Thermophilic Chloroflexi Dominate in the Microbial Community Associated with Coal-Fire Gas Vents in the Kuznetsk Coal Basin, Russia
Abstract
1. Introduction
2. Materials and Methods
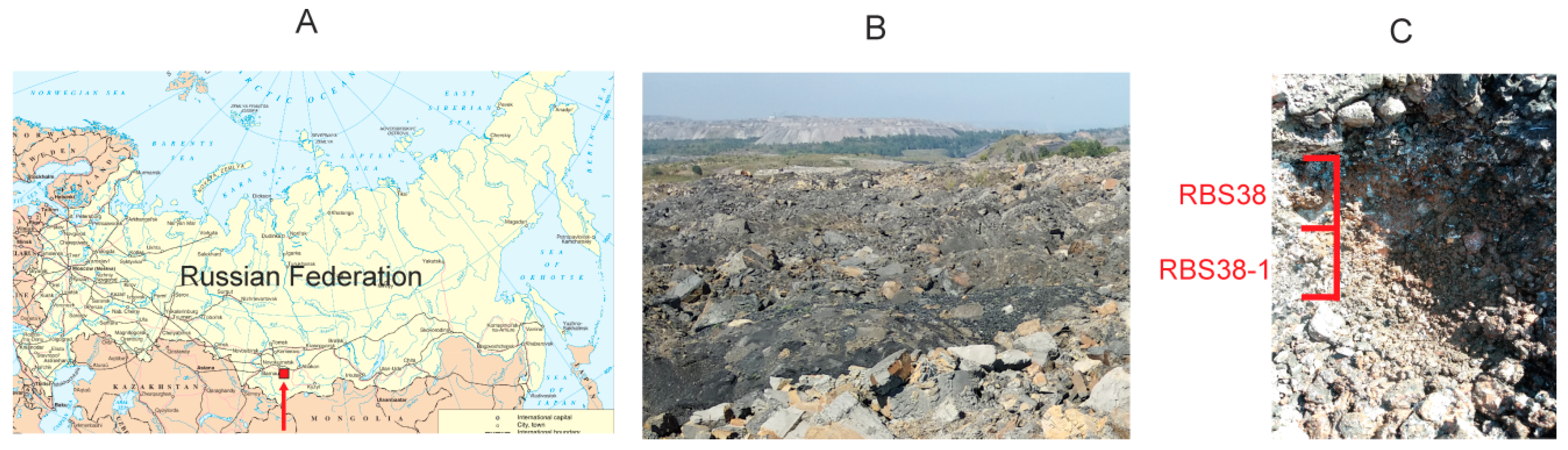
2.1. Study Site, Sampling, and Chemical Analyses
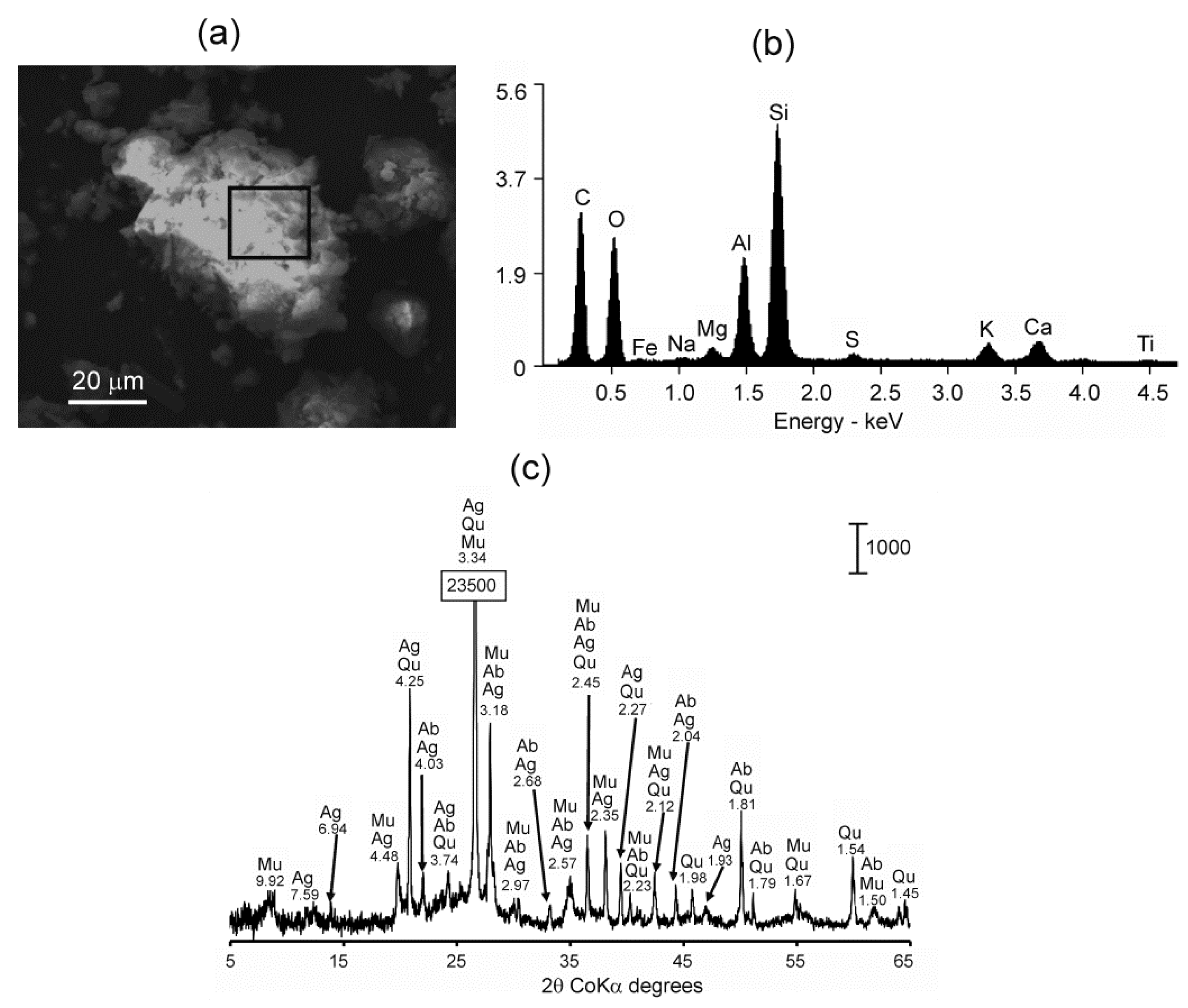
2.2. Mineralogical and Elemental Analysis
2.3. 16S rRNA Gene Sequencing and Analysis
2.4. Nucleotide Sequence Accession Numbers
3. Results and Discussion
3.1. Physicochemical and Mineralogical Characteristics of Ground Samples
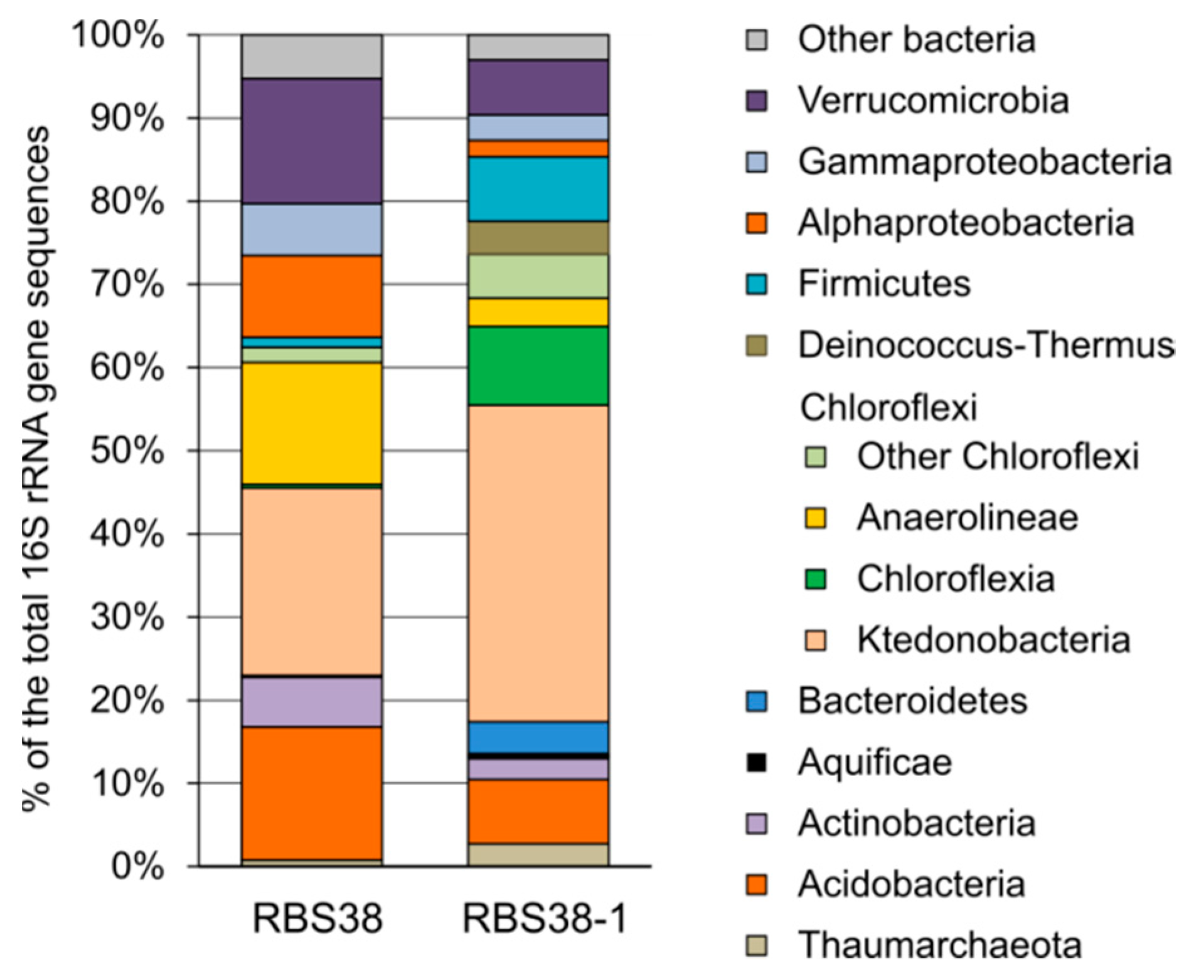
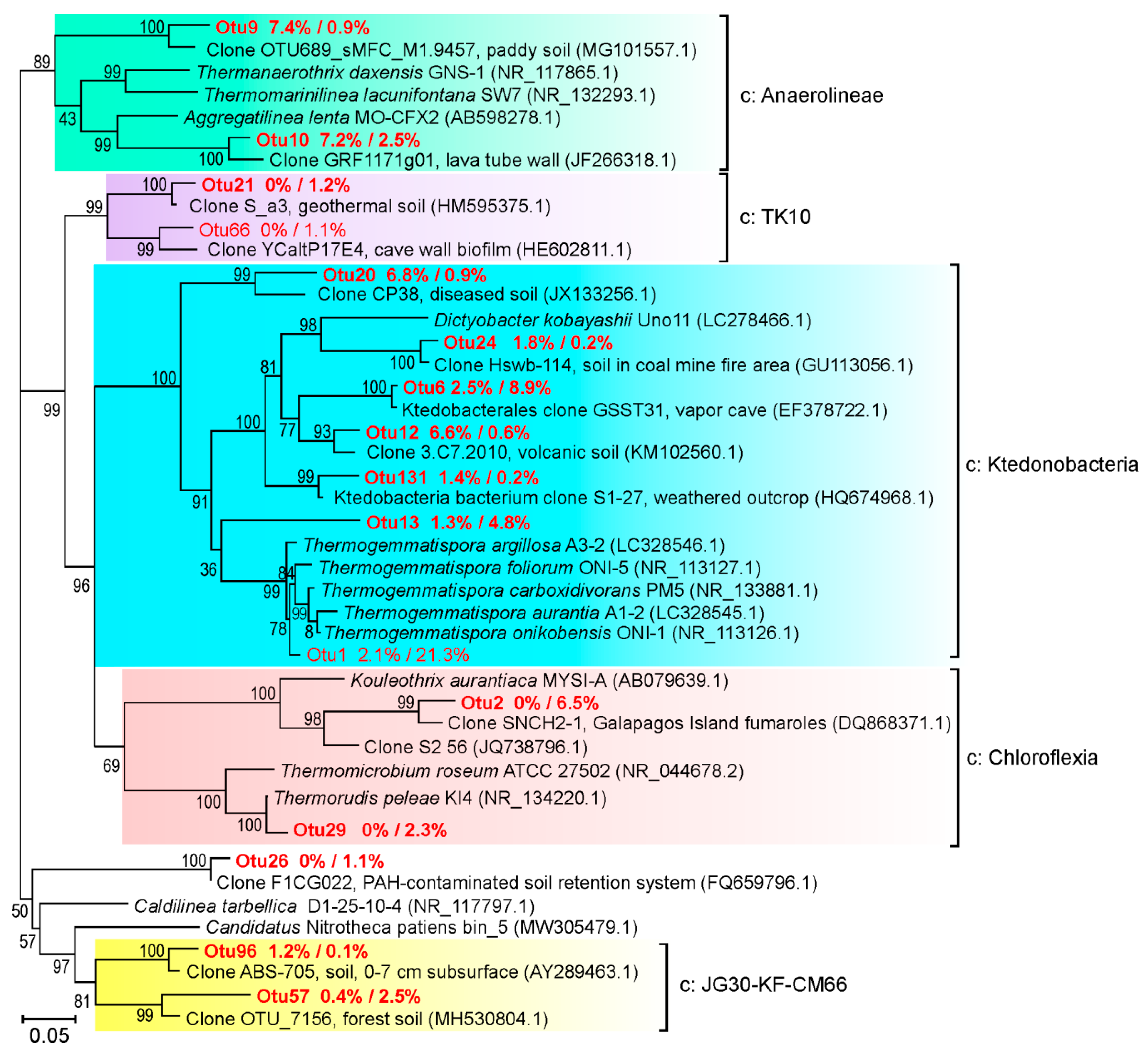
3.2. Microbial Community Structure Revealed by 16S rRNA Profiling
3.3. Comparison with Other Coal Fire Sites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Counts, J.A.; Zeldes, B.M.; Lee, L.L.; Straub, C.T.; Adams, M.W.W.; Kelly, R.M. Physiological, metabolic and biotechnological features of extremely thermophilic microorganisms. Wiley Interdiscip. Rev. Syst. Biol. Med. 2017, 9, e1377. [Google Scholar] [CrossRef] [PubMed]
- Urbieta, M.S.; Donati, E.R.; Chan, K.G.; Shahar, S.; Sin, L.L.; Goh, K.M. Thermophiles in the genomic era: Biodiversity, science, and applications. Biotechnol. Adv. 2015, 33, 633–647. [Google Scholar] [CrossRef]
- Stracher, G.B.; Taylor, T.P. Coal fires burning out of control around the world: Thermodynamic recipe for environmental catastrophe. Int. J. Coal Geol. 2004, 59, 7–17. [Google Scholar] [CrossRef]
- Rattigan, J.H. Phenomena about Burning Mountain, Wingen, NSW. Aust. J. Sci. 1967, 30, 183–184. [Google Scholar]
- Shafirovich, E.; Varma, A. Underground coal gasification: A brief review of current status. Ind. Eng. Res. 2009, 48, 7865–7875. [Google Scholar] [CrossRef]
