Degradation Products of Complex Arabinoxylans by Bacteroides intestinalis Enhance the Host Immune Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Ethical Statement
2.3. Bacterial Strains
2.4. Arabinoxylan Substrates
2.5. Bacterial Growth Conditions
2.6. Cell Culture
2.7. Treatment of Intestinal Caco-2 and HIEC-6 Cells
2.8. Isolation and Treatment of Dendritic Cells
2.9. Isolation and Analysis of Mesenteric Lymph Node Cells and Spleen Cells
2.10. Blood Collection
2.11. Flow Cytometry Analysis
2.12. Statistical Analysis
3. Results
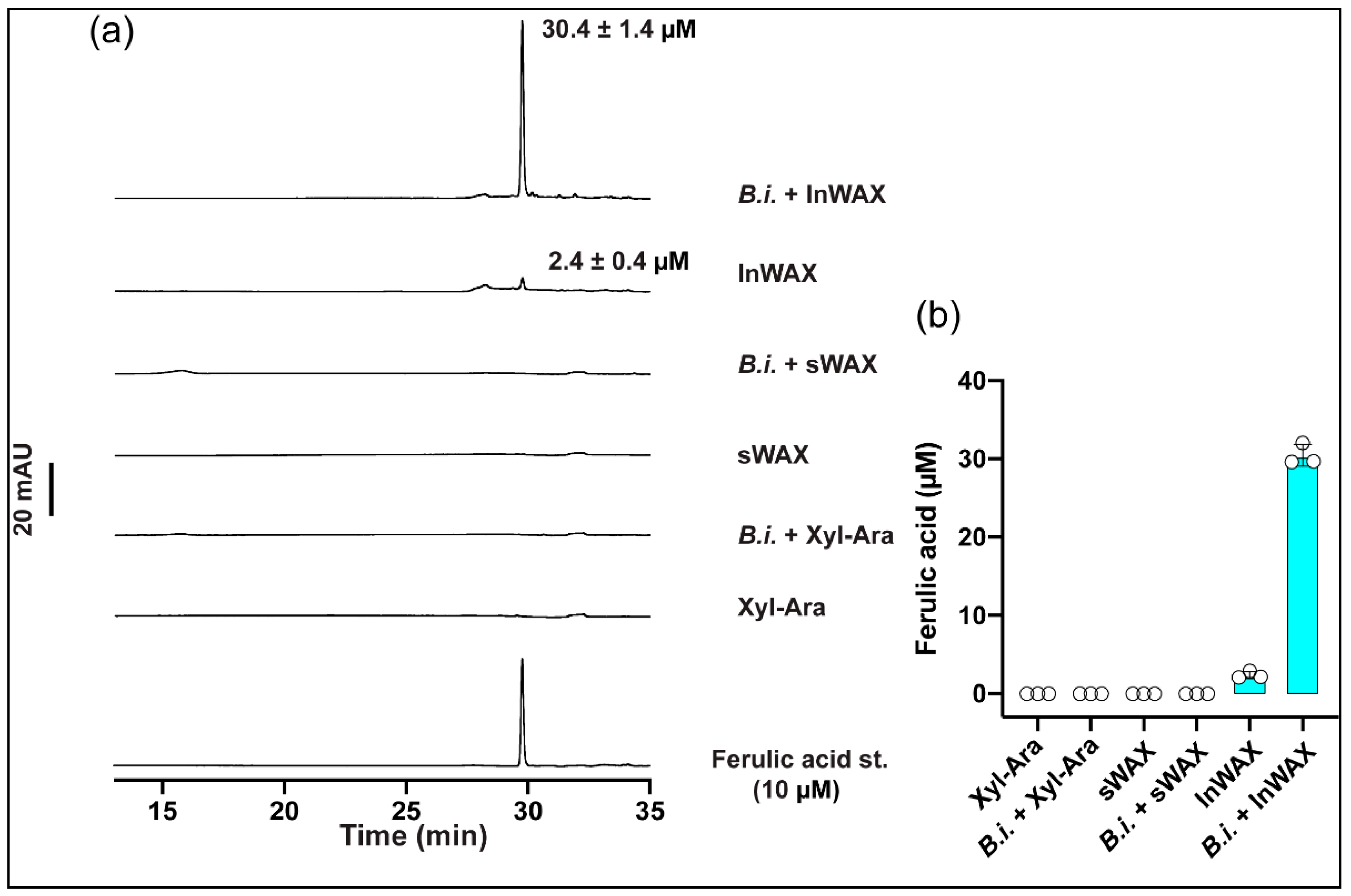
3.1. High Concentration of Ferulic Acid in Culture Supernatant from Bacteria Grown in the Presence of Insoluble Arabinoxylans
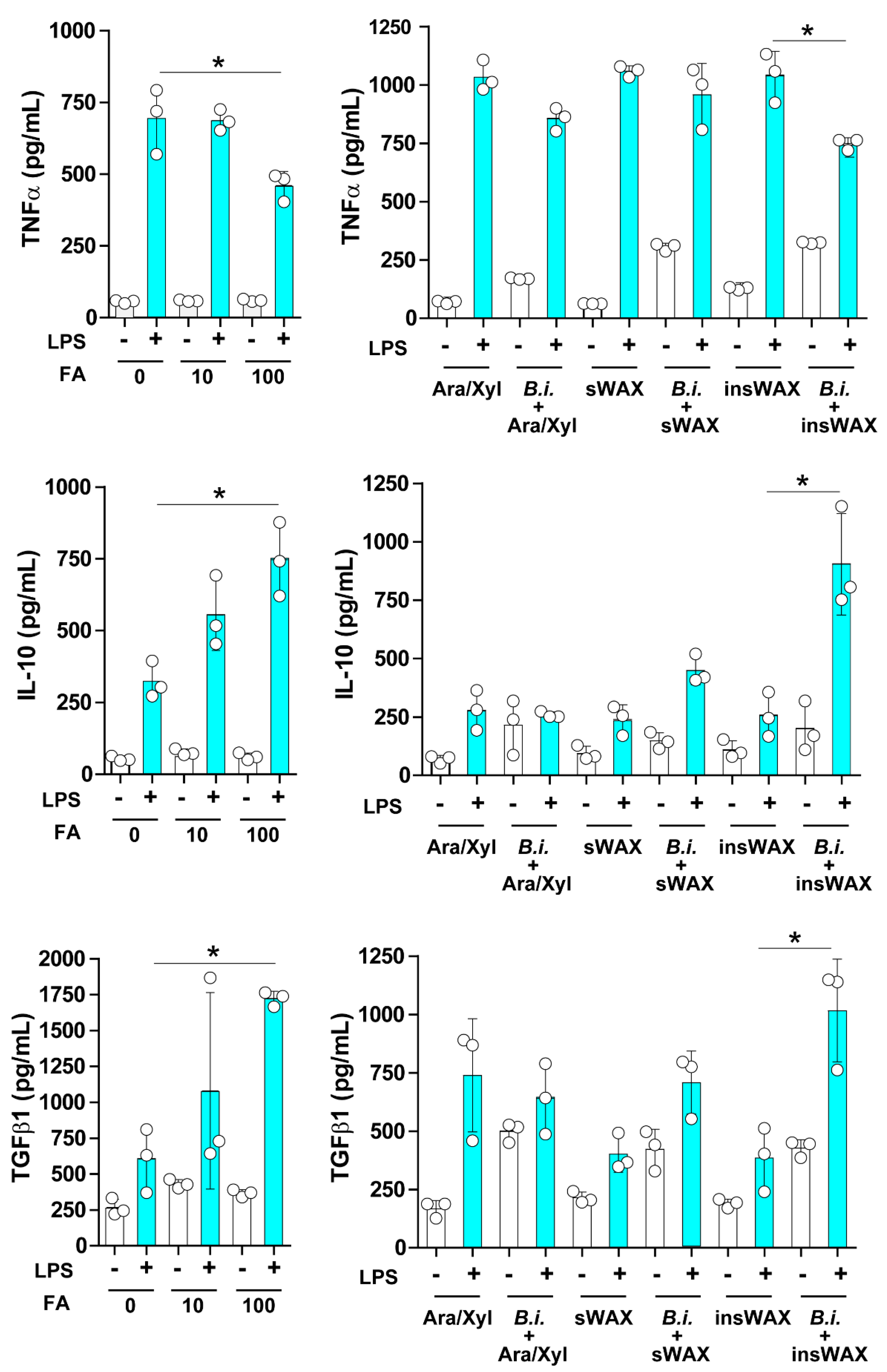
3.2. The Culture Supernatant from Bacteroides Intestinalis Modulates the Immune Response under Inflammatory Conditions
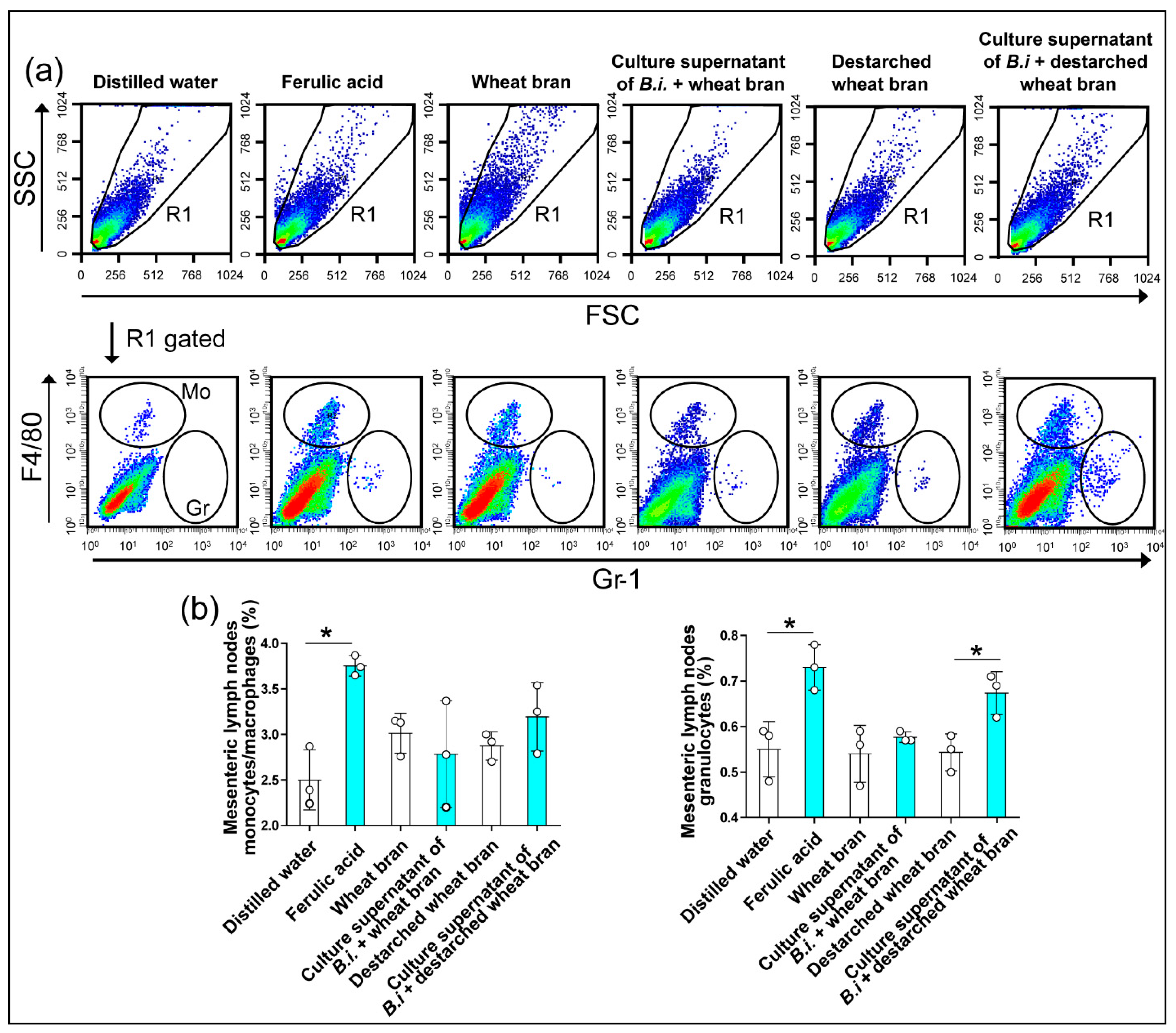
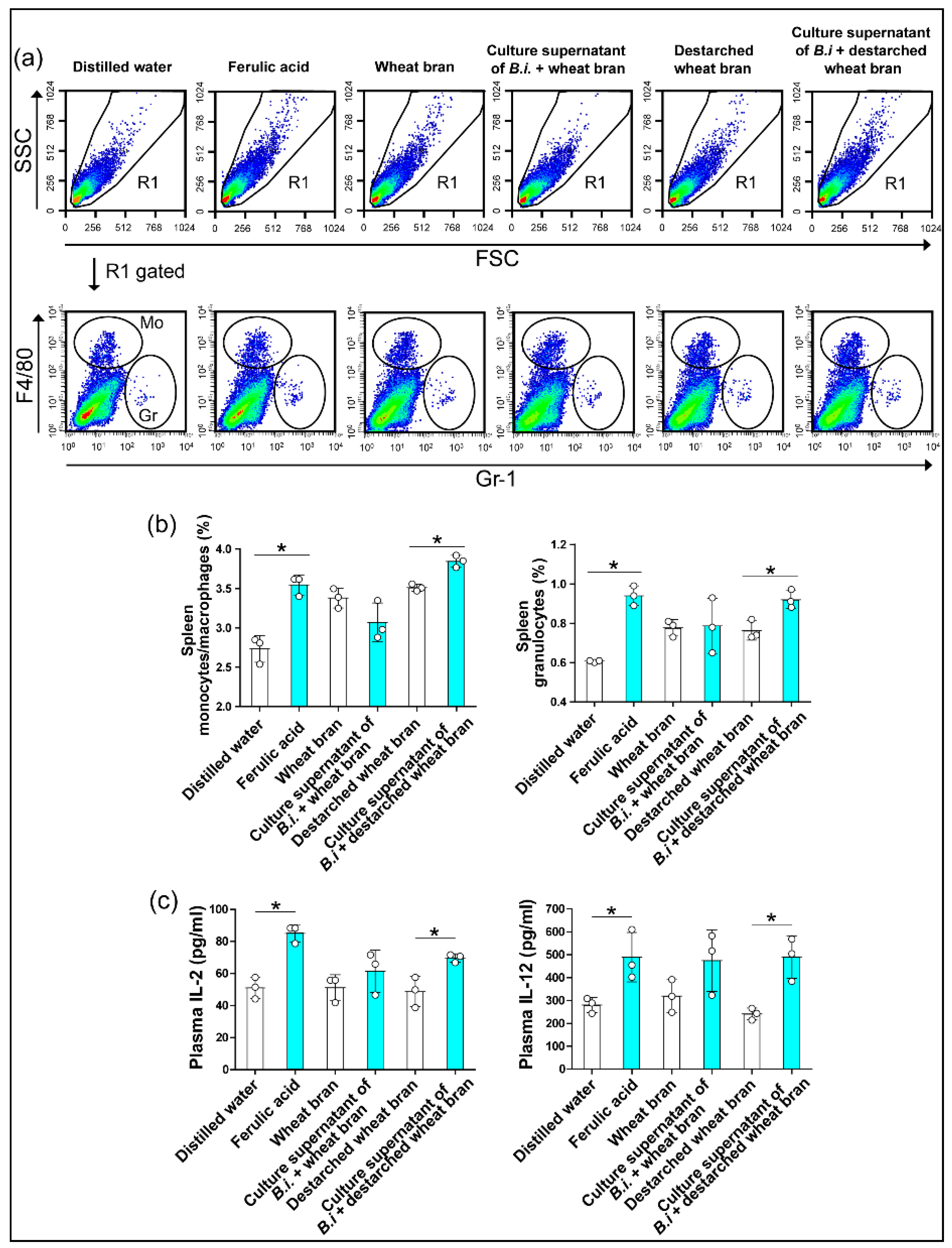
3.3. The Culture Supernatant from Bacteroides Intestinalis Modulates the Immune Response under Physiological Conditions
3.4. The Culture Supernatant from Bacteroides Intestinalis Modulates the Immune State of Colonic Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Barrett, E.M.; Batterham, M.J.; Ray, S.; Beck, E.J. Whole grain, bran and cereal fibre consumption and CVD: A systematic review. Br. J. Nutr. 2019, 121, 914–937. [Google Scholar] [CrossRef]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef] [Green Version]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fibre in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.; Xu, M.; Lee, A.; Cho, S.; Qi, L. Consumption of whole grains and cereal fiber and total and cause-specific mortality: Prospective analysis of 367,442 individuals. BMC Med. 2015, 13, 59. [Google Scholar]
