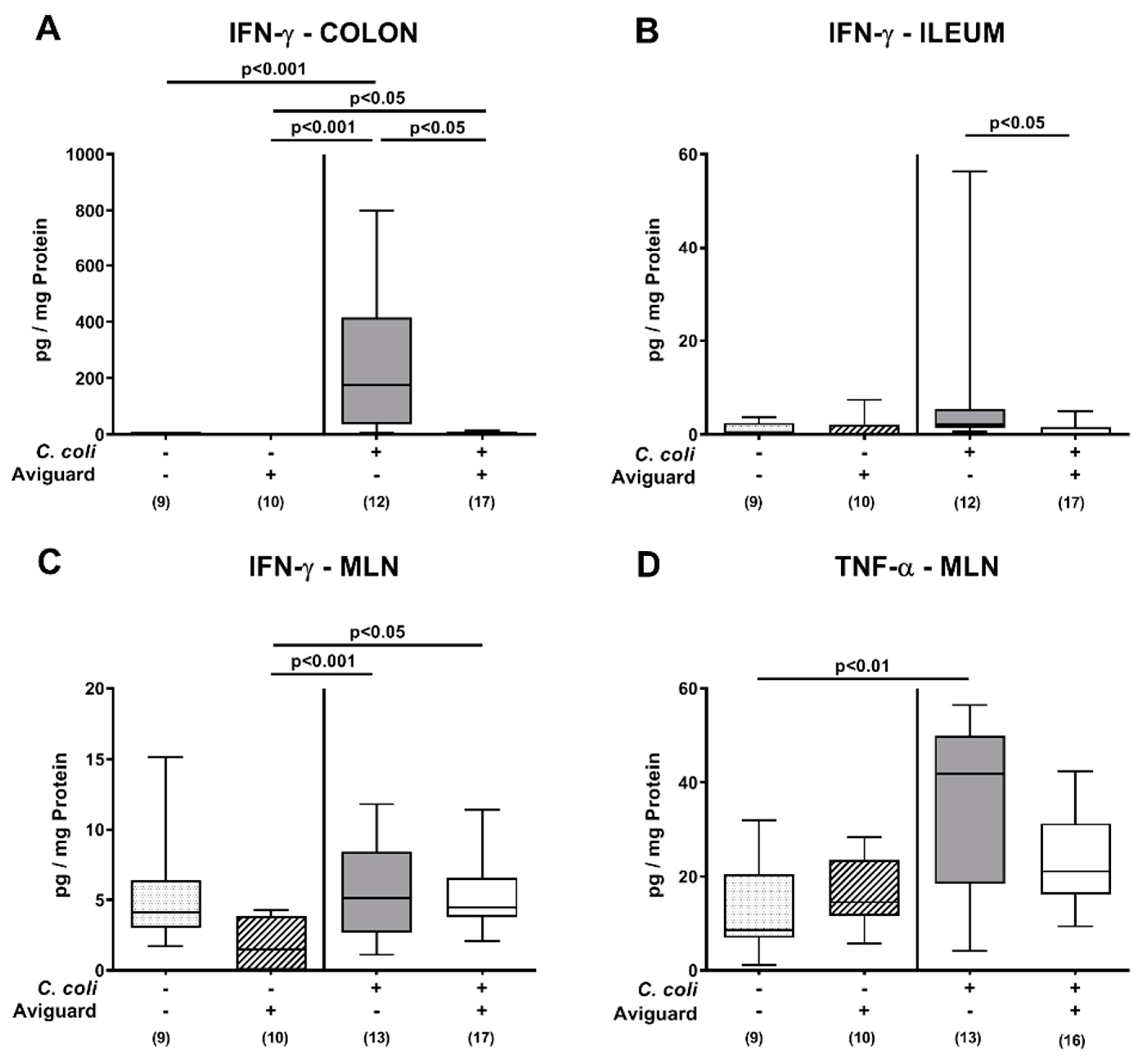
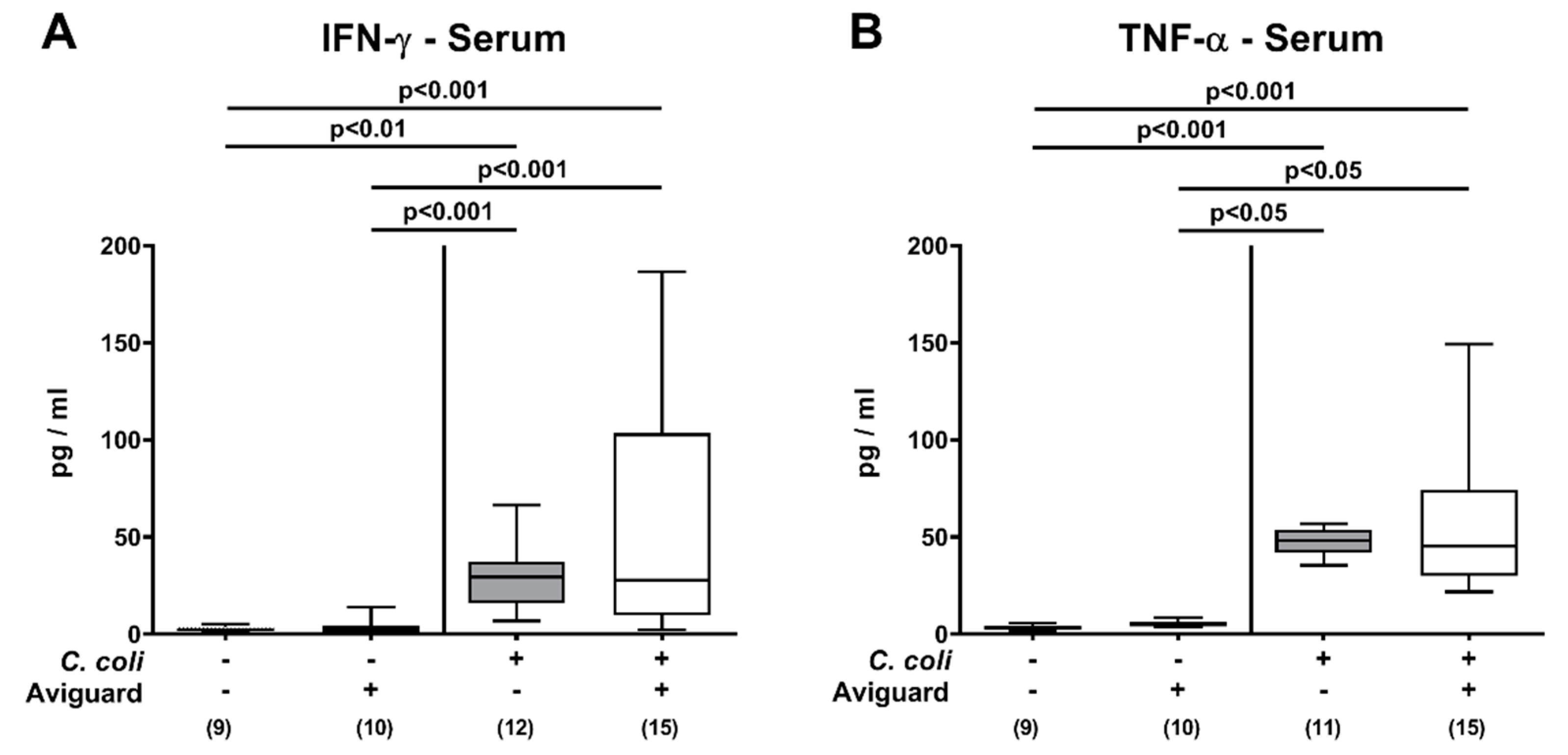
3.4. Intestinal and Systemic Proinflammatory Immune Responses Following Oral Aviguard® Treatment of C. coli-Infected Secondary Abiotic IL-10−/− Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organisation. Campylobacter. Available online: https://www.who.int/news-room/fact-sheets/detail/campylobacter (accessed on 4 June 2020).
