Detection of Human Cytomegalovirus Proteins in Paraffin-Embedded Breast Cancer Tissue Specimens—A Novel, Automated Immunohistochemical Staining Protocol
Abstract
:1. Introduction
1.1. Human Onco-Viruses
1.2. Human Cytomegalovirus (HCMV)
1.3. HCMV in Cancer
1.4. HCMV Diagnostics
2. Materials and Methods
2.1. Patient Samples
2.2. Reagents
2.3. Immunohistochemical Staining
2.4. DNA Extraction and Quantitative PCR (qPCR)
2.5. Viral Infection of Breast Cancer Cells in Culture
2.6. RNA Extraction and Quantitative Reverse Transcription PCR (RT-qPCR)
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McGuire, A.; Brown, J.A.; Malone, C.; McLaughlin, R.; Kerin, M.J. Effects of age on the detection and management of breast cancer. Cancers 2015, 7, 908–929. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.S.; Zhao, Z.; Yang, Z.N.; Xu, F.; Lu, H.J.; Zhu, Z.Y.; Shi, W.; Jiang, J.; Yao, P.P.; Zhu, H.P. Risk Factors and Preventions of Breast Cancer. Int. J. Biol. Sci. 2017, 13, 1387–1397. [Google Scholar] [CrossRef] [Green Version]
- Howell, A.; Anderson, A.S.; Clarke, R.B.; Duffy, S.W.; Evans, D.G.; Garcia-Closas, M.; Gescher, A.J.; Key, T.J.; Saxton, J.M.; Harvie, M.N. Risk determination and prevention of breast cancer. Breast Cancer Res. 2014, 16, 446. [Google Scholar] [CrossRef] [PubMed]
- Geisler, J.; Touma, J.; Rahbar, A.; Soderberg-Naucler, C.; Vetvik, K. A Review of the Potential Role of Human Cytomegalovirus (HCMV) Infections in Breast Cancer Carcinogenesis and Abnormal Immunity. Cancers 2019, 11, 1842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, A.K.; Walker, L.C.; Cox, B.; Rollag, H.; Robinson, B.A.; Morrin, H.; Pearson, J.F.; Potter, J.D.; Paterson, M.; Surcel, H.M.; et al. Breast cancer and cytomegalovirus. Clin. Transl. Oncol. 2020, 22, 585–602. [Google Scholar] [CrossRef]
- Bishop, R.K.; Valle Oseguera, C.A.; Spencer, J.V. Human Cytomegalovirus interleukin-10 promotes proliferation and migration of MCF-7 breast cancer cells. Cancer Cell Microenviron 2015, 2. [Google Scholar] [CrossRef] [Green Version]
- Mui, U.N.; Haley, C.T.; Vangipuram, R.; Tyring, S.K. Human oncoviruses: Mucocutaneous manifestations, pathogenesis, therapeutics, and prevention: Hepatitis viruses, human T-cell leukemia viruses, herpesviruses, and Epstein-Barr virus. J. Am. Acad. Dermatol. 2019, 81, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Lawson, J.S.; Salmons, B.; Glenn, W.K. Oncogenic Viruses and Breast Cancer: Mouse Mammary Tumor Virus (MMTV), Bovine Leukemia Virus (BLV), Human Papilloma Virus (HPV), and Epstein-Barr Virus (EBV). Front. Oncol. 2018, 8, 1. [Google Scholar] [CrossRef]
- Gaglia, M.M.; Munger, K. More than just oncogenes: Mechanisms of tumorigenesis by human viruses. Curr. Opin. Virol. 2018, 32, 48–59. [Google Scholar] [CrossRef] [PubMed]
- El Shazly, D.F.; Bahnassey, A.A.; Omar, O.S.; Elsayed, E.T.; Al-Hindawi, A.; El-Desouky, E.; Youssef, H.; Zekri, A.N. Detection of Human Cytomegalovirus in Malignant and Benign Breast Tumors in Egyptian Women. Clin. Breast Cancer 2018, 18, e629–e642. [Google Scholar] [CrossRef]