- Prasadi, V.P.; Joye, I.J. Dietary Fibre from Whole Grains and Their Benefits on Metabolic Health. Nutrients 2020, 12, 3045. [Google Scholar]
- McCartney, L.; Marcus, S.E.; Knox, J.P. Monoclonal antibodies to plant cell wall xylans and arabinoxylans. J. Histochem. Cytochem. 2005, 53, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.V.; Abdel-Hamid, A.M.; Dutta, S.; D’Alessandro-Gabazza, C.N.; Wefers, D.; Farris, J.A.; Bajaj, S.; Wawrzak, Z.; Atomi, H.; Mackie, R.I.; et al. Degradation of complex arabinoxylans by human colonic Bacteroidetes. Nat. Commun. 2021, 12, 459. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.S.; Muralikrishna, G. Water soluble feruloyl arabinoxylans from rice and ragi: Changes upon malting and their consequence on antioxidant activity. Phytochemistry 2006, 67, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Basak, P.; Dutta, S.; Chowdhury, S.; Sil, P.C. New insights into the ameliorative effects of ferulic acid in pathophysiological conditions. Food Chem. Toxicol. 2017, 103, 41–55. [Google Scholar] [CrossRef]
- Fadel, A.; Mahmoud, A.M.; Ashworth, J.J.; Li, W.; Ng, Y.L.; Plunkett, A. Health-related effects and improving extractability of cereal arabinoxylans. Int. J. Biol. Macromol. 2018, 109, 819–831. [Google Scholar] [CrossRef] [Green Version]
- Holck, J.; Fredslund, F.; Moller, M.S.; Brask, J.; Krogh, K.; Lange, L.; Welner, D.H.; Svensson, B.; Meyer, A.S.; Wilkens, C. A carbohydrate-binding family 48 module enables feruloyl esterase action on polymeric arabinoxylan. J. Biol. Chem. 2019, 294, 17339–17353. [Google Scholar] [CrossRef] [Green Version]