- Engle, M.A.; Radke, L.F.; Heffern, E.L.; O’Keefe, J.M.; Hower, J.C.; Smeltzer, C.D.; Hower, J.M.; Olea, R.A.; Eatwell, R.J.; Blake, D.R.; et al. Gas emissions, minerals, and tars associated with three coal fires, Powder River Basin, USA. Sci. Total Environ. 2012, 420, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xu, J.; Zeng, J.; Lou, K. Diversity of prokaryotes associated with soils around coal-fire gas vents in MaNasi county of Xinjiang, China. Antonie Van Leeuwenhoek 2013, 103, 23–36. [Google Scholar] [CrossRef]
- Pone, J.D.N.; Hein, K.A.; Stracher, G.B.; Annegarn, H.J.; Finkleman, R.B.; Blake, D.R.; McCormack, J.K.; Schroeder, P. The spontaneous combustion of coal and its by-products in the Witbank and Sasolburg coalfields of South Africa. Int. J. Coal Geol. 2007, 72, 124–140. [Google Scholar] [CrossRef]
- Tobin-Janzen, T.; Shade, A.; Marshall, L.; Torres, K.; Beblo, C.; Janzen, C.; Lenig, J.; Martinez, A.; Ressler, D. Nitrogen changes and domain bacteria ribotype diversity in soils overlying the Centralia, Pennsylvania underground coal mine fire. Soil Sci. 2005, 170, 191–201. [Google Scholar] [CrossRef]
- Eremin, M.; Esterhuizen, G.; Smolin, I. Numerical simulation of roof cavings in several Kuzbass mines using finite-difference continuum damage mechanics approach. Int. J. Min. Sci. Technol. 2020, 30, 157–166. [Google Scholar] [CrossRef]
- Sokolyanskaya, L.O.; Ivanov, M.V.; Ikkert, O.P.; Kalinina, A.E.; Evseev, V.A.; Glukhova, L.B.; Karnachuk, O.V. Copper precipitation as insoluble oxalates by thermotolerant Aspergillus spp. from burning wastes of coal mining. Microbiology 2020, 89, 498–501. [Google Scholar] [CrossRef]
