Characterization of Cold-Tolerant Saccharomyces cerevisiae Cheongdo Using Phenotype Microarray
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Fermentation
2.2. Phenotype Microarray
2.3. DNA Sequencing
2.4. High-Performance Liquid Chromatography (HPLC) Analysis
3. Results
3.1. Phenotype Microarray Analysis
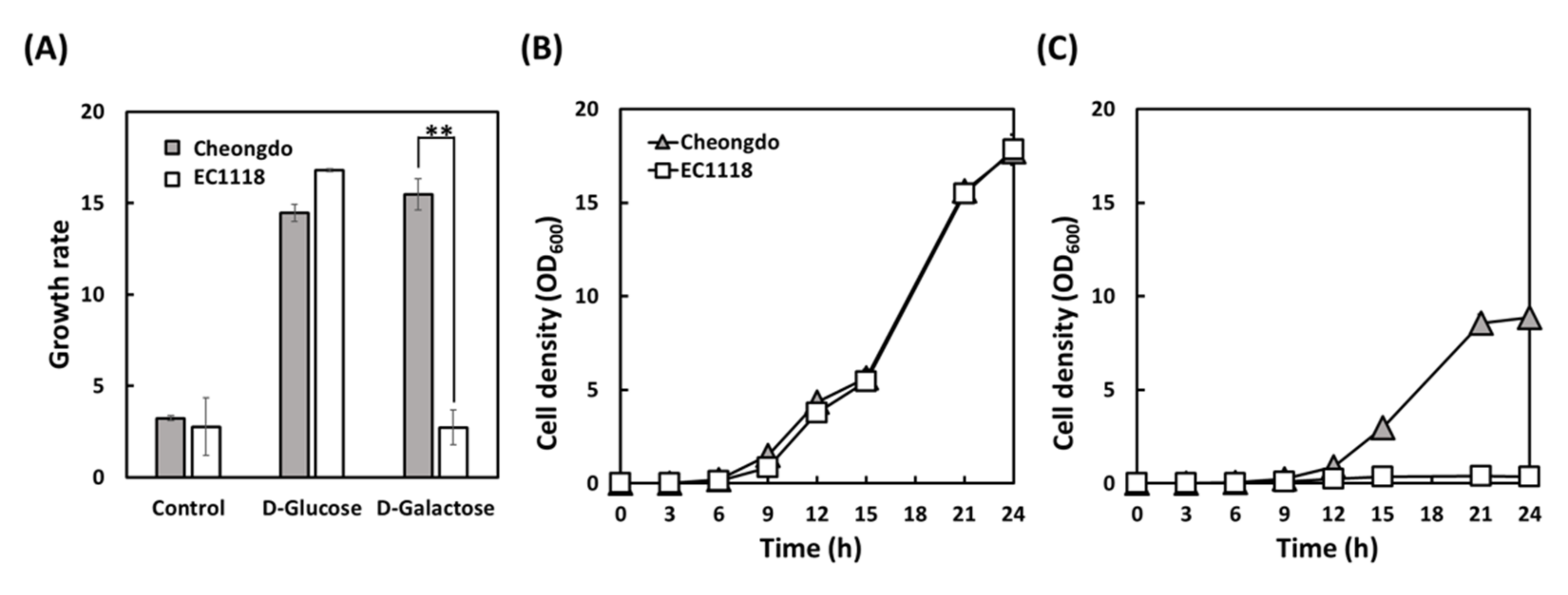
3.2. Validation of Phenotype Microarray Results by Flask Fermentation
3.3. Molecular Mechanism of the Difference in Galactose Fermentation
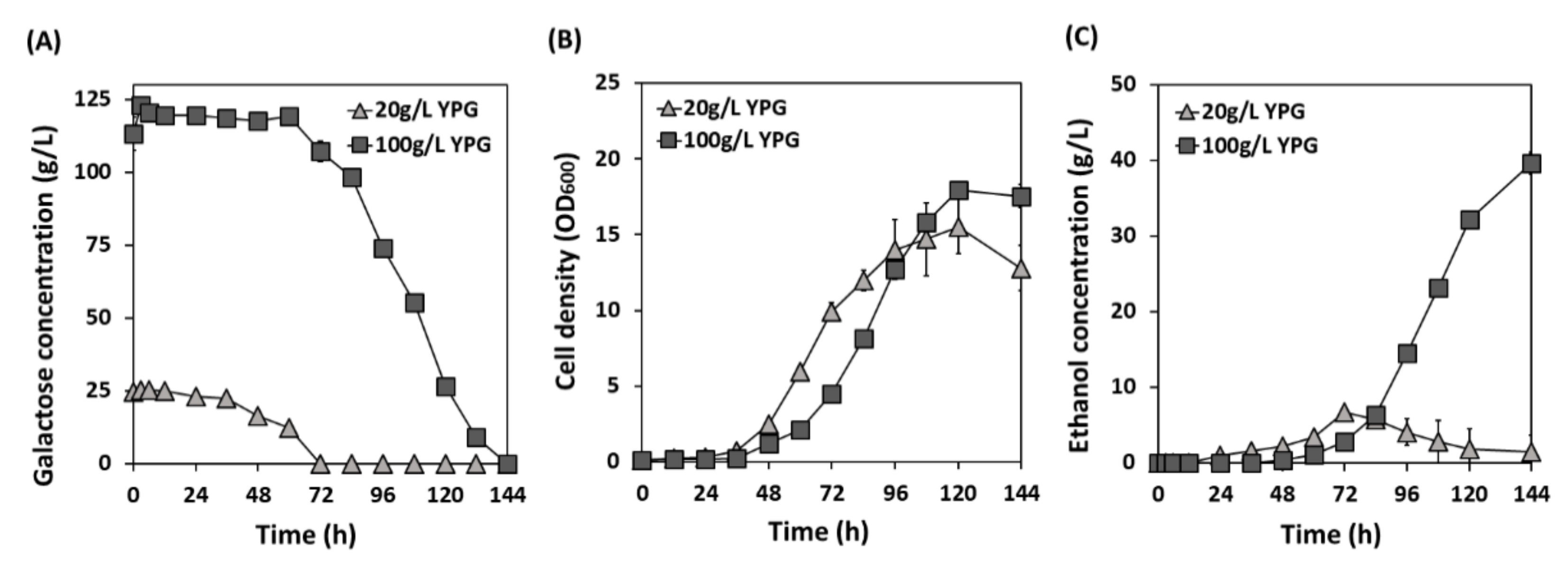
3.4. Galactose Fermentation at a Low Temperature
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bochner, B.R.; Gadzinski, P.; Panomitros, E. Phenotype MicroArrays for high-throughput phenotypic testing and assay of gene function. Genome Res. 2001, 11, 1246–1255. [Google Scholar] [CrossRef]
- Bochner, B.R. New technologies to assess genotype–phenotype relationships. Nat. Rev. Genet. 2003, 4, 309–314. [Google Scholar] [CrossRef]
- Warringer, J.; Blomberg, A. Automated screening in environmental arrays allows analysis of quantitative phenotypic profiles in Saccharomyces cerevisiae. Yeast 2003, 20, 53–67. [Google Scholar] [CrossRef]
- Vaas, L.A.I.; Sikorski, J.; Hofner, B.; Fiebig, A.; Buddruhs, N.; Klenk, H.P.; Goker, M. opm: An R package for analysing OmniLog((R)) phenotype microarray data. Bioinformatics 2013, 29, 1823–1824. [Google Scholar] [CrossRef]
- Warringer, J.; Ericson, E.; Fernandez, L.; Nerman, O.; Blomberg, A. High-resolution yeast phenomics resolves different physiological features in the saline response. Proc. Natl. Acad. Sci. USA 2003, 100, 15724–15729. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Ricaud, L.; Warringer, J.; Ericson, E.; Pylvänäinen, I.; Kemp, G.J.L.; Nerman, O.; Blomberg, A. PROPHECY—A database for high-resolution phenomics. Nucleic Acids Res. 2005, 33, D369–D373. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baryshnikova, A.; Costanzo, M.; Kim, Y.; Ding, H.; Koh, J.; Toufighi, K.; Youn, J.-Y.; Ou, J.; San Luis, B.-J.; Bandyopadhyay, S.; et al. Quantitative analysis of fitness and genetic interactions in yeast on a genome scale. Nat. Methods 2010, 7, 1017–1024. [Google Scholar] [CrossRef]