- Glenwright, A.J.; Pothula, K.R.; Bhamidimarri, S.P.; Chorev, D.S.; Basle, A.; Firbank, S.J.; Zheng, H.; Robinson, C.V.; Winterhalter, M.; Kleinekathofer, U.; et al. Structural basis for nutrient acquisition by dominant members of the human gut microbiota. Nature 2017, 541, 407–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leth, M.L.; Ejby, M.; Workman, C.; Ewald, D.A.; Pedersen, S.S.; Sternberg, C.; Bahl, M.I.; Licht, T.R.; Aachmann, F.L.; Westereng, B.; et al. Differential bacterial capture and transport preferences facilitate co-growth on dietary xylan in the human gut. Nat. Microbiol. 2018, 3, 570–580. [Google Scholar] [CrossRef]
- Koropatkin, N.M.; Cameron, E.A.; Martens, E.C. How glycan metabolism shapes the human gut microbiota. Nat. Rev. Microbiol. 2012, 10, 323–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turnbaugh, P.J.; Backhed, F.; Fulton, L.; Gordon, J.I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 2008, 3, 213–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Chekan, J.R.; Dodd, D.; Hong, P.Y.; Radlinski, L.; Revindran, V.; Nair, S.K.; Mackie, R.I.; Cann, I. Xylan utilization in human gut commensal bacteria is orchestrated by unique modular organization of polysaccharide-degrading enzymes. Proc. Natl. Acad. Sci. USA 2014, 111, E3708–E3717. [Google Scholar] [CrossRef] [Green Version]
- El Kaoutari, A.; Armougom, F.; Gordon, J.I.; Raoult, D.; Henrissat, B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Microbiol. 2013, 11, 497–504. [Google Scholar] [CrossRef]
