Immunobiotic Feed Developed with Lactobacillus delbrueckii subsp. delbrueckii TUA4408L and the Soymilk By-Product Okara Improves Health and Growth Performance in Pigs
Abstract
:1. Introduction
2. Materials and Methods
2.1. L. delbrueckii subsp. delbrueckii TUA4408L and Fermentation of Soymilk By-Product
2.2. Animals and Treatments
2.3. Evaluation of Meat Quality
2.4. Evaluation of Meat Lipids
2.5. Plasma Determinations
2.6. Blood Leukocytes
2.7. Detection of Pathogenic Escherichia coli in Feces
2.8. Intestinal Microbiota
2.9. Immune Factor Expression in Intestinal Tissue
2.10. Statistical Analysis
3. Results
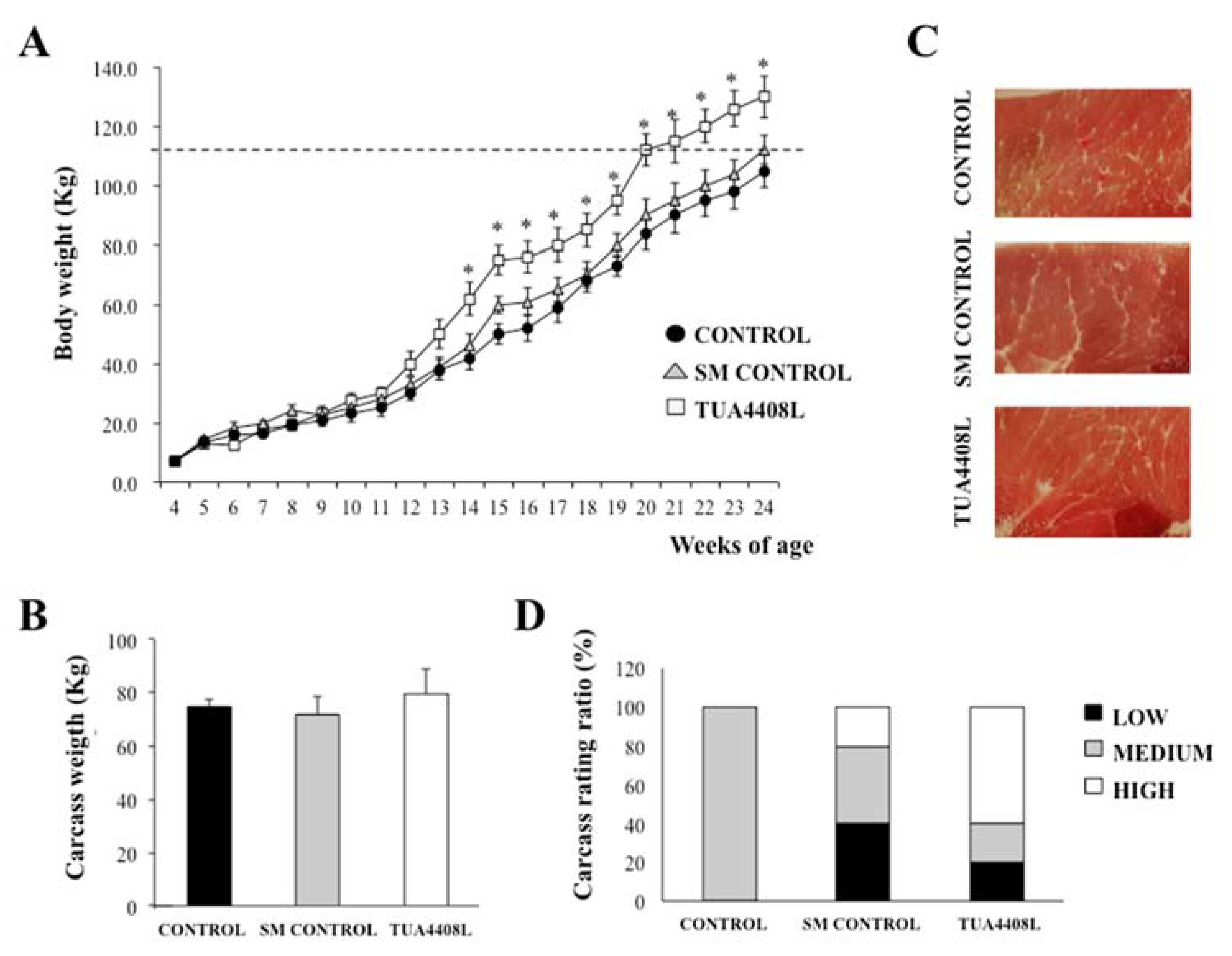
3.1. Immunobiotic TUA4408L-Fermented Okara Feed Improves Growth Performance and Meat Quality of Pigs
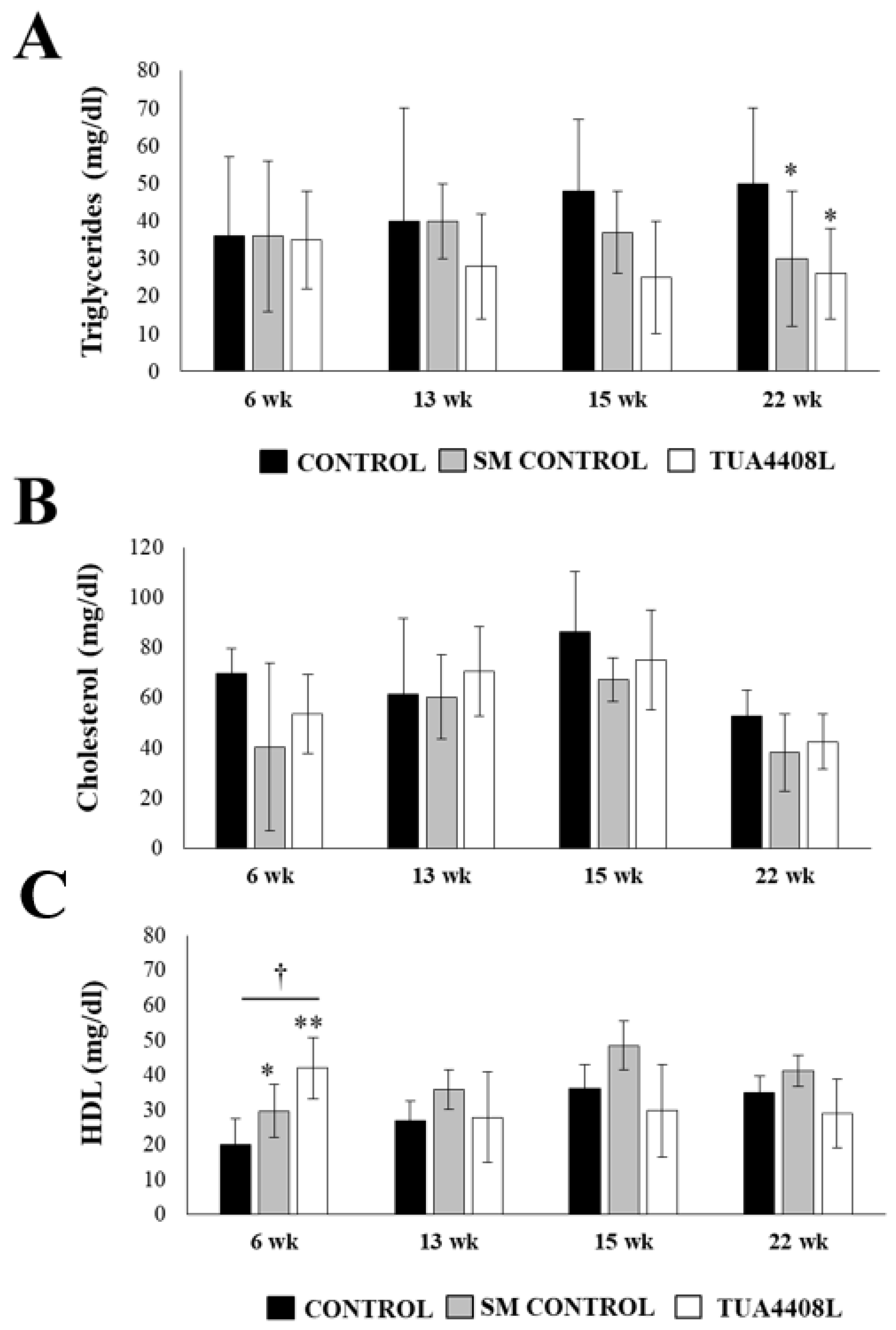
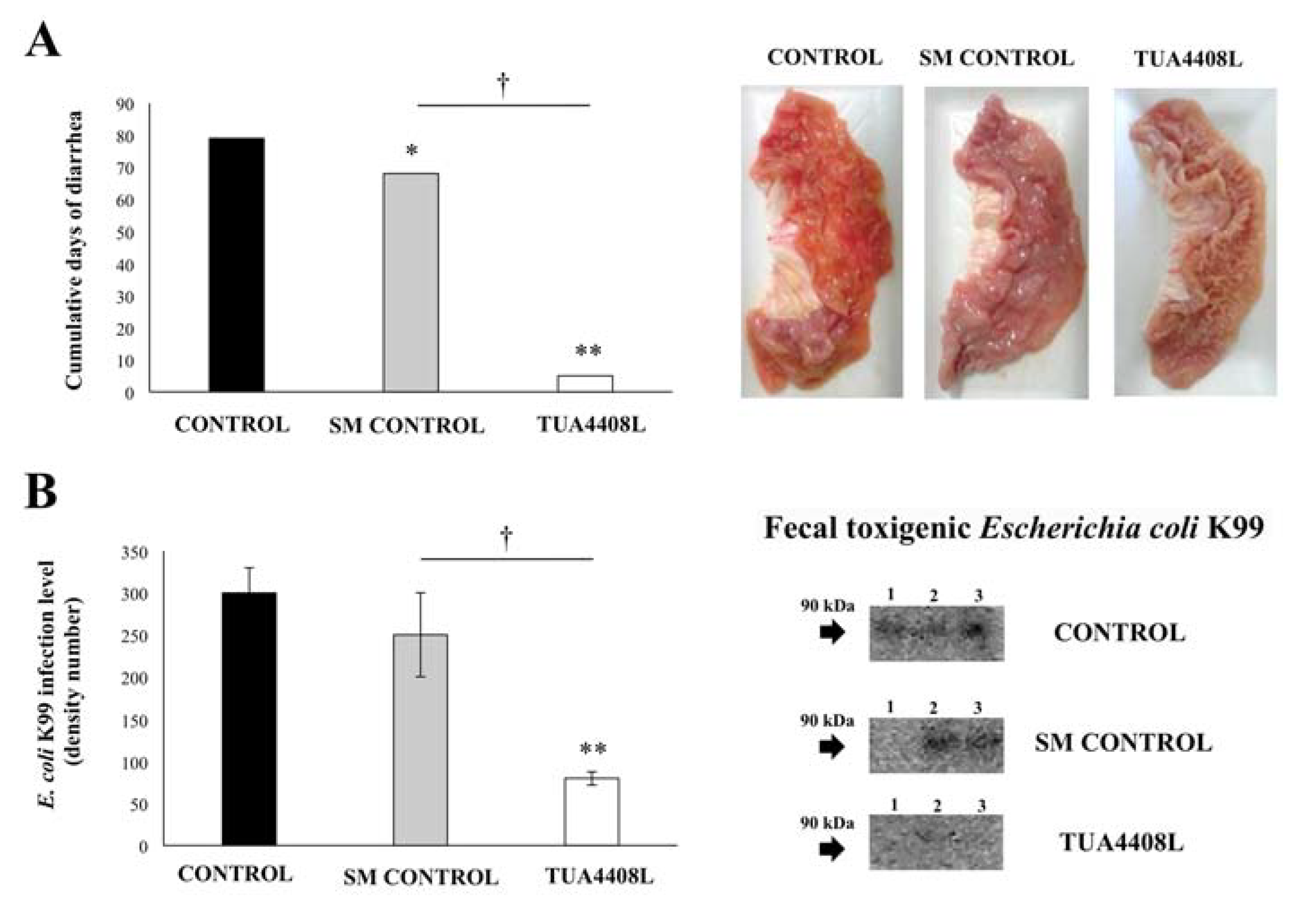
3.2. Immunobiotic TUA4408L-Fermented Okara Feed Improves the Health of Pigs
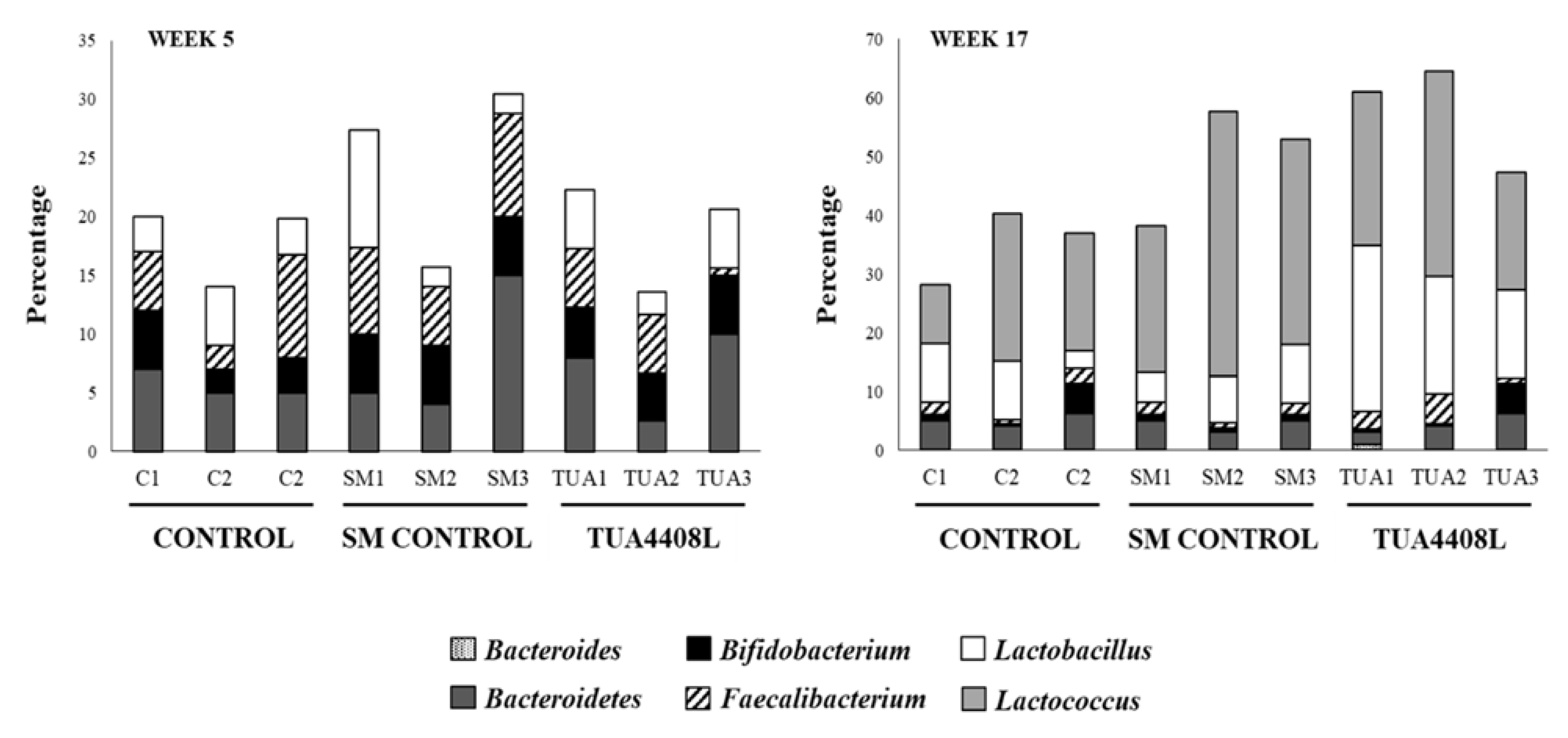
3.3. Immunobiotic TUA4408L-Fermented Okara Feed Modulates the Composition of the Intestinal Microbiota of Pigs
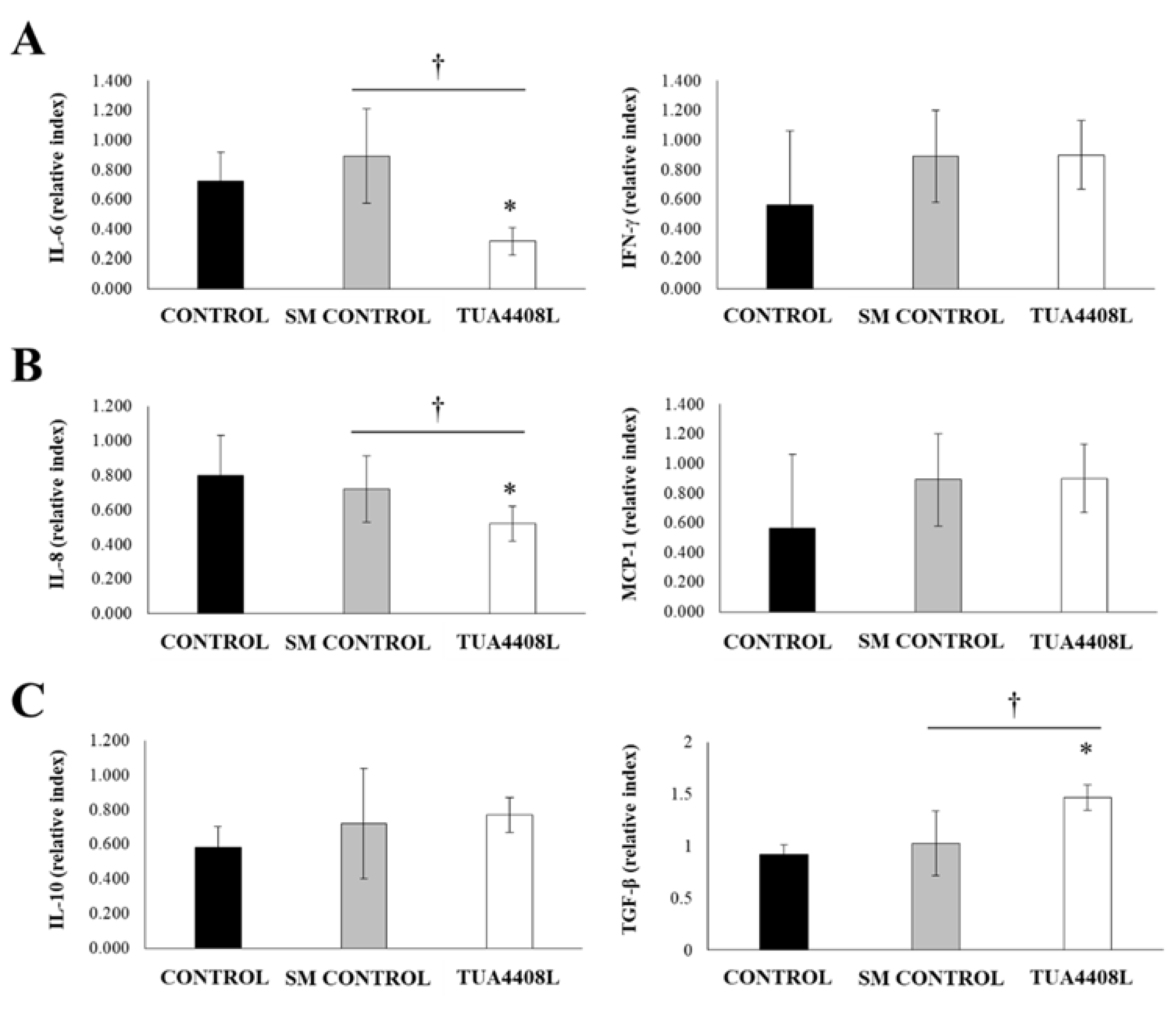
3.4. Immunobiotic TUA4408L-Fermented Okara Feed Modulates the Immunity of Pigs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blavi, L.; Solà-Oriol, D.; Llonch, P.; López-Vergé, S.; Martín-Orúe, S.M.; Pérez, J.F. Management and feeding strategies in early life to increase piglet performance and welfare around weaning: A review. Animals 2021, 11, 302. [Google Scholar] [CrossRef]
- Villena, J.; Kitazawa, H. Modulation of intestinal TLR4-inflammatory signalling pathways by probiotic microorganisms: Lessons learned from Lactobacillus jensenii TL2937. Front. Immunol. 2014, 4, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Gardiner, G.E.; Metzler-Zebeli, B.U.; Lawlor, P.G. Impact of intestinal microbiota on growth and feed efficiency in pigs: A review. Microorganisms 2020, 8, 1886. [Google Scholar] [CrossRef]
- Wang, W.; Gänzle, M. Toward rational selection criteria for selection of probiotics in pigs. Adv Appl Microbiol 2019, 107, 83–112. [Google Scholar]
- Stanojevic, S.P.; Barac, M.B.; Pesic, M.B.; Jankovic, V.S.; Vucelic-Radovic, B.V. Bioactive proteins and energy value of okara as a byproduct in hydrothermal processing of soy milk. J. Agric. Food Chem. 2013, 61, 9210–9219. [Google Scholar] [CrossRef]