- Skelly, D.A.; Merrihew, G.E.; Riffle, M.; Connelly, C.F.; Kerr, E.O.; Johansson, M.; Jaschob, D.; Graczyk, B.; Shulman, N.J.; Wakefield, J.; et al. Integrative phenomics reveals insight into the structure of phenotypic diversity in budding yeast. Genome Res. 2013, 23, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Märtens, K.; Hallin, J.; Warringer, J.; Liti, G.; Parts, L. Predicting quantitative traits from genome and phenome with near perfect accuracy. Nat. Commun. 2016, 7, 11512. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Bergdahl, B.; Machado, D.; Dato, L.; Han, T.L.; Li, J.; Villas-Boas, S.; Herrgard, M.J.; Forster, J.; Panagiotou, G. Linking genetic, metabolic, and phenotypic diversity among Saccharomyces cerevisiae strains using multi-omics associations. Gigascience 2019, 8, giz015. [Google Scholar] [CrossRef]
- Hyma, K.E.; Saerens, S.M.; Verstrepen, K.J.; Fay, J.C. Divergence in wine characteristics produced by wild and domesticated strains of Saccharomyces cerevisiae. FEMS Yeast Res. 2011, 11, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Peter, J.; De Chiara, M.; Friedrich, A.; Yue, J.X.; Pflieger, D.; Bergstrom, A.; Sigwalt, A.; Barre, B.; Freel, K.; Llored, A.; et al. Genome evolution across 1011 Saccharomyces cerevisiae isolates. Nature 2018, 556, 339. [Google Scholar] [CrossRef]
- Legras, J.L.; Galeote, V.; Bigey, F.; Camarasa, C.; Marsit, S.; Nidelet, T.; Sanchez, I.; Couloux, A.; Guy, J.; Franco-Duarte, R.; et al. Adaptation of S. cerevisiae to Fermented Food Environments Reveals Remarkable Genome Plasticity and the Footprints of Domestication. Mol. Biol. Evol. 2018, 35, 1712–1727. [Google Scholar] [CrossRef] [PubMed]