- Wang, K.; Pereira, G.V.; Cavalcante, J.J.; Zhang, M.; Mackie, R.; Cann, I. Bacteroides intestinalis DSM 17393, a member of the human colonic microbiome, upregulates multiple endoxylanases during growth on xylan. Sci. Rep. 2016, 6, 34360. [Google Scholar] [CrossRef] [Green Version]
- Martens, E.C.; Lowe, E.C.; Chiang, H.; Pudlo, N.A.; Wu, M.; McNulty, N.P.; Abbott, D.W.; Henrissat, B.; Gilbert, H.J.; Bolam, D.N.; et al. Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PLoS Biol. 2011, 9, e1001221. [Google Scholar] [CrossRef]
- Bartolome, B.; Faulds, C.B.; Kroon, P.A.; Waldron, K.; Gilbert, H.J.; Hazlewood, G.; Williamson, G. An Aspergillus niger esterase (ferulic acid esterase III) and a recombinant Pseudomonas fluorescens subsp. cellulosa esterase (Xy1D) release a 5-5’ ferulic dehydrodimer (diferulic acid) from barley and wheat cell walls. Appl. Environ. Microbiol. 1997, 63, 208–212. [Google Scholar] [CrossRef] [Green Version]
- Couteau, D.; McCartney, A.L.; Gibson, G.R.; Williamson, G.; Faulds, C.B. Isolation and characterization of human colonic bacteria able to hydrolyse chlorogenic acid. J. Appl. Microbiol. 2001, 90, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Ahmadifar, E.; Moghadam, M.S.; Dawood, M.A.O.; Hoseinifar, S.H. Lactobacillus fermentum and/or ferulic acid improved the immune responses, antioxidative defence and resistance against Aeromonas hydrophila in common carp (Cyprinus carpio) fingerlings. Fish Shellfish Immunol. 2019, 94, 916–923. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, M.; Medina, S.; Baeza, I.; Jimenez, L. Improvement of leucocyte functions in mature and old mice after 15 and 30 weeks of diet supplementation with polyphenol-rich biscuits. Eur. J. Nutr. 2011, 50, 563–573. [Google Scholar] [CrossRef] [PubMed]