- Li, Y.; Hao, J.; Cheng, Y.; Zhao, R.; Yin, L.; Li, L. Improvement of okara mouthfeel by Aspergillus niger and Aspergillus oryzae fermentation. Trans. Chin. Soc. Agric. Eng. 2012, 28, 248–253. [Google Scholar] [CrossRef]
- Vong, W.C.; Liu, S.Q. Biovalorisation of okara (soybean residue) for food and nutrition. Trends Food Sci. Technol. 2016, 52, 139–147. [Google Scholar] [CrossRef]
- Jimenez-Escrig, A.; Tenorio, M.D.; Espinosa-Martos, I.; Ruperez, P. Health-promoting effects of a dietary fiber concentrate from the soybean byproduct okara in rats. J. Agric. Food Chem. 2008, 56, 7495–7501. [Google Scholar] [CrossRef]
- Song, D.; Chang, S.K.C.; Ibrahim, A.A. Effect of fermentation substrates on enzyme production and degradation of oligosaccharides in pinto bean flour as affected by particle size. J. Food Process. Pres. 2009, 33, 527–546. [Google Scholar] [CrossRef]
- Bedani, R.; Rossi, E.A.; Isay Saad, S.M. Impact of inulin and okara on Lactobacillus acidophilus La-5 and Bifidobacterium animalis Bb-12 viability in a fermented soy product and probiotic survival under in vitro simulated gastrointestinal conditions. Food Microbiol. 2013, 34, 382–389. [Google Scholar] [CrossRef] [Green Version]
- Bedani, R.; Campos, M.M.; Castro, I.A.; Rossi, E.A.; Saad, S.M.I. Incorporation of soybean by-product okara and inulin in a probiotic soy yoghurt: Texture profile and sensory acceptance. J. Sci. Food Agric. 2014, 94, 119–125. [Google Scholar] [CrossRef]
- Quintana, G.; Gerbino, E.; Gómez-Zavaglia, A. Okara: A nutritionally valuable by-product able to stabilize Lactobacillus plantarum during freeze-drying, spray-drying, and storage. Front. Microbiol. 2017, 8, 641. [Google Scholar] [CrossRef] [Green Version]
- Laiño, J.; Villena, J.; Kanmani, P.; Kitazawa, H. Immunoregulatory effects of lactic acid bacteria exopolysaccharides: New insights into molecular interactions with intestinal epithelial cells. Special Issue: Probiotic Microorganisms: An Intimate Gaze. Microorganisms 2016, 4, 27. [Google Scholar] [CrossRef] [Green Version]
- Villena, J.; Aso, H.; Rutten, V.P.M.G.; Takahashi, H.; van Eden, W.; Kitazawa, H. Immunobiotics for the bovine host: Their interaction with intestinal epithelial cells and their effect on antiviral immunity. Front. Immunol. 2018, 9, 326. [Google Scholar] [CrossRef] [Green Version]