- Parapouli, M.; Vasileiadis, A.; Afendra, A.-S.; Hatziloukas, E. Saccharomyces cerevisiae and its industrial applications. Aims Microbiol. 2020, 6, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Lengeler, K.B.; Stovicek, V.; Fennessy, R.T.; Katz, M.; Förster, J. Never Change a Brewing Yeast? Why Not, There Are Plenty to Choose From. Front. Genet. 2020, 11, 582789. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.-M.; Lee, Y.; Kim, J.-w.; Seol, J.; Jung, Y.H.; Kim, S.R. Low-temperature alcoholic fermentation for the production of high quality vinegar using peach. KSBB J. 2018, 33, 95–103. [Google Scholar] [CrossRef]
- Jeong, D.; Park, H.; Bae, E.-Y.; Seol, M.-K.; Ju, Y.-B.; Kim, J.-S.; Jang, B.-K.; Akhmadjon, S.; Kim, B.-O.; Cho, Y.-J.; et al. Source of contamination, detection techniques, and control methods of spoilage yeast in fermented foods. KSBB J. 2020, 35, 23–33. [Google Scholar] [CrossRef]
- Liszkowska, W.; Berlowska, J. Yeast Fermentation at Low Temperatures: Adaptation to Changing Environmental Conditions and Formation of Volatile Compounds. Molecules 2021, 26, 1035. [Google Scholar] [CrossRef]
- Magalhães, F.; Calton, A.; Heiniö, R.-L.; Gibson, B. Frozen-dough baking potential of psychrotolerant Saccharomyces species and derived hybrids. Food Microbiol. 2021, 94, 103640. [Google Scholar] [CrossRef]
- Aguilera, J.; Randez-Gil, F.; Prieto, J.A. Cold response in Saccharomyces cerevisiae: New functions for old mechanisms. Fems Microbiol. Rev. 2007, 31, 327–341. [Google Scholar] [CrossRef]
- Feng, L.; Jia, H.; Qin, Y.; Song, Y.; Tao, S.; Liu, Y. Rapid Identification of Major QTLS Associated With Near- Freezing Temperature Tolerance in Saccharomyces cerevisiae. Front. Microbiol. 2018, 9, 2110. [Google Scholar] [CrossRef] [PubMed]
- Libkind, D.; Hittinger, C.T.; Valério, E.; Gonçalves, C.; Dover, J.; Johnston, M.; Gonçalves, P.; Sampaio, J.P. Microbe domestication and the identification of the wild genetic stock of lager-brewing yeast. Proc. Natl. Acad. Sci. USA 2011, 108, 14539–14544. [Google Scholar] [CrossRef]