- Soderberg-Naucler, C. Treatment of cytomegalovirus infections beyond acute disease to improve human health. Expert Rev. Anti Infect. Ther. 2014, 12, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Steininger, C. Clinical relevance of cytomegalovirus infection in patients with disorders of the immune system. Clin. Microbiol. Infect. 2007, 13, 953–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Söderberg-Nauclér, C.; Fish, K.N.; Nelson, J.A. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell 1997, 91, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Reeves, M.; Sissons, P.; Sinclair, J. Reactivation of human cytomegalovirus in dendritic cells. Discov. Med. 2005, 5, 170–174. [Google Scholar]
- Ljungman, P.; Hakki, M.; Boeckh, M. Cytomegalovirus in hematopoietic stem cell transplant recipients. Infect. Dis. Clin. North Am. 2010, 24, 319–337. [Google Scholar] [CrossRef] [Green Version]
- Hamprecht, K.; Maschmann, J.; Vochem, M.; Dietz, K.; Speer, C.P.; Jahn, G. Epidemiology of transmission of cytomegalovirus from mother to preterm infant by breastfeeding. Lancet 2001, 357, 513–518. [Google Scholar] [CrossRef]
- Kumari, P.; Saha, I.; Narayanan, A.; Narayanan, S.; Takaoka, A.; Kumar, N.S.; Tailor, P.; Kumar, H. Essential role of HCMV deubiquitinase in promoting oncogenesis by targeting anti-viral innate immune signaling pathways. Cell Death Dis. 2017, 8, e3078. [Google Scholar] [CrossRef] [Green Version]
- Castillo, J.P.; Kowalik, T.F. Human cytomegalovirus immediate early proteins and cell growth control. Gene 2002, 290, 19–34. [Google Scholar] [CrossRef]
- Van Damme, E.; Van Loock, M. Functional annotation of human cytomegalovirus gene products: An update. Front. Microbiol. 2014, 5, 218. [Google Scholar] [CrossRef]
- Reeves, M.B. Chromatin-mediated regulation of cytomegalovirus gene expression. Virus Res. 2011, 157, 134–143. [Google Scholar] [CrossRef] [Green Version]
- Soroceanu, L.; Matlaf, L.; Bezrookove, V.; Harkins, L.; Martinez, R.; Greene, M.; Soteropoulos, P.; Cobbs, C.S. Human cytomegalovirus US28 found in glioblastoma promotes an invasive and angiogenic phenotype. Cancer Res. 2011, 71, 6643–6653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baryawno, N.; Rahbar, A.; Wolmer-Solberg, N.; Taher, C.; Odeberg, J.; Darabi, A.; Khan, Z.; Sveinbjornsson, B.; FuskevAg, O.M.; Segerstrom, L.; et al. Detection of human cytomegalovirus in medulloblastomas reveals a potential therapeutic target. J. Clin. Investig. 2011, 121, 4043–4055. [Google Scholar] [CrossRef] [Green Version]
- Tafvizi, F.; Fard, Z.T. Detection of human cytomegalovirus in patients with colorectal cancer by nested-PCR. Asian Pac. J. Cancer Prev. 2014, 15, 1453–1457. [Google Scholar] [CrossRef] [Green Version]
- Samanta, M.; Harkins, L.; Klemm, K.; Britt, W.J.; Cobbs, C.S. High prevalence of human cytomegalovirus in prostatic intraepithelial neoplasia and prostatic carcinoma. J. Urol. 2003, 170, 998–1002. [Google Scholar] [CrossRef]
- Mehravaran, H.; Makvandi, M.; Samarbaf Zade, A.; Neisi, N.; Kiani, H.; Radmehr, H.; Shahani, T.; Hoseini, S.Z.; Ranjbari, N.; Nahid Samiei, R. Association of Human Cytomegalovirus with Hodgkin’s Disease and Non-Hodgkin’s lymphomas. Asian Pac. J. Cancer Prev. 2017, 18, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Taher, C.; de Boniface, J.; Mohammad, A.A.; Religa, P.; Hartman, J.; Yaiw, K.C.; Frisell, J.; Rahbar, A.; Soderberg-Naucler, C. High prevalence of human cytomegalovirus proteins and nucleic acids in primary breast cancer and metastatic sentinel lymph nodes. PLoS ONE 2013, 8, e56795. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.W.; Rådestad, A.F.; Söderberg-Naucler, C.; Rahbar, A. Human cytomegalovirus in high grade serous ovarian cancer possible implications for patients survival. Medicine 2018, 97, e9685. [Google Scholar] [CrossRef]