- European Food Safety Authority; European Centre for Disease. Prevention Control. The European Union One Health 2018 Zoonoses Report. EFSA J. 2019, 17, e05926. [Google Scholar] [CrossRef]
- Vandamme, P.; De Ley, J. Proposal for a new family, Campylobacteraceae. Int. J. Syst. Evol. Microbiol. 1991, 41, 451–455. [Google Scholar] [CrossRef]
- Silva, J.; Leite, D.; Fernandes, M.; Mena, C.; Gibbs, P.A.; Teixeira, P. Campylobacter spp. as a foodborne pathogen: A review. Front. Microbiol. 2011, 2, 200. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.I.; Caldwell, M.B.; Lee, E.C.; Guerry, P.; Trust, T.J.; Ruiz-Palacios, G.M. Pathophysiology of Campylobacter enteritis. Microbiol. Rev. 1986, 50, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Kaakoush, N.O.; Castaño-Rodríguez, N.; Mitchell, H.M.; Man, S.M. Global Epidemiology of Campylobacter Infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef] [PubMed]
- Cody, A.J.; Maiden, M.C.; Strachan, N.J.; McCarthy, N.D. A systematic review of source attribution of human campylobacteriosis using multilocus sequence typing. Eurosurveillance 2019, 24, 1800696. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention Control. European Centre for Disease Prevention Control. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018, 16, e05500. [Google Scholar] [CrossRef]
- Bull, S.; Allen, V.; Domingue, G.; Jørgensen, F.; Frost, J.; Ure, R.; Whyte, R.; Tinker, D.; Corry, J.; Gillard-King, J. Sources of Campylobacter spp. colonizing housed broiler flocks during rearing. Appl. Environ. Microbiol. 2006, 72, 645–652. [Google Scholar] [CrossRef]
- Powell, L.; Lawes, J.; Clifton-Hadley, F.; Rodgers, J.; Harris, K.; Evans, S.; Vidal, A. The prevalence of Campylobacter spp. in broiler flocks and on broiler carcases, and the risks associated with highly contaminated carcases. Epidemiol. Infect. 2012, 140, 2233–2246. [Google Scholar] [CrossRef]
- Newell, D.G.; Mughini-Gras, L.; Kalupahana, R.S.; Wagenaar, J.A. Campylobacter epidemiology—Sources and routes of transmission for human infection. In Campylobacter; Elsevier: Amsterdam, The Netherlands, 2017; pp. 85–110. [Google Scholar]
- Wagenaar, J.A.; French, N.P.; Havelaar, A.H. Preventing Campylobacter at the source: Why is it so difficult? Clin. Infect. Dis. 2013, 57, 1600–1606. [Google Scholar] [CrossRef]
- Peyrat, M.; Soumet, C.; Maris, P.; Sanders, P. Recovery of Campylobacter jejuni from surfaces of poultry slaughterhouses after cleaning and disinfection procedures: Analysis of a potential source of carcass contamination. Int. J. Food Microbiol. 2008, 124, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Kist, M.; Bereswill, S. Campylobacter jejuni. Contrib. Microbiol. 2001, 8, 150–165. [Google Scholar] [CrossRef]
- Young, K.T.; Davis, L.M.; Dirita, V.J. Campylobacter jejuni: Molecular biology and pathogenesis. Nat. Rev. Microbiol. 2007, 5, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Backert, S.; Tegtmeyer, N.; Cróinín, T.Ó.; Boehm, M.; Heimesaat, M.M. Human campylobacteriosis. In Campylobacter; Klein, G., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 1–25. [Google Scholar] [CrossRef]
- Allos, B.M. Association between Campylobacter infection and Guillain-Barre syndrome. J. Infect. Dis 1997, 176 (Suppl. 2), S125–S128. [Google Scholar] [CrossRef]
- Hsieh, Y.-H.; Sulaiman, I.M. Campylobacteriosis: An Emerging Infectious Foodborne Disease. In Foodborne Diseases; Elsevier: Amsterdam, The Netherlands, 2018; pp. 119–155. [Google Scholar]
- Mortensen, N.P.; Kuijf, M.L.; Ang, C.W.; Schiellerup, P.; Krogfelt, K.A.; Jacobs, B.C.; van Belkum, A.; Endtz, H.P.; Bergman, M.P. Sialylation of Campylobacter jejuni lipo-oligosaccharides is associated with severe gastro-enteritis and reactive arthritis. Microbes Infect. 2009, 11, 988–994. [Google Scholar] [CrossRef]