- Ortiz-Tovar, G.; Minebois, R.; Barrio, E.; Querol, A.; Pérez-Torrado, R. Aroma production and fermentation performance of S. cerevisiae × S. kudriavzevii natural hybrids under cold oenological conditions. Int. J. Food Microbiol. 2019, 297, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Kopsahelis, N.; Nisiotou, A.; Kourkoutas, Y.; Panas, P.; Nychas, G.J.E.; Kanellaki, M. Molecular characterization and molasses fermentation performance of a wild yeast strain operating in an extremely wide temperature range. Bioresour. Technol. 2009, 100, 4854–4862. [Google Scholar] [CrossRef]
- Paget, C.M.; Schwartz, J.-M.; Delneri, D. Environmental systems biology of cold-tolerant phenotype in Saccharomyces species adapted to grow at different temperatures. Mol. Ecol. 2014, 23, 5241–5257. [Google Scholar] [CrossRef] [PubMed]
- Bou Zeidan, M.; Zara, G.; Viti, C.; Decorosi, F.; Mannazzu, I.; Budroni, M.; Giovannetti, L.; Zara, S. l-histidine inhibits biofilm formation and FLO11-associated phenotypes in Saccharomyces cerevisiae flor yeasts. PLoS ONE 2014, 9, e112141. [Google Scholar] [CrossRef]
- Borglin, S.; Joyner, D.; DeAngelis, K.M.; Khudyakov, J.; D’haeseleer, P.; Joachimiak, M.P.; Hazen, T. Application of phenotypic microarrays to environmental microbiology. Curr. Opin. Biotechnol. 2012, 23, 41–48. [Google Scholar] [CrossRef]
- Dulermo, R.; Legras, J.L.; Brunel, F.; Devillers, H.; Sarilar, V.; Neuveglise, C.; Nguyen, H.V. Truncation of Gal4p explains the inactivation of the GAL/MEL regulon in both Saccharomyces bayanus and some Saccharomyces cerevisiae wine strains. FEMS Yeast Res. 2016, 16, fow070. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhou, P.; Xu, N.; Yang, Z.; Du, Y.; Yue, C.; Ye, L. Directed evolution of the transcription factor Gal4 for development of an improved transcriptional regulation system in Saccharomyces cerevisiae. Enzym. Microb. Technol. 2020, 142, 109675. [Google Scholar] [CrossRef] [PubMed]
- Otero, J.M.; Vongsangnak, W.; Asadollahi, M.A.; Olivares-Hernandes, R.; Maury, J.; Farinelli, L.; Barlocher, L.; Osterås, M.; Schalk, M.; Clark, A.; et al. Whole genome sequencing of Saccharomyces cerevisiae: From genotype to phenotype for improved metabolic engineering applications. BMC Genom. 2010, 11, 723. [Google Scholar] [CrossRef]