- Ikkert, O.P.; Gerasimchuk, A.L.; Bukhtiyarova, P.A.; Tuovinen, O.H.; Karnachuk, O.V. Characterization of precipitates formed by H2S-producing, Cu-resistant Firmicute isolates of Tissierella from human gut and Desulfosporosinus from mine waste. Antonie Van Leeuwenhoek 2013, 103, 1221–1234. [Google Scholar] [CrossRef] [PubMed]
- Frey, B.; Rime, T.; Phillips, M.; Stierli, B.; Hajdas, I.; Widmer, F.; Hartmann, M. Microbial diversity in European alpine permafrost and active layers. FEMS Microbiol. Ecol. 2016, 92, fiw018. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2—approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- Kahlenberg, V.; Braun, D.E.; Krüger, H.; Schmidmair, D.; Orlova, M. Temperature-and moisture-dependent studies on alunogen and the crystal structure of meta-alunogen determined from laboratory powder diffraction data. Phys. Chem. Miner. 2017, 44, 95–107. [Google Scholar] [CrossRef]
- Abby, S.S.; Melcher, M.; Kerou, M.; Krupovic, M.; Stieglmeier, M.; Rossel, C.; Pfeifer, K.; Schleper, C. Candidatus Nitrosocaldus cavascurensis, an ammonia oxidizing, extremely thermophilic archaeon with a highly mobile genome. Front. Microbiol. 2018, 9, 28. [Google Scholar] [CrossRef]
- De la Torre, J.R.; Walker, C.B.; Ingalls, A.E.; Könneke, M.; Stahl, D.A. Cultivation of a thermophilic ammonia oxidizing archaeon synthesizing crenarchaeol. Environ. Microbiol. 2008, 10, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Hansel, C.M.; Fendorf, S.; Jardine, P.M.; Francis, C.A. Changes in bacterial and archaeal community structure and functional diversity along a geochemically variable soil profile. Appl. Environ. Microbiol. 2008, 74, 1620–1633. [Google Scholar] [CrossRef] [PubMed]