- Wachi, S.; Kanmani, P.; Tomosada, Y.; Kobayashi, H.; Yuri, T.; Egusa, S.; Shimazu, T.; Suda, Y.; Aso, H.; Sugawara, M.; et al. Lactobacillus delbrueckii TUA4408L and its extracellular polysaccharides attenuate enterotoxigenic Escherichia coli-induced inflammatory response in porcine intestinal epitheliocytes via Toll-like receptor-2 and 4. Mol. Nutr. Food Res. 2014, 58, 2080–2093. [Google Scholar] [CrossRef]
- Kanmani, P.; Albarracin, L.; Kobayashi, H.; Hebert, E.M.; Saavedra, L.; Komatsu, R.; Gatica, B.; Miyazaki, A.; Ikeda-Ohtsubo, W.; Suda, Y.; et al. Genomic characterization of Lactobacillus delbrueckii TUA4408L and evaluation of the antiviral activities of its extracellular polysaccharides in porcine intestinal epithelial cells. Front. Immunol 2018, 9, 2178. [Google Scholar] [CrossRef]
- Li, S.; Zhu, D.; Li, K.; Yang, Y.; Lei, Z.; Zhang, Z. Soybean curd residue: Composition, utilization, and related limiting factors. ISRN Ind. Eng. 2013, 13, 1–8. [Google Scholar] [CrossRef]
- Tian, Z.; Deng, D.; Cui, Y.; Chen, W.; Yu, M.; Ma, X. Diet supplemented with fermented okara improved growth performance, meat quality, and amino acid profiles in growing pigs. Food Sci. Nutr. 2020, 8, 5650–5659. [Google Scholar] [CrossRef]
- Suda, Y.; Villena, J.; Takahashi, Y.; Hosoya, S.; Tomosada, Y.; Tsukida, K.; Shimazu, T.; Aso, H.; Tohno, M.; Ishida, M.; et al. Immunobiotic Lactobacillus jensenii as immune-health promoting factor to improve growth performance and productivity in post-weaning pigs. BMC Immunol 2014, 15, 24. [Google Scholar] [CrossRef] [Green Version]
- Suda, Y.; Shinohara, H.; Yamaguchi, T.; Matsuda, T.; Nakagawa, K.; Ohtomo, Y.; Nishida, A.; Yamagishi, T. Change of fatty acid composition of white adipose tissue with increasing age in Syrian hamster fed alfalfa or cereal based diet. Anim. Sci. J. 2004, 75, 43–47. [Google Scholar] [CrossRef]
- Kibe, R.; Sakamoto, M.; Yokota, H.; Ishikawa, H.; Aiba, Y.; Koga, Y.; Benno, Y. Movement and fixation of intestinal microbiota after administration of human feces to germfree mice. Appl. Environ. Microbiol. 2005, 71, 3171–3178. [Google Scholar] [CrossRef] [Green Version]