- Nurmi, E.; Rantala, M. New aspects of Salmonella infection in broiler production. Nature 1973, 241, 210–211. [Google Scholar] [CrossRef]
- Rantala, M.; Nurmi, E. Prevention of the growth of Salmonella infantis in chicks by the flora of the alimentary tract of chickens. Br. Poult. Sci. 1973, 14, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Mountzouris, K.C.; Balaskas, C.; Xanthakos, I.; Tzivinikou, A.; Fegeros, K. Effects of a multi-species probiotic on biomarkers of competitive exclusion efficacy in broilers challenged with Salmonella enteritidis. Br. Poult. Sci. 2009, 50, 467–478. [Google Scholar] [CrossRef]
- Ducatelle, R.; Eeckhaut, V.; Haesebrouck, F.; Van Immerseel, F. A review on prebiotics and probiotics for the control of dysbiosis: Present status and future perspectives. Animal 2015, 9, 43–48. [Google Scholar] [CrossRef]
- Nakamura, A.; Ota, Y.; Mizukami, A.; Ito, T.; Ngwai, Y.B.; Adachi, Y. Evaluation of aviguard, a commercial competitive exclusion product for efficacy and after-effect on the antibody response of chicks to Salmonella. Poult. Sci. 2002, 81, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Abudabos, A.M. Use of a Competitive Exclusion Product (Aviguard®) to Prevent Clostridium perfringens Colonization in Broiler Chicken under Induced Challenge. Pak. J. Zool. 2013, 45, 371–376. [Google Scholar]
- Haag, L.M.; Fischer, A.; Otto, B.; Plickert, R.; Kuhl, A.A.; Gobel, U.B.; Bereswill, S.; Heimesaat, M.M. Campylobacter jejuni induces acute enterocolitis in gnotobiotic IL-10-/- mice via Toll-like-receptor-2 and -4 signaling. PLoS ONE 2012, 7, e40761. [Google Scholar] [CrossRef]
- Mousavi, S.; Bereswill, S.; Heimesaat, M.M. Novel Clinical Campylobacter jejuni Infection Models Based on Sensitization of Mice to Lipooligosaccharide, a Major Bacterial Factor Triggering Innate Immune Responses in Human Campylobacteriosis. Microorganisms 2020, 8, 482. [Google Scholar] [CrossRef] [PubMed]
- Kløve, S.; Genger, C.; Mousavi, S.; Weschka, D.; Bereswill, S.; Heimesaat, M.M. Toll-Like Receptor-4 Dependent Intestinal and Systemic Sequelae Following Peroral Campylobacter coli Infection of IL10 Deficient Mice Harboring a Human Gut Microbiota. Pathogens 2020, 9, 386. [Google Scholar] [CrossRef] [PubMed]
- Kløve, S.; Genger, C.; Weschka, D.; Mousavi, S.; Bereswill, S.; Heimesaat, M.M. Toll-Like Receptor-4 Is Involved in Mediating Intestinal and Extra-Intestinal Inflammation in Campylobacter coli-Infected Secondary Abiotic IL-10-/-Mice. Microorganisms 2020, 8, 1882. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Bereswill, S.; Fischer, A.; Fuchs, D.; Struck, D.; Niebergall, J.; Jahn, H.K.; Dunay, I.R.; Moter, A.; Gescher, D.M.; et al. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J. Immunol. 2006, 177, 8785–8795. [Google Scholar] [CrossRef] [PubMed]
- Bereswill, S.; Fischer, A.; Plickert, R.; Haag, L.M.; Otto, B.; Kuhl, A.A.; Dasti, J.I.; Zautner, A.E.; Munoz, M.; Loddenkemper, C.; et al. Novel murine infection models provide deep insights into the “menage a trois” of Campylobacter jejuni, microbiota and host innate immunity. PLoS ONE 2011, 6, e20953. [Google Scholar] [CrossRef]
- Ekmekciu, I.; von Klitzing, E.; Fiebiger, U.; Escher, U.; Neumann, C.; Bacher, P.; Scheffold, A.; Kuhl, A.A.; Bereswill, S.; Heimesaat, M.M. Immune Responses to Broad-Spectrum Antibiotic Treatment and Fecal Microbiota Transplantation in Mice. Front. Immunol. 2017, 8, 397. [Google Scholar] [CrossRef]
- Lallemand Animal Nutrition. Aviguard®. Available online: https://svitagro.com.ua/aviguard-eng/ (accessed on 4 June 2020).