- de Melo, T.S.; Lima, P.R.; Carvalho, K.M.; Fontenele, T.M.; Solon, F.R.; Tome, A.R.; de Lemos, T.L.; da Cruz Fonseca, S.G.; Santos, F.A.; Rao, V.S.; et al. Ferulic acid lowers body weight and visceral fat accumulation via modulation of enzymatic, hormonal and inflammatory changes in a mouse model of high-fat diet-induced obesity. Braz J. Med. Biol. Res. 2017, 50, e5630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naowaboot, J.; Piyabhan, P.; Munkong, N.; Parklak, W.; Pannangpetch, P. Ferulic acid improves lipid and glucose homeostasis in high-fat diet-induced obese mice. Clin. Exp. Pharmacol. Physiol. 2016, 43, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Lopez, N.J.; Astiazaran-Garcia, H.; Gonzalez-Aguilar, G.A.; Loarca-Pina, G.; Ezquerra-Brauer, J.M.; Dominguez Avila, J.A.; Robles-Sanchez, M. Ferulic Acid on Glucose Dysregulation, Dyslipidemia, and Inflammation in Diet-Induced Obese Rats: An Integrated Study. Nutrients 2017, 9, 675. [Google Scholar] [CrossRef] [Green Version]
- Senaphan, K.; Kukongviriyapan, U.; Sangartit, W.; Pakdeechote, P.; Pannangpetch, P.; Prachaney, P.; Greenwald, S.E.; Kukongviriyapan, V. Ferulic Acid Alleviates Changes in a Rat Model of Metabolic Syndrome Induced by High-Carbohydrate, High-Fat Diet. Nutrients 2015, 7, 6446–6464. [Google Scholar] [CrossRef]