- Nakanishi, Y.; Murashima, K.; Ohara, H.; Suzuki, T.; Hayashi, H.; Sakamoto, M.; Fukasawa, T.; Kubota, H.; Hosono, A.; Kono, T.; et al. Increase in terminal restriction fragments of Bacteroidetes-derived 16S rRNA genes after administration of short-chain fructooligosaccharides. Appl. Environ. Microbiol. 2006, 72, 6271–6276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albarracin, L.; Kobayashi, H.; Iida, H.; Sato, N.; Nochi, T.; Aso, H.; Salva, S.; Alvarez, S.; Kitazawa, H.; Villena, J. Transcriptomic analysis of the innate antiviral immune response in porcine intestinal epithelial cells: Influence of immunobiotic lactobacilli. Front. Immunol. 2017, 8, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albarracin, L.; Garcia-Castillo, V.; Masumizu, Y.; Indo, Y.; Islam, M.A.; Suda, Y.; Garcia-Cancino, A.; Aso, H.; Takahashi, H.; Kitazawa, H.; et al. Efficient selection of new immunobiotic strains with antiviral effects in local and distal mucosal sites by using porcine intestinal epitheliocytes. Front. Immunol. 2020, 11, 543. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Lee, J.; Chen, W.N. Analysis of improved nutritional composition of potential functional food (Okara) after probiotic Solid-State fermentation. J. Agric. Food Chem. 2018, 66, 5373–5381. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, R.; Chakraborty, R.; Dutta, A. Role of fermentation in improving nutritional quality of soybean meal—a review. Asian-Australas J. Anim. Sci. 2016, 29, 1523–1529. [Google Scholar] [CrossRef] [Green Version]
- Jeong, D.W.; Choi, Y.M.; Lee, S.H.; Choe, J.H.; Hong, K.C.; Park, H.C.; Kim, B.C. Correlations of trained panel sensory values of cooked pork with fatty acid composition, muscle fiber type, and pork quality characteristics in Berkshire pigs. Meat Sci. 2010, 86, 607–615. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef]
- Dugan, M.E.; Vahmani, P.; Turner, T.D.; Mapiye, C.; Juarez, M.; Prieto, N.; Beaulieu, A.D.; Zijlstra, R.T.; Patience, J.F.; Aalhus, J.L. Pork as a Source of Omega-3 (n-3) Fatty Acids. J. Clin. Med. 2015, 4, 1999–2011. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. N−3 fatty acids and human health: Defining strategies for public policy. Lipids 2001, 36, 83–89. [Google Scholar] [CrossRef]
- Robinson, L.E.; Buchholz, A.C.; Mazurak, V.C. Inflammation, obesity, and fatty acid metabolism: Influence of n-3 polyunsaturated fatty acids on factors contributing to metabolic syndrome. Appl. Physiol. Nutr. Metab. 2007, 32, 1008–1024. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef] [PubMed]