- Rausch, S.; Held, J.; Fischer, A.; Heimesaat, M.M.; Kühl, A.A.; Bereswill, S.; Hartmann, S. Small intestinal nematode infection of mice is associated with increased enterobacterial loads alongside the intestinal tract. PLoS ONE 2013, 8, e74026. [Google Scholar] [CrossRef] [PubMed]
- Heimesaat, M.M.; Alutis, M.; Grundmann, U.; Fischer, A.; Tegtmeyer, N.; Bohm, M.; Kuhl, A.A.; Gobel, U.B.; Backert, S.; Bereswill, S. The role of serine protease HtrA in acute ulcerative enterocolitis and extra-intestinal immune responses during Campylobacter jejuni infection of gnotobiotic IL-10 deficient mice. Front. Cell Infect. Microbiol. 2014, 4, 77. [Google Scholar] [CrossRef] [PubMed]
- Erben, U.; Loddenkemper, C.; Doerfel, K.; Spieckermann, S.; Haller, D.; Heimesaat, M.M.; Zeitz, M.; Siegmund, B.; Kühl, A.A. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int. J. Clin. Exp. Pathol. 2014, 7, 4557. [Google Scholar] [PubMed]
- Heimesaat, M.M.; Giladi, E.; Kuhl, A.A.; Bereswill, S.; Gozes, I. The octapetide NAP alleviates intestinal and extra-intestinal anti-inflammatory sequelae of acute experimental colitis. Peptides 2018, 101, 1–9. [Google Scholar] [CrossRef]
- Lee, D.H.; Zo, Y.G.; Kim, S.J. Nonradioactive method to study genetic profiles of natural bacterial communities by PCR-single-strand-conformation polymorphism. Appl. Environ. Microbiol. 1996, 62, 3112–3120. [Google Scholar] [CrossRef]
- Kühbacher, T.; Ott, S.J.; Helwig, U.; Mimura, T.; Rizzello, F.; Kleessen, B.; Gionchetti, P.; Blaut, M.; Campieri, M.; Fölsch, U.R.; et al. Bacterial and fungal microbiota in relation to probiotic therapy (VSL#3) in pouchitis. Gut 2006, 55, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Rinttilä, T.; Kassinen, A.; Malinen, E.; Krogius, L.; Palva, A. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J. Appl. Microbiol. 2004, 97, 1166–1177. [Google Scholar] [CrossRef] [PubMed]
- Heilig, H.G.; Zoetendal, E.G.; Vaughan, E.E.; Marteau, P.; Akkermans, A.D.; de Vos, W.M. Molecular diversity of Lactobacillus spp. and other lactic acid bacteria in the human intestine as determined by specific amplification of 16S ribosomal DNA. Appl. Environ. Microbiol. 2002, 68, 114–123. [Google Scholar] [CrossRef]
- Walter, J.; Hertel, C.; Tannock, G.W.; Lis, C.M.; Munro, K.; Hammes, W.P. Detection of Lactobacillus, Pediococcus, Leuconostoc, and Weissella species in human feces by using group-specific PCR primers and denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 2001, 67, 2578–2585. [Google Scholar] [CrossRef]
- Matsuki, T.; Watanabe, K.; Fujimoto, J.; Miyamoto, Y.; Takada, T.; Matsumoto, K.; Oyaizu, H.; Tanaka, R. Development of 16S rRNA-gene-targeted group-specific primers for the detection and identification of predominant bacteria in human feces. Appl. Environ. Microbiol. 2002, 68, 5445–5451. [Google Scholar] [CrossRef]
- Bartosch, S.; Fite, A.; Macfarlane, G.T.; McMurdo, M.E.T. Characterization of bacterial communities in feces from healthy elderly volunteers and hospitalized elderly patients by using real-time PCR and effects of antibiotic treatment on the fecal microbiota. Appl. Environ. Microbiol. 2004, 70, 3575–3581. [Google Scholar] [CrossRef]