- Goshima, T.; Tsuji, M.; Inoue, H.; Yano, S.; Hoshino, T.; Matsushika, A. Bioethanol Production from Lignocellulosic Biomass by a Novel Kluyveromyces marxianus Strain. Biosci. Biotechnol. Biochem. 2013, 77, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-S.; Hong, M.-E.; Jung, S.-C.; Ha, S.-J.; Yu, B.J.; Koo, H.M.; Park, S.M.; Seo, J.-H.; Kweon, D.-H.; Park, J.C.; et al. Improved galactose fermentation of Saccharomyces cerevisiae through inverse metabolic engineering. Biotechnol. Bioeng. 2011, 108, 621–631. [Google Scholar] [CrossRef]
- Quarterman, J.; Skerker, J.M.; Feng, X.; Liu, I.Y.; Zhao, H.; Arkin, A.P.; Jin, Y.-S. Rapid and efficient galactose fermentation by engineered Saccharomyces cerevisiae. J. Biotechnol. 2016, 229, 13–21. [Google Scholar] [CrossRef]
- Kim, J.H.; Ryu, J.; Huh, I.Y.; Hong, S.-K.; Kang, H.A.; Chang, Y.K. Ethanol production from galactose by a newly isolated Saccharomyces cerevisiae KL17. Bioprocess. Biosyst. Eng. 2014, 37, 1871–1878. [Google Scholar] [CrossRef]
- Ostergaard, S.; Olsson, L.; Johnston, M.; Nielsen, J. Increasing galactose consumption by Saccharomyces cerevisiae through metabolic engineering of the GAL gene regulatory network. Nat. Biotechnol. 2000, 18, 1283–1286. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.-F.; Shi, J.-Y.; Yin, Q.; Zhang, R.-P.; Han, P.-J.; Wang, Q.-M.; Bai, F.-Y. Reverse Evolution of a Classic Gene Network in Yeast Offers a Competitive Advantage. Curr. Biol. 2019, 29, 1126–1136.e1125. [Google Scholar] [CrossRef] [PubMed]
- Kouamé, C.; Loiseau, G.; Grabulos, J.; Boulanger, R.; Mestres, C. Development of a model for the alcoholic fermentation of cocoa beans by a Saccharomyces cerevisiae strain. Int. J. Food Microbiol. 2021, 337, 108917. [Google Scholar] [CrossRef]