- De Tonnac, A.; Mourot, J. Effect of dietary sources of n-3 fatty acids on pig performance and technological, nutritional and sensory qualities of pork. Animal 2018, 12, 1527–1535. [Google Scholar] [CrossRef] [PubMed]
- Corino, C.; Rossi, R.; Cannata, S.; Ratti, S. Effect of dietary linseed on the nutritional value and quality of pork and pork products: Systematic review and meta-analysis. Meat Sci. 2014, 98, 679–688. [Google Scholar] [CrossRef]
- Rey, A.I.; Puig, P.; Cardozo, P.W.; Hechavarría, T. Supplementation effect of oleuropein extract combined with betaine, magnesium, and vitamin E on pigs’ performance and meat quality characteristics. Animals 2021, 11, 443. [Google Scholar] [CrossRef] [PubMed]
- Komprda, T.; Jůzl, M.; Matejovičová, M.; Levá, L.; Piechowiczová, M.; Nedomová, Š.; Popelková, V.; Vymazalová, P. Effect of high dietary level (8%) of fish oil on long-chain polyunsaturated fatty acid n-3 content in pig tissues and plasma biochemical parameters. Animals 2020, 10, 1657. [Google Scholar] [CrossRef]
- Duan, Y.; Duan, Y.; Li, F.; Li, Y.; Guo, Q.; Ji, Y.; Tan, B.; Li, T.; Yin, Y. Effects of supplementation with branched-chain amino acids to low-protein diets on expression of genes related to lipid metabolism in skeletal muscle of growing pigs. Amino Acids. 2016, 48, 2131–2144. [Google Scholar] [CrossRef]
- Lalle’s, J.P.; Bosia, P.; Smidta, H.; Stokes, C.R. Nutritional management of gut health in pigs around weaning. Proceed. Nutr. Soc. 2007, 66, 260–268. [Google Scholar] [CrossRef]
- Nowland, T.L.; Plush, K.J.; Barton, M.; Kirkwood, R.N. Development and function of the intestinal microbiome and potential implications for pig production. Animals 2019, 9, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaukroger, C.H.; Stewart, C.J.; Edwards, S.A.; Walshaw, J.; Adams, I.P.; Kyriazakis, I. Changes in faecal microbiota profiles associated with performance and birthweight of piglets. Front. Microbiol. 2020, 11, 917. [Google Scholar] [CrossRef]