- Van Dyke, M.I.; McCarthy, A.J. Molecular Biological Detection and Characterization of Clostridium; Populations in Municipal Landfill Sites. Appl. Environ. Microbiol. 2002, 68, 2049–2053. [Google Scholar] [CrossRef]
- Wise, M.G.; Siragusa, G.R. Quantitative analysis of the intestinal bacterial community in one- to three-week-old commercially reared broiler chickens fed conventional or antibiotic-free vegetable-based diets. J. Appl. Microbiol. 2007, 102, 1138–1149. [Google Scholar] [CrossRef] [PubMed]
- Beery, J.T.; Hugdahl, M.B.; Doyle, M.P. Colonization of gastrointestinal tracts of chicks by Campylobacter jejuni. Appl. Environ. Microbiol. 1988, 54, 2365–2370. [Google Scholar] [CrossRef] [PubMed]
- Meinersmann, R.J.; Rigsby, W.E.; Stern, N.J.; Kelley, L.C.; Hill, J.E.; Doyle, M.P. Comparative study of colonizing and noncolonizing Campylobacter jejuni. Am. J. Vet. Res. 1991, 52, 1518–1522. [Google Scholar] [PubMed]
- Stern, N.J. Mucosal competitive exclusion to diminish colonization of chickens by Campylobacter jejuni. Poult. Sci. 1994, 73, 402–407. [Google Scholar] [CrossRef]
- Stern, N.J.; Bailey, J.S.; Blankenship, L.; Cox, N.; McHan, F. Colonization characteristics of Campylobacter jejuni in chick ceca. Avian Dis. 1988, 32, 330–334. [Google Scholar] [CrossRef]
- Stern, N.; Cox, N.; Bailey, J.; Berrang, M.; Musgrove, M. Comparison of mucosal competitive exclusion and competitive exclusion treatment to reduce Salmonella and Campylobacter spp. colonization in broiler chickens. Poult. Sci. 2001, 80, 156–160. [Google Scholar]
- Heimesaat, M.M.; Dunay, I.R.; Schulze, S.; Fischer, A.; Grundmann, U.; Alutis, M.; Kuhl, A.A.; Tamas, A.; Toth, G.; Dunay, M.P.; et al. Pituitary adenylate cyclase-activating polypeptide ameliorates experimental acute ileitis and extra-intestinal sequelae. PLoS ONE 2014, 9, e108389. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Reifenberger, G.; Vicena, V.; Illes, A.; Horvath, G.; Tamas, A.; Fulop, B.D.; Bereswill, S.; Reglodi, D. Intestinal Microbiota Changes in Mice Lacking Pituitary Adenylate Cyclase Activating Polypeptide (PACAP) - Bifidobacteria Make the Difference. Eur. J. Microbiol. Immunol. 2017, 7, 187–199. [Google Scholar] [CrossRef]
- Tojo, R.; Suárez, A.; Clemente, M.G.; de los Reyes-Gavilán, C.G.; Margolles, A.; Gueimonde, M.; Ruas-Madiedo, P. Intestinal microbiota in health and disease: Role of bifidobacteria in gut homeostasis. World J. Gastroenterol. 2014, 20, 15163–15176. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Weschka, D.; Kløve, S.; Genger, C.; Biesemeier, N.; Mousavi, S.; Bereswill, S. Microbiota composition and inflammatory immune responses upon peroral application of the commercial competitive exclusion product Aviguard® to microbiota-depleted wildtype mice. Eur. J. Microbiol. Immunol. 2020, 10, 139–146. [Google Scholar] [CrossRef]