- Presky, D.H.; Yang, H.; Minetti, L.J.; Chua, A.O.; Nabavi, N.; Wu, C.Y.; Gately, M.K.; Gubler, U. A functional interleukin 12 receptor complex is composed of two beta-type cytokine receptor subunits. Proc. Natl. Acad. Sci. USA 1996, 93, 14002–14007. [Google Scholar] [CrossRef] [Green Version]
- Vignali, D.A.; Kuchroo, V.K. IL-12 family cytokines: Immunological playmakers. Nat. Immunol. 2012, 13, 722–728. [Google Scholar] [CrossRef] [Green Version]
- Boxx, G.M.; Cheng, G. The Roles of Type I Interferon in Bacterial Infection. Cell Host Microbe 2016, 19, 760–769. [Google Scholar] [CrossRef] [Green Version]
- Crouse, J.; Kalinke, U.; Oxenius, A. Regulation of antiviral T cell responses by type I interferons. Nat. Rev. Immunol. 2015, 15, 231–242. [Google Scholar] [CrossRef] [PubMed]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Galluzzi, L.; Kepp, O.; Smyth, M.J.; Kroemer, G. Type I interferons in anticancer immunity. Nat. Rev. Immunol. 2015, 15, 405–414. [Google Scholar] [CrossRef] [PubMed]


| Gene | Direction | Sequence (5’ to 3’) | Length | Product Size | Cycles |
|---|---|---|---|---|---|
| GAPDH | Sense Antisense | GGAGCGAGATCCCTCCAAAAT GGCTGTTGTCATACTTCTCATGG | 21 23 | 197 bp | 26 |
| IL-12 p35 | Sense Antisense | CCTTGCACTTCTGAAGAGATTGA ACAGGGCCATCATAAAAGAGGT | 23 22 | 181 bp | 35 |
| IFNα | Sense Antisense | GACTCCATCYTGGCTGTGA TGATTTCTGCTCTGACAACCT | 19 21 | 103 bp | 33 |
| IFNβ | Sense Antisense | GCTTGGATTCCTACAAAGAAGCA ATAGATGGTCAATGCGGCGTC | 23 21 | 166 bp | 33 |
| Treatment | Dendritic Cells (%) | Total Lymphocytes (%) | B Cells (%) | CD4+ T Cells (%) | CD8+ T Cells (%) | Natural Killer Cells (%) | Natural Killer T Cells (%) |
|---|---|---|---|---|---|---|---|
| Distilled water | 2.44 ± 0.33 | 77.36 ± 3.59 | 56.63 ± 0.75 | 11.30 ± 2.69 | 7.75 ± 1.37 | 0.29 ± 0.05 | 0.09 ± 0.02 |