- Liu, B.; Wang, W.; Zhu, X.; Sun, X.; Xiao, J.; Li, D.; Cui, Y.; Wang, C.; Shi, Y. Response of gut microbiota to dietary fiber and metabolic interaction with SCFAs in piglets. Front. Microbiol. 2018, 9, 2344. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Q.; Wu, X.; Pan, Y.; Wang, L.; Cui, C.; Guo, Y.; Zhu, L.; Peng, J.; Wei, H. Early-life intervention using fecal microbiota combined with probiotics promotes gut microbiota maturation, regulates immune system development, and alleviates weaning stress in piglets. Int. J. Mol. Sci. 2020, 21, 503. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Ji, H.; Wang, S.; Liu, H.; Zhang, W.; Zhang, D.; Wang, Y. Probiotic Lactobacillus plantarum promotes intestinal barrier function by strengthening the epithelium and modulating gut microbiota. Front. Microbiol. 2018, 9, 1953. [Google Scholar] [CrossRef] [Green Version]
- Cao, G.; Tao, F.; Hu, Y.; Li, Z.; Zhang, Y.; Deng, B. Positive effects of a Clostridium butyricum-based compound probiotic on growth performance, immune responses, intestinal morphology, hypothalamic neurotransmitters, and colonic microbiota in weaned piglets. Food Function 2019, 10, 2926–2934. [Google Scholar] [CrossRef]
- Shin, D.; Chang, S.Y.; Bogere, P.; Won, K.; Choi, J.Y.; Choi, Y.J.; Lee, H.K.; Hur, J.; Park, B.; Kim, Y.; et al. Beneficial roles of probiotics on the modulation of gut microbiota and immune response in pigs. PLoS ONE 2019, 14, e0220843. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Zhang, N.; Zhou, M.; Tu, Y.; Deng, K.; Diao, Q. Effects of dietary probiotics on growth performance, faecal microbiota and serum profiles in weaned piglets. Anim. Prod. Sci. 2014, 54, 616–621. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; King, D.E.; Willing, B.P.; Band, M.R.; Beever, J.E.; Lane, A.B.; Loor, J.J.; Marini, J.C.; Rund, L.A.; Schook, L.B.; et al. Transcriptome profiling of the small intestinal epithelium in germfree versus conventional piglets. BMC Genomics 2007, 8, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimazu, T.; Villena, J.; Tohno, M.; Fujie, H.; Hosoya, S.; Shimosato, T.; Aso, H.; Suda, Y.; Kawai, Y.; Saito, T.; et al. Immunobiotic Lactobacillus jensenii elicits anti-inflammatory activity in porcine intestinal epithelial cells by modulating negative regulators of the Toll-like receptor signaling pathway. Infect Immun. 2012, 80, 276–288. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, H.; Albarracin, L.; Sato, N.; Kanmani, P.; Kober, A.K.; Ikeda-Ohtsubo, W.; Suda, Y.; Nochi, T.; Aso, H.; Makino, S.; et al. Modulation of porcine intestinal epitheliocytes immunetranscriptome response by Lactobacillus jensenii TL2937. Benef. Microbes 2016, 7, 769–782. [Google Scholar] [CrossRef]