- Wolmer-Solberg, N.; Baryawno, N.; Rahbar, A.; Fuchs, D.; Odeberg, J.; Taher, C.; Wilhelmi, V.; Milosevic, J.; Mohammad, A.A.; Martinsson, T.; et al. Frequent detection of human cytomegalovirus in neuroblastoma: A novel therapeutic target? Int. J. Cancer 2013, 133, 2351–2361. [Google Scholar] [CrossRef] [PubMed]
- Taher, C.; Frisk, G.; Fuentes, S.; Religa, P.; Costa, H.; Assinger, A.; Vetvik, K.K.; Bukholm, I.R.; Yaiw, K.C.; Smedby, K.E.; et al. High prevalence of human cytomegalovirus in brain metastases of patients with primary breast and colorectal cancers. Transl. Oncol. 2014, 7, 732–740. [Google Scholar] [CrossRef] [Green Version]
- Rahbar, A.; Touma, J.; Costa, H.; Davoudi, B.; Bukholm, I.R.; Sauer, T.; Vetvik, K.; Geisler, J.; Soderberg-Naucler, C. Low Expression of Estrogen Receptor-alpha and Progesterone Receptor in Human Breast Cancer Tissues Is Associated With High-Grade Human Cytomegalovirus Protein Expression. Clin. Breast Cancer 2017, 17, 526–535.e521. [Google Scholar] [CrossRef] [Green Version]
- El-Shinawi, M.; Mohamed, H.T.; El-Ghonaimy, E.A.; Tantawy, M.; Younis, A.; Schneider, R.J.; Mohamed, M.M. Human cytomegalovirus infection enhances NF-κB/p65 signaling in inflammatory breast cancer patients. PLoS ONE 2013, 8, e55755. [Google Scholar] [CrossRef]
- Kumar, A.; Tripathy, M.K.; Pasquereau, S.; Al Moussawi, F.; Abbas, W.; Coquard, L.; Khan, K.A.; Russo, L.; Algros, M.P.; Valmary-Degano, S.; et al. The Human Cytomegalovirus Strain DB Activates Oncogenic Pathways in Mammary Epithelial Cells. EBioMedicine 2018, 30, 167–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smolarz, B.; Wilczynski, J.; Nowakowska, D. DNA repair mechanisms and human cytomegalovirus (HCMV) infection. Folia Microbiol. 2015, 60, 199–209. [Google Scholar] [CrossRef] [Green Version]
- Herbein, G. The Human Cytomegalovirus, from Oncomodulation to Oncogenesis. Viruses 2018, 10, 408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michaelis, M.; Doerr, H.W.; Cinatl, J. The story of human cytomegalovirus and cancer: Increasing evidence and open questions. Neoplasia 2009, 11, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basta, S.; Bennink, J.R. A survival game of hide and seek: Cytomegaloviruses and MHC class I antigen presentation pathways. Viral Immunol. 2003, 16, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Pasquereau, S.; Al Moussawi, F.; Karam, W.; Diab Assaf, M.; Kumar, A.; Herbein, G. Cytomegalovirus, Macrophages and Breast Cancer. Open Virol. J. 2017, 11, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Shenk, T.; Alwine, J.C. Human Cytomegalovirus: Coordinating Cellular Stress, Signaling, and Metabolic Pathways. Annu. Rev. Virol. 2014, 1, 355–374. [Google Scholar] [CrossRef]
- Naucler, C.S.; Geisler, J.; Vetvik, K. The emerging role of human cytomegalovirus infection in human carcinogenesis: A review of current evidence and potential therapeutic implications. Oncotarget 2019, 10, 4333–4347. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Oberstein, A.; Shenk, T. Cellular responses to human cytomegalovirus infection: Induction of a mesenchymal-to-epithelial transition (MET) phenotype. Proc. Natl. Acad. Sci. USA 2017, 114, E8244–E8253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soroceanu, L.; Cobbs, C.S. Is HCMV a tumor promoter? Virus Res. 2011, 157, 193–203. [Google Scholar] [CrossRef]