- Bressani, A.P.P.; Martinez, S.J.; Evangelista, S.R.; Dias, D.R.; Schwan, R.F. Characteristics of fermented coffee inoculated with yeast starter cultures using different inoculation methods. LWT 2018, 92, 212–219. [Google Scholar] [CrossRef]
- Barišić, V.; Kopjar, M.; Jozinović, A.; Flanjak, I.; Ačkar, Đ.; Miličević, B.; Šubarić, D.; Jokić, S.; Babić, J. The Chemistry behind Chocolate Production. Molecules 2019, 24, 3163. [Google Scholar] [CrossRef]
- Redgwell, R.J.; Curti, D.; Rogers, J.; Nicolas, P.; Fischer, M. Changes to the galactose/mannose ratio in galactomannans during coffee bean (Coffea arabica L.) development: Implications for in vivo modification of galactomannan synthesis. Planta 2003, 217, 316–326. [Google Scholar] [CrossRef]
- Kim, J.-w.; Jang, J.H.; Yeo, H.J.; Seol, J.; Kim, S.R.; Jung, Y.H. Lactic Acid Production from a Whole Slurry of Acid-Pretreated Spent Coffee Grounds by Engineered Saccharomyces cerevisiae. Appl. Biochem. Biotechnol. 2019, 189, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Machado, E.M.S.; Carneiro, L.M.; Teixeira, J.A. Sugars metabolism and ethanol production by different yeast strains from coffee industry wastes hydrolysates. Appl. Energy 2012, 92, 763–768. [Google Scholar] [CrossRef]
- Domingues, L.; Guimarães, P.M.R.; Oliveira, C. Metabolic engineering of Saccharomyces cerevisiae for lactose/whey fermentation. Bioeng Bugs 2010, 1, 164–171. [Google Scholar] [CrossRef]
- Turner, T.L.; Kim, E.; Hwang, C.; Zhang, G.-C.; Liu, J.-J.; Jin, Y.-S. Short communication: Conversion of lactose and whey into lactic acid by engineered yeast. J. Dairy Sci. 2017, 100, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Ha, S.-J.; Wei, N.; Oh, E.J.; Jin, Y.-S. Simultaneous co-fermentation of mixed sugars: A promising strategy for producing cellulosic ethanol. Trends Biotechnol. 2012, 30, 274–282. [Google Scholar] [CrossRef]
- Kim, D.H.; Liu, J.J.; Lee, J.W.; Pelton, J.G.; Yun, E.J.; Yu, S.; Jin, Y.S.; Kim, K.H. Biological upgrading of 3,6-anhydro-l-galactose from agarose to a new platform chemical. Green Chem. 2020, 22, 1776–1785. [Google Scholar] [CrossRef]