- Bereswill, S.; Ekmekciu, I.; Escher, U.; Fiebiger, U.; Stingl, K.; Heimesaat, M.M. Lactobacillus johnsonii ameliorates intestinal, extra-intestinal and systemic pro-inflammatory immune responses following murine Campylobacter jejuni infection. Sci. Rep. 2017, 7, 2138. [Google Scholar] [CrossRef] [PubMed]
- Ekmekciu, I.; Fiebiger, U.; Stingl, K.; Bereswill, S.; Heimesaat, M.M. Amelioration of intestinal and systemic sequelae of murine Campylobacter jejuni infection by probiotic VSL#3 treatment. Gut Pathog. 2017, 9, 17. [Google Scholar] [CrossRef] [PubMed]



| Target | Reference Strain | Primer Sequence (5′ to 3′) and Orientation b | Reference |
|---|---|---|---|
| Domain Bacteria (targets 16S V3 region) | Escherichia coli ATCC 25922 | F:CGGYCCAGACTCCTACGGG, R:TTACCGCGGCTGCTGGCAC | [38] |
| γ-Proteobacteria/Enterobacteriaceae | Escherichia coli ATCC 25922 | F: AAACTCAAATGAATTGACGG, R: CTTTTGCAACCCACTCC | [39] |
| Enterococcus genus | Enterococcus faecalis DSM 20478 | F: CCTTATTGTTAGTTGCCATCATT, R: ACTCGTTGTACTTCCCATTGT | [40] |
| Lactobacillus group f | Lactobacillus acidophilus DSM 20079 | F: CACCGCTACACATGGAG, R: AGCAGTAGGGAATCTTCCA | [41,42] |
| Bifidobacterium genus | Bifidobacterium sp. (murine origin) | F: CTCCTGGAAACGGGTGG, R: GGTGTTCTTCCCGATATCTACA | [41,42,43] |
| Bacteroides group e | Bacteroides ovatus DSMZ 1896 | F: GAAGGTCCCCCACATTG, R: CAATCGGAGTTCTTCGTG | [44] |
| Clostridium coccoides–Eubacterium rectale subgroup d | Clostridium coccoides DSMZ 935 | F: AAATGACGGTACCTGACTAA, R: CTTTGAGTTTCATTCTTGCGAA | [43] |
| Clostridium leptum subgroup c | Clostridium leptum DSMZ 753 | F: TTACTGGGTGTAAAGGG, R: TAGAGTGCTCTTGCGTA | [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weschka, D.; Mousavi, S.; Biesemeier, N.; Bereswill, S.; Heimesaat, M.M. Survey of Pathogen-Lowering and Immuno-Modulatory Effects Upon Treatment of Campylobacter coli-Infected Secondary Abiotic IL-10−/− Mice with the Probiotic Formulation Aviguard®. Microorganisms 2021, 9, 1127. https://doi.org/10.3390/microorganisms9061127
Weschka D, Mousavi S, Biesemeier N, Bereswill S, Heimesaat MM. Survey of Pathogen-Lowering and Immuno-Modulatory Effects Upon Treatment of Campylobacter coli-Infected Secondary Abiotic IL-10−/− Mice with the Probiotic Formulation Aviguard®. Microorganisms. 2021; 9(6):1127. https://doi.org/10.3390/microorganisms9061127
Chicago/Turabian StyleWeschka, Dennis, Soraya Mousavi, Nina Biesemeier, Stefan Bereswill, and Markus M. Heimesaat. 2021. "Survey of Pathogen-Lowering and Immuno-Modulatory Effects Upon Treatment of Campylobacter coli-Infected Secondary Abiotic IL-10−/− Mice with the Probiotic Formulation Aviguard®" Microorganisms 9, no. 6: 1127. https://doi.org/10.3390/microorganisms9061127
APA StyleWeschka, D., Mousavi, S., Biesemeier, N., Bereswill, S., & Heimesaat, M. M. (2021). Survey of Pathogen-Lowering and Immuno-Modulatory Effects Upon Treatment of Campylobacter coli-Infected Secondary Abiotic IL-10−/− Mice with the Probiotic Formulation Aviguard®. Microorganisms, 9(6), 1127. https://doi.org/10.3390/microorganisms9061127