- Cavaletti, L.; Monciardini, P.; Bamonte, R.; Schumann, P.; Rohde, M.; Sosio, M.; Donadio, S. New lineage of filamentous, spore-forming, gram-positive bacteria from soil. Appl. Environ. Microbiol. 2006, 72, 4360–4369. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.; Vera-Gargallo, B.; Calabi-Floody, M.; King, G.M.; Conrad, R.; Tebbe, C.C. Reconstructing genomes of carbon monoxide oxidisers in volcanic deposits including members of the class Ktedonobacteria. Microorganisms 2020, 8, 1880. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Li, P.; Jiang, D.; Dai, X.; Zhang, R.; Wang, Y.; Wang, Y. Microbial community structure and arsenic biogeochemistry in an acid vapor-formed spring in Tengchong geothermal area, China. PLoS ONE 2016, 11, e0146331. [Google Scholar] [CrossRef] [PubMed]
- de Miera, L.E.S.; Arroyo, P.; de Luis Calabuig, E.; Ansola, G. Effects of varying CO2 flows on bacterial communities in mesocosms created from two soils. Int. J. Greenhouse Gas Control. 2014, 46, 205–214. [Google Scholar] [CrossRef]
- de Miera, L.E.S.; Arroyo, P.; de Luis Calabuig, E.; Falagán, J.; Ansola, G. High-throughput sequencing of 16S RNA genes of soil bacterial communities from a naturally occurring CO2 gas vent. Int. J. Greenhouse Gas Control. 2014, 29, 176–184. [Google Scholar] [CrossRef]
- Arce-Rodríguez, A.; Puente-Sánchez, F.; Avendaño, R.; Martínez-Cruz, M.; de Moor, J.M.; Pieper, D.H.; Chavarria, M. Thermoplasmatales and sulfur-oxidizing bacteria dominate the microbial community at the surface water of a CO2-rich hydrothermal spring located in Tenorio Volcano National Park, Costa Rica. Extremophiles 2019, 23, 177–187. [Google Scholar] [CrossRef]
- Yabe, S.; Aiba, Y.; Sakai, Y.; Hazaka, M.; Yokota, A. Thermogemmatispora onikobensis gen. nov., sp. nov. and Thermogemmatispora foliorum sp. nov., isolated from fallen leaves on geothermal soils, and description of Thermogemmatisporaceae fam. nov. and Thermogemmatisporales ord. nov. within the class Ktedonobacteria. Int. J. Syst. Evol. Microbiol. 2011, 61, 903–910. [Google Scholar]