- Villena, J.; Suzuki, R.; Fujie, H.; Chiba, E.; Takahashi, T.; Tomosada, Y.; Shimazu, T.; Aso, H.; Ohwada, S.; Suda, Y.; et al. Immunobiotic Lactobacillus jensenii modulates the Toll-like receptor 4-induced inflammatory response via negative regulation in porcine antigen-presenting cells. Clin Vaccine Immunol. 2012, 19, 1038–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsukida, K.; Takahashi, T.; Iida, H.; Kanmani, P.; Suda, Y.; Nochi, T.; Ohwada, S.; Aso, H.; Ohkawara, S.; Makino, S.; et al. Immunoregulatory effects triggered by immunobiotic Lactobacillus jensenii TL2937 strain involve efficient phagocytosis in porcine antigen presenting cells. BMC Immunol. 2016, 17, 21. [Google Scholar] [CrossRef] [Green Version]
- Marranzino, G.; Villena, J.; Salva, S.; Alvarez, S. Stimulation of macrophages by immunobiotic Lactobacillus strains: Influence beyond the intestinal tract. Microbiol. Immunol. 2012, 56, 771–781. [Google Scholar] [CrossRef]
- Salva, S.; Villena, J.; Alvarez, S. Immunomodulatory activity of Lactobacillus rhamnosus strains isolated from goat milk: Impact on intestinal and respiratory infections. Int. J. Food Microbiol. 2010, 141, 82–89. [Google Scholar] [CrossRef]
- Villena, J.; Chiba, E.; Tomosada, Y.; Salva, S.; Marranzino, G.; Kitazawa, H.; Alvarez, S. Orally administered Lactobacillus rhamnosus modulates the respiratory immune response triggered by the viral pathogen-associated molecular pattern poly(I:C). BMC Immunol 2012, 53, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritz, J.H.; Le Bourhis, L.; Magalhaes, J.G.; Philpott, D.J. Innate immune recognition at the epithelial barrier drives adaptive immunity: APCs take the back seat. Trends Immunol. 2007, 29, 41–49. [Google Scholar] [CrossRef] [PubMed]
- McEntee, C.P.; Gunaltay, S.; Travis, M.A. Regulation of barrier immunity and homeostasis by integrin-mediated transforming growth factor β activation. Immunology 2020, 160, 139–148. [Google Scholar] [CrossRef] [Green Version]

| Fatty Acid | Control | SM Control | TUA4408L |
|---|---|---|---|
| Myristic acid | 2.1 | 1.1 * | 1.0 * |
| Palmitic acid | 27.6 | 24.9 | 23.9 |