- Lee, H.-J.; Kim, S.-J.; Yoon, J.-J.; Kim, K.H.; Seo, J.-H.; Park, Y.-C. Evolutionary engineering of Saccharomyces cerevisiae for efficient conversion of red algal biosugars to bioethanol. Bioresour. Technol. 2015, 191, 445–451. [Google Scholar] [CrossRef]
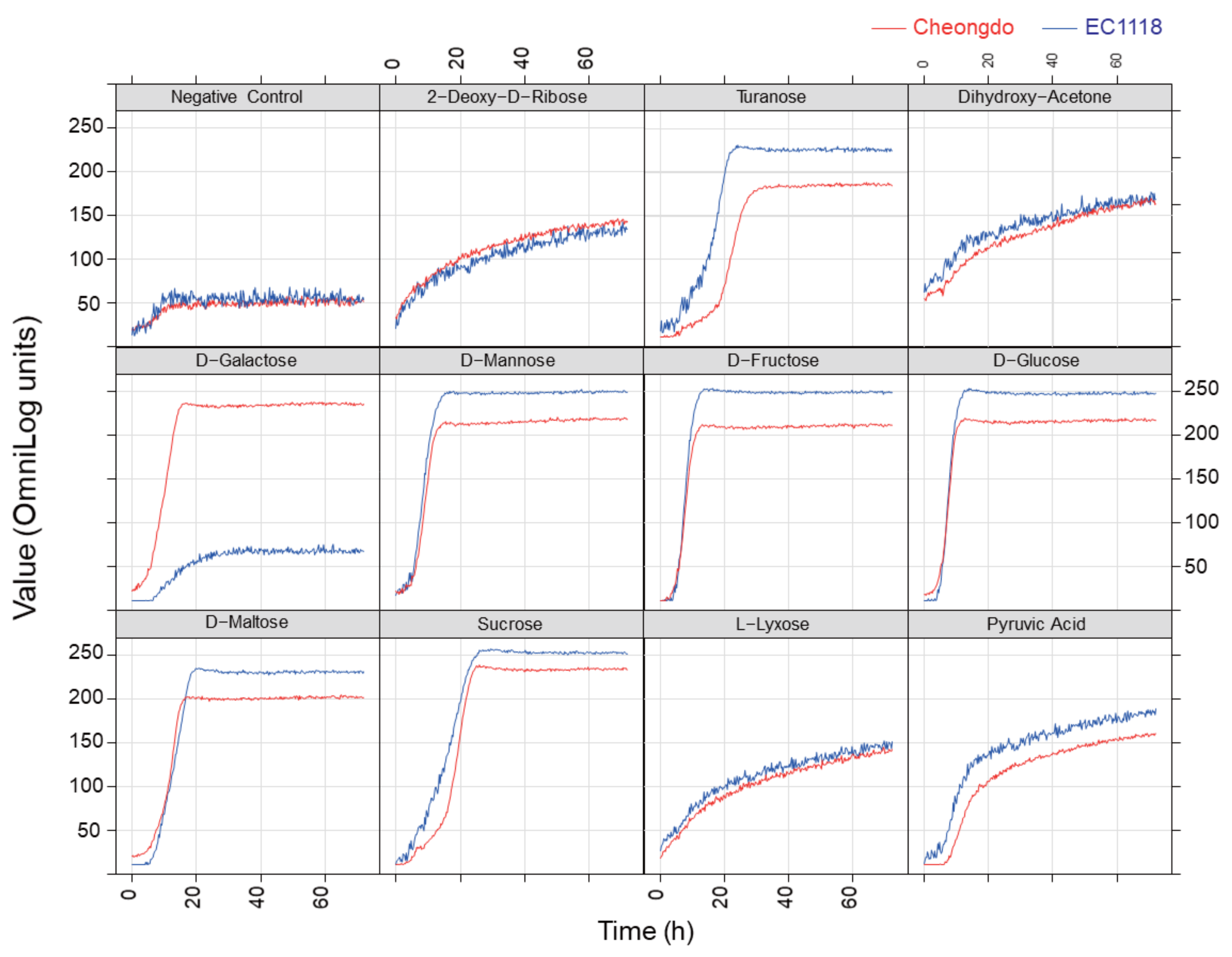


| Carbon Source 2 | Cheongdo | EC1118 | Difference | p-Value 3 |
|---|---|---|---|---|
| Negative Control | 3.23 ± 0.14 | 2.77 ± 1.56 | 0.47 | 0.74 |
| d-Galactose | 15.47 ± 0.85 | 2.73 ± 0.94 | 12.73 | 0.01 |
| l-Pyroglutamic Acid | 1.43 ± 0.33 | 4.83 ± 0.24 | 3.40 | 0.01 |
| 3-Hydroxy-2-Butanone | 2.93 ± 0.19 | 5.53 ± 0.28 | 2.60 | 0.01 |
| N-Acetyl-l-Glutamic Acid | 1.33 ± 0.19 | 3.73 ± 0.28 | 2.40 | 0.02 |
| 2,3-Butanedione | 1.87 ± 0.19 | 4.27 ± 0.38 | 2.40 | 0.03 |
| Butyric Acid | 2.30 ± 0.14 | 4.57 ± 0.33 | 2.27 | 0.04 |
| Palatinose | 1.97 ± 0.24 | 4.00 ± 0.19 | 2.03 | 0.01 |
| d,l-Carnitine | 1.33 ± 0.09 | 3.33 ± 0.19 | 2.00 | 0.02 |
| Putrescine | 1.03 ± 0.33 | 2.83 ± 0.33 | 1.80 | 0.03 |
| 3-0-β-d-Galactopyranosyl-d-Arabinose | 1.30 ± 0.14 | 3.07 ± 0.19 | 1.77 | 0.01 |
| l-Phenylalanine | 1.20 ± 0.19 | 2.97 ± 0.24 | 1.77 | 0.02 |
| d-Serine | 2.67 ± 0.00 | 1.00 ± 0.09 | 1.67 | 0.03 |
| β-Hydroxy Butyric Acid | 1.20 ± 0.09 | 2.73 ± 0.19 | 1.53 | 0.02 |
| l-Lysine | 1.43 ± 0.05 | 2.90 ± 0.05 | 1.47 | 0.00 |
| β-Methyl-d-Xyloside | 2.07 ± 0.09 | 3.53 ± 0.00 | 1.47 | 0.03 |
| Glycine | 0.80 ± 0.00 | 2.23 ± 0.05 | 1.43 | 0.01 |
| l-Alaninamide | 1.27 ± 0.19 | 2.67 ± 0.28 | 1.40 | 0.04 |
| l-Glutamic Acid | 3.43 ± 0.24 | 4.80 ± 0.19 | 1.37 | 0.03 |
| l-Arginine | 1.37 ± 0.24 | 2.73 ± 0.19 | 1.37 | 0.03 |
| d-Ribono-1,4-Lactone | 0.77 ± 0.05 | 2.10 ± 0.14 | 1.33 | 0.03 |
| d-Tartaric Acid | 1.63 ± 0.05 | 2.90 ± 0.05 | 1.27 | 0.00 |
| Hydroxy-l-Proline | 1.53 ± 0.09 | 2.77 ± 0.14 | 1.23 | 0.01 |
| Sorbic Acid | 1.27 ± 0.09 | 2.47 ± 0.19 | 1.20 | 0.03 |
| γ-Cyclodextrin | 3.03 ± 0.24 | 1.87 ± 0.19 | 1.17 | 0.04 |
| N-Acetyl-d-Glucosaminitol | 0.83 ± 0.14 | 1.97 ± 0.24 | 1.13 | 0.04 |