- King, C.E.; King, G.M. Description of Thermogemmatispora carboxidivorans sp. nov., a carbon-monoxide-oxidizing member of the class Ktedonobacteria isolated from a geothermally heated biofilm, and analysis of carbon monoxide oxidation by members of the class Ktedonobacteria. Int. J. Syst. Evol. Microbiol. 2014, 64, 1244–1251. [Google Scholar] [CrossRef]
- Zheng, Y.; Saitou, A.; Wang, C.M.; Toyoda, A.; Minakuchi, Y.; Sekiguchi, Y.; Ueda, K.; Takano, H.; Sakai, Y.; Abe, K.; et al. Genome features and secondary metabolites biosynthetic potential of the class Ktedonobacteria. Front. Microbiol. 2019, 10, 893. [Google Scholar] [CrossRef] [PubMed]
- Islam, Z.F.; Cordero, P.R.; Feng, J.; Chen, Y.J.; Bay, S.K.; Jirapanjawat, T.; Gleadow, R.M.; Carere, C.R.; Stott, M.B.; Chiri, E.; et al. Two Chloroflexi classes independently evolved the ability to persist on atmospheric hydrogen and carbon monoxide. ISME J. 2019, 13, 1801–1813. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Sekiguchi, Y.; Hanada, S.; Imachi, H.; Ohashi, A.; Harada, H.; Kamagata, Y. Anaerolinea thermolimosa sp. nov., Levilinea saccharolytica gen. nov., sp. nov. and Leptolinea tardivitalis gen. nov., sp. nov., novel filamentous anaerobes, and description of the new classes Anaerolineae classis nov. and Caldilineae classis nov. in the bacterial phylum Chloroflexi. Int. J. Syst. Evol. Microbiol. 2006, 56, 1331–1340. [Google Scholar] [PubMed]
- Mayhew, L.E.; Geist, D.J.; Childers, S.E.; Pierson, J.D. Microbial community comparisons as a function of the physical and geochemical conditions of Galápagos Island fumaroles. Geomicrobiol. J. 2007, 24, 615–625. [Google Scholar] [CrossRef]