| Palmitoleic acid | 1.2 | 1.3 | 1.1 |
| Stearic acid | 15.6 | 14.9 | 10.9 * |
| Oleic acid | 40.2 | 41.0 | 45.3 |
| Linoleic acid | 8.2 | 10.9 | 15.9 * |
| Linolenic acid | 0.8 | 1.0 | 0.8 |
| Saturated fatty acids | 45.3 | 40.9 | 35.8 * |
| Unsaturated fatty acid | 50.4 | 54.2 | 63.1 * |
| Monounsaturated fatty acids | 41.4 | 42.3 | 46.4 |
| Δ9index | 0.7 | 0.7 | 0.8 |
| Group | Registered Diseases |
|---|---|
| Control | Drowning, cough, mycoplasma pneumonia, eczema, diarrhea, kidney disease, liver disease |
| SM Control | Drowning, cough, mycoplasma pneumonia, diarrhea |
| TUA4408L | Mild diarrhea |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suda, Y.; Sasaki, N.; Kagawa, K.; Elean, M.; Zhou, B.; Tomokiyo, M.; Islam, M.A.; Rajoka, M.S.R.; Kober, A.K.M.H.; Shimazu, T.; et al. Immunobiotic Feed Developed with Lactobacillus delbrueckii subsp. delbrueckii TUA4408L and the Soymilk By-Product Okara Improves Health and Growth Performance in Pigs. Microorganisms 2021, 9, 921. https://doi.org/10.3390/microorganisms9050921
Suda Y, Sasaki N, Kagawa K, Elean M, Zhou B, Tomokiyo M, Islam MA, Rajoka MSR, Kober AKMH, Shimazu T, et al. Immunobiotic Feed Developed with Lactobacillus delbrueckii subsp. delbrueckii TUA4408L and the Soymilk By-Product Okara Improves Health and Growth Performance in Pigs. Microorganisms. 2021; 9(5):921. https://doi.org/10.3390/microorganisms9050921
Chicago/Turabian StyleSuda, Yoshihito, Nana Sasaki, Kyoma Kagawa, Mariano Elean, Binghui Zhou, Mikado Tomokiyo, Md. Aminul Islam, Muhammad Shahid Riaz Rajoka, A. K. M. Humayun Kober, Tomoyuki Shimazu, and et al. 2021. "Immunobiotic Feed Developed with Lactobacillus delbrueckii subsp. delbrueckii TUA4408L and the Soymilk By-Product Okara Improves Health and Growth Performance in Pigs" Microorganisms 9, no. 5: 921. https://doi.org/10.3390/microorganisms9050921
APA StyleSuda, Y., Sasaki, N., Kagawa, K., Elean, M., Zhou, B., Tomokiyo, M., Islam, M. A., Rajoka, M. S. R., Kober, A. K. M. H., Shimazu, T., Egusa, S., Terashima, Y., Aso, H., Ikeda-Ohtsubo, W., Villena, J., & Kitazawa, H. (2021). Immunobiotic Feed Developed with Lactobacillus delbrueckii subsp. delbrueckii TUA4408L and the Soymilk By-Product Okara Improves Health and Growth Performance in Pigs. Microorganisms, 9(5), 921. https://doi.org/10.3390/microorganisms9050921