| 3-Methyl Glucose | 2.27 ± 0.09 | 3.30 ± 0.05 | 1.03 | 0.02 |
| l-Homoserine | 0.80 ± 0.09 | 1.83 ± 0.05 | 1.03 | 0.02 |
| δ-Amino Valeric Acid | 1.23 ± 0.05 | 2.23 ± 0.05 | 1.00 | 0.00 |
| d-Fucose | 2.23 ± 0.05 | 3.20 ± 0.00 | 0.97 | 0.02 |
| Sebacic Acid | 1.27 ± 0.09 | 2.20 ± 0.09 | 0.93 | 0.01 |
| l-Methionine | 1.43 ± 0.05 | 2.30 ± 0.05 | 0.87 | 0.00 |
| Citraconic Acid | 1.93 ± 0.19 | 2.77 ± 0.14 | 0.83 | 0.04 |
| γ-Amino Butyric Acid | 1.33 ± 0.00 | 1.97 ± 0.05 | 0.63 | 0.03 |
| l-Sorbose | 1.60 ± 0.09 | 2.20 ± 0.09 | 0.60 | 0.02 |
| Carbon Source 1 | 10 °C 2 | 30 °C 3 | ||
|---|---|---|---|---|
| Cheongdo | EC1118 | Cheongdo | EC1118 | |
| d-Glucose 20 g/L | 0.10 ± 0.00 | 0.11 ± 0.00 | 0.66 ± 0.06 | 0.55 ± 0.02 |
| d-Glucose 200 g/L | 0.60 ± 0.00 * | 0.52 ± 0.00 | 3.33 ± 0.01 | 3.38 ± 0.03 |
| d-Galactose 20 g/L | 0.09 ± 0.01 | No growth | 0.59 ± 0.01 | No growth |
| d-Galactose 100 g/L | 0.29 ± 0.00 | No growth | 1.76 ± 0.17 | No growth |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, K.-M.; Park, J.; Jang, J.; Jung, S.-H.; Lee, S.H.; Kim, S.R. Characterization of Cold-Tolerant Saccharomyces cerevisiae Cheongdo Using Phenotype Microarray. Microorganisms 2021, 9, 982. https://doi.org/10.3390/microorganisms9050982
Jung K-M, Park J, Jang J, Jung S-H, Lee SH, Kim SR. Characterization of Cold-Tolerant Saccharomyces cerevisiae Cheongdo Using Phenotype Microarray. Microorganisms. 2021; 9(5):982. https://doi.org/10.3390/microorganisms9050982
Chicago/Turabian StyleJung, Kyung-Mi, Jongbeom Park, Jueun Jang, Seok-Hwa Jung, Sang Han Lee, and Soo Rin Kim. 2021. "Characterization of Cold-Tolerant Saccharomyces cerevisiae Cheongdo Using Phenotype Microarray" Microorganisms 9, no. 5: 982. https://doi.org/10.3390/microorganisms9050982
APA StyleJung, K.-M., Park, J., Jang, J., Jung, S.-H., Lee, S. H., & Kim, S. R. (2021). Characterization of Cold-Tolerant Saccharomyces cerevisiae Cheongdo Using Phenotype Microarray. Microorganisms, 9(5), 982. https://doi.org/10.3390/microorganisms9050982