| Ferulic acid | 3.23 ± 0.19 * | 86.06 ± 1.34 * | 53.03 ± 0.98 * | 18.87 ± 0.46 * | 11.25 ± 0.38 * | 0.48 ± 0.03 * | 0.13 ± 0.02 * |
| Wheat bran | 2.39 ± 0.11 | 82.93 ± 0.73 | 55.62 ± 0.36 | 15.49 ± 0.86 | 10.23 ± 0.29 | 0.31 ± 0.05 | 0.13 ± 0.01 |
| Culture supernatant of B.i + wheat bran | 2.97 ± 0.21 † | 84.31 ± 0.70 | 50.86 ± 0.93 † | 16.33 ± 0.58 | 15.19 ± 1.22 † | 0.52 ± 0.04 † | 0.11 ± 0.02 |
| Destarched bran | 3.08 ± 0.18 | 81.40 ± 1.26 | 52.71 ± 0.63 | 14.17 ± 1.13 | 12.79 ± 0.60 | 0.46 ± 0.06 | 0.13 ± 0.03 |
| Culture supernatant of B.i + destarched wheat bran | 3.14 ± 0.24 | 82.25 ± 1.38 | 49.88 ± 0.82 ‡ | 16.24 ± 1.86 | 14.01 ± 0.35 ‡ | 0.64 ± 0.07 ‡ | 0.12 ± 0.01 |
| Treatment | Dendritic Cells (%) | Total Lymphocytes (%) | B Cells (%) | CD4+ T Cells (%) | CD8+ T Cells (%) | Natural Killer Cells (%) | Natural Killer T Cells (%) |
|---|---|---|---|---|---|---|---|
| Distilled water | 3.08 ± 0.06 | 61.84 ± 2.58 | 37.12 ± 1.51 | 11.95 ± 1.02 | 10.90 ± 0.45 | 0.42 ± 0.04 | 0.15 ± 0.03 |
| Ferulic acid | 3.94 ± 0.14 * | 61.19 ± 1.42 | 33.32 ± 0.66 * | 13.77 ± 0.79 * | 12.01 ± 0.21 * | 0.61 ± 0.09 * | 0.18 ± 0.05 * |
| Wheat bran | 3.19 ± 0.17 | 57.05 ± 1.88 | 33.24 ± 0.70 | 11.32 ± 0.83 | 10.55 ± 0.42 | 0.65 ± 0.02 | 0.12 ± 0.04 |
| Culture supernatant of B.i + wheat bran | 3.19 ± 0.27 | 57.41 ± 0.48 | 31.67 ± 0.45 † | 12.57 ± 0.11 † | 11.08 ± 0.62 | 0.66 ± 0.01 | 0.12 ± 0.02 |
| Destarched bran | 3.40 ± 0.16 | 56.47 ± 0.35 | 30.10 ± 0.55 | 13.72 ± 0.66 | 10.77 ± 0.09 | 0.61 ± 0.08 | 0.12 ± 0.01 |