- Steven, B.; Chen, M.Q.; Greer, C.W.; Whyte, L.G.; Niederberger, T.D. Tumebacillus permanentifrigoris gen. nov., sp. nov., an aerobic, spore-forming bacterium isolated from Canadian high Arctic permafrost. Int. J. Syst. Evol. Microbiol. 2008, 58, 1497–1501. [Google Scholar] [CrossRef]
- Prasad, R.V.; Bhumika, V.; Kumar, P.A.; Srinivas, N.R.T. Tumebacillus lipolyticus sp. nov., isolated from river water. Int. J. Syst. Evol. Microbiol. 2015, 65, 4363–4368. [Google Scholar] [CrossRef]
- Klenk, H.P.; Lapidus, A.; Chertkov, O.; Copeland, A.; Del Rio, T.G.; Nolan, M.; Lucas, S.; Chen, F.; Tice, H.; Cheng, J.-F.; et al. Complete genome sequence of the thermophilic, hydrogen-oxidizing Bacillus tusciae type strain (T2 T) and reclassification in the new genus, Kyrpidia gen. nov. as Kyrpidia tusciae comb. nov. and emendation of the family Alicyclobacillaceae da Costa and Rainey, 2010. Stand. Genomic Sci. 2011, 5, 121–134. [Google Scholar]
- Kadnikov, V.V.; Mardanov, A.V.; Ivasenko, D.A.; Antsiferov, D.V.; Beletsky, A.V.; Karnachuk, O.V.; Ravin, N.V. Lignite coal burning seam in the remote Altai Mountains harbors a hydrogen-driven thermophilic microbial community. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Yu, T.T.; Yin, Y.R.; Zhang, Y.G.; Yao, J.C.; Klenk, H.P.; Wang, H.F.; Ming, H.; Zhou, E.M.; Li, W.J. Meiothermus terrae sp. nov., isolated from a geothermally heated soil sample. Int. J. Syst. Evol. Microbiol. 2014, 64, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Q.; Zhang, W.J.; Wei, B.P.; Xu, X.W.; Zhu, X.F.; Wu, M. Meiothermus cateniformans sp. nov., a slightly thermophilic species from north-eastern China. Int. J. Syst. Evol. Microbiol. 2010, 60, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Kawasumi, T.; Igarashi, Y.; Kodama, T.; Minoda, Y. Hydrogenobacter thermophilus gen. nov., sp. nov., an extremely thermophilic, aerobic, hydrogen-oxidizing bacterium. Int. J. Syst. Evol. Microbiol. 1984, 34, 5–10. [Google Scholar]