| Culture supernatant of B.i + destarched wheat bran | 3.36 ± 0.12 | 56.66 ± 1.64 | 30.46 ± 1.00 | 12.52 ± 0.17 ‡ | 11.48 ± 0.74 | 0.71 ± 0.06 | 0.15 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasuma, T.; Toda, M.; Abdel-Hamid, A.M.; D’Alessandro-Gabazza, C.; Kobayashi, T.; Nishihama, K.; D’Alessandro, V.F.; Pereira, G.V.; Mackie, R.I.; Gabazza, E.C.; et al. Degradation Products of Complex Arabinoxylans by Bacteroides intestinalis Enhance the Host Immune Response. Microorganisms 2021, 9, 1126. https://doi.org/10.3390/microorganisms9061126
Yasuma T, Toda M, Abdel-Hamid AM, D’Alessandro-Gabazza C, Kobayashi T, Nishihama K, D’Alessandro VF, Pereira GV, Mackie RI, Gabazza EC, et al. Degradation Products of Complex Arabinoxylans by Bacteroides intestinalis Enhance the Host Immune Response. Microorganisms. 2021; 9(6):1126. https://doi.org/10.3390/microorganisms9061126
Chicago/Turabian StyleYasuma, Taro, Masaaki Toda, Ahmed M. Abdel-Hamid, Corina D’Alessandro-Gabazza, Tetsu Kobayashi, Kota Nishihama, Valeria Fridman D’Alessandro, Gabriel V. Pereira, Roderick I. Mackie, Esteban C. Gabazza, and et al. 2021. "Degradation Products of Complex Arabinoxylans by Bacteroides intestinalis Enhance the Host Immune Response" Microorganisms 9, no. 6: 1126. https://doi.org/10.3390/microorganisms9061126
APA StyleYasuma, T., Toda, M., Abdel-Hamid, A. M., D’Alessandro-Gabazza, C., Kobayashi, T., Nishihama, K., D’Alessandro, V. F., Pereira, G. V., Mackie, R. I., Gabazza, E. C., & Cann, I. (2021). Degradation Products of Complex Arabinoxylans by Bacteroides intestinalis Enhance the Host Immune Response. Microorganisms, 9(6), 1126. https://doi.org/10.3390/microorganisms9061126