- Anders, H.; Dunfield, P.F.; Lagutin, K.; Houghton, K.M.; Power, J.F.; MacKenzie, A.D.; Vyssotski, M.; Ryan, J.L.J.; Hanssen, E.G.; Moreau, J.W.; et al. Thermoflavifilum aggregans gen. nov., sp. nov., a thermophilic and slightly halophilic filamentous bacterium from the phylum Bacteroidetes. Int. J. Syst. Evol. Microbiol. 2014, 64, 1264–1270. [Google Scholar] [CrossRef]
- Lebedeva, E.V.; Off, S.; Zumbrägel, S.; Kruse, M.; Shagzhina, A.; Lücker, S.; Maixner, F.; Lipski, A.; Daims, H.; Spieck, E. Isolation and characterization of a moderately thermophilic nitrite-oxidizing bacterium from a geothermal spring. FEMS Microbiol. Ecol. 2011, 75, 195–204. [Google Scholar] [CrossRef][Green Version]
- Hanson, R.S.; Hanson, T.E. Methanotrophic bacteria. Microbiol. Rev. 1996, 60, 439–471. [Google Scholar] [CrossRef] [PubMed]
- Brewer, T.E.; Handley, K.M.; Carini, P.; Gilbert, J.A.; Fierer, N. Genome reduction in an abundant and ubiquitous soil bacterium “Candidatus Udaeobacter copiosus”. Nat. Microbiol. 2016, 2, 16198. [Google Scholar] [CrossRef] [PubMed]
- Willms, I.M.; Rudolph, A.Y.; Göschel, I.; Bolz, S.H.; Schneider, D.; Penone, C.; Poehlein, A.; Schöning, I.; Nacke, H. Globally abundant “Candidatus Udaeobacter” benefits from release of antibiotics in soil and potentially performs trace gas scavenging. Msphere 2020, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Sorensen, J.W.; Grady, K.L.; Tobin, T.C.; Shade, A. Divergent extremes but convergent recovery of bacterial and archaeal soil communities to an ongoing subterranean coal mine fire. ISME J. 2017, 11, 1447. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, J.W.; Dunivin, T.K.; Tobin, T.C.; Shade, A. Ecological selection for small microbial genomes along a temperate-to-thermal soil gradient. Nat. Microbial. 2019, 4, 55. [Google Scholar] [CrossRef] [PubMed]
| Parameter | RBS38 | RBS38-1 |
|---|---|---|
| OTU number | 67 | 92 |
| Chao1 index | 67.8 | 92.5 |
| Shannon_e index | 3.36 | 3.21 |
| Simpson index | 0.0534 | 0.0763 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadnikov, V.V.; Mardanov, A.V.; Beletsky, A.V.; Grigoriev, M.A.; Karnachuk, O.V.; Ravin, N.V. Thermophilic Chloroflexi Dominate in the Microbial Community Associated with Coal-Fire Gas Vents in the Kuznetsk Coal Basin, Russia. Microorganisms 2021, 9, 948. https://doi.org/10.3390/microorganisms9050948
Kadnikov VV, Mardanov AV, Beletsky AV, Grigoriev MA, Karnachuk OV, Ravin NV. Thermophilic Chloroflexi Dominate in the Microbial Community Associated with Coal-Fire Gas Vents in the Kuznetsk Coal Basin, Russia. Microorganisms. 2021; 9(5):948. https://doi.org/10.3390/microorganisms9050948
Chicago/Turabian StyleKadnikov, Vitaly V., Andrey V. Mardanov, Alexey V. Beletsky, Mikhail A. Grigoriev, Olga V. Karnachuk, and Nikolai V. Ravin. 2021. "Thermophilic Chloroflexi Dominate in the Microbial Community Associated with Coal-Fire Gas Vents in the Kuznetsk Coal Basin, Russia" Microorganisms 9, no. 5: 948. https://doi.org/10.3390/microorganisms9050948
APA StyleKadnikov, V. V., Mardanov, A. V., Beletsky, A. V., Grigoriev, M. A., Karnachuk, O. V., & Ravin, N. V. (2021). Thermophilic Chloroflexi Dominate in the Microbial Community Associated with Coal-Fire Gas Vents in the Kuznetsk Coal Basin, Russia. Microorganisms, 9(5), 948. https://doi.org/10.3390/microorganisms9050948