- Stragliotto, G.; Pantalone, M.R.; Rahbar, A.; Bartek, J.; Soderberg-Naucler, C. Valganciclovir as Add-on to Standard Therapy in Glioblastoma Patients. Clin. Cancer Res. 2020, 26, 4031–4039. [Google Scholar] [CrossRef]
- Stragliotto, G.; Pantalone, M.R.; Rahbar, A.; Söderberg-Nauclér, C. Valganciclovir as Add-On to Standard Therapy in Secondary Glioblastoma. Microorganisms 2020, 8, 1471. [Google Scholar] [CrossRef] [PubMed]
- Batich, K.A.; Mitchell, D.A.; Healy, P.; Herndon, J.E., II; Sampson, J.H. Once, Twice, Three Times a Finding: Reproducibility of Dendritic Cell Vaccine Trials Targeting Cytomegalovirus in Glioblastoma. Clin. Cancer Res. 2020, 26, 5297–5303. [Google Scholar] [CrossRef] [PubMed]
- Razonable, R.R.; Humar, A.; Practice, A.S.T.I.D.C.o. Cytomegalovirus in solid organ transplantation. Am. J. Transplant. 2013, 13 (Suppl. S4), 93–106. [Google Scholar] [CrossRef]
- Ljungman, P.; Boeckh, M.; Hirsch, H.H.; Josephson, F.; Lundgren, J.; Nichols, G.; Pikis, A.; Razonable, R.R.; Miller, V.; Griffiths, P.D.; et al. Definitions of Cytomegalovirus Infection and Disease in Transplant Patients for Use in Clinical Trials. Clin. Infect. Dis. 2017, 64, 87–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, S.A.; Novak, Z.; Pati, S.; Boppana, S.B. Overview of the diagnosis of cytomegalovirus infection. Infect Disord Drug Targets 2011, 11, 466–474. [Google Scholar] [CrossRef]
- Kotton, C.N.; Kumar, D.; Caliendo, A.M.; Huprikar, S.; Chou, S.; Danziger-Isakov, L.; Humar, A.; The Transplantation Society International, C.M.V.C.G. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation. Transplantation 2018, 102, 900–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duraiyan, J.; Govindarajan, R.; Kaliyappan, K.; Palanisamy, M. Applications of immunohistochemistry. J. Pharm. Bioallied Sci. 2012, 4, S307–S309. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.A.; Ahmed, A.; Palmer, A.L.; Michaels, M.G.; Sanchez, P.J.; Bernstein, D.I.; Tolan, R.W., Jr.; Novak, Z.; Chowdhury, N.; Fowler, K.B.; et al. Detection of congenital cytomegalovirus infection by real-time polymerase chain reaction analysis of saliva or urine specimens. J. Infect. Dis. 2014, 210, 1415–1418. [Google Scholar] [CrossRef] [PubMed]
- Razonable, R.R.; Inoue, N.; Pinninti, S.G.; Boppana, S.B.; Lazzarotto, T.; Gabrielli, L.; Simonazzi, G.; Pellett, P.E.; Schmid, D.S. Clinical Diagnostic Testing for Human Cytomegalovirus Infections. J. Infect. Dis. 2020, 221, S74–S85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabiraj, A.; Gupta, J.; Khaitan, T.; Bhattacharya, P.T. Principle and Techniques of Immunohistochemistry—A Review. Int. J. Biol. Med Res. 2015, 6, 5204–5210. [Google Scholar]
- Cobbs, C.S.; Harkins, L.; Samanta, M.; Gillespie, G.Y.; Bharara, S.; King, P.H.; Nabors, L.B.; Cobbs, C.G.; Britt, W.J. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res. 2002, 62, 3347–3350. [Google Scholar]
- Harkins, L.; Volk, A.L.; Samanta, M.; Mikolaenko, I.; Britt, W.J.; Bland, K.I.; Cobbs, C.S. Specific localisation of human cytomegalovirus nucleic acids and proteins in human colorectal cancer. Lancet 2002, 360, 1557–1563. [Google Scholar] [CrossRef]
- Cobbs, C.S.; Matlaf, L.; Harkins, L.E. Methods for the detection of cytomegalovirus in glioblastoma cells and tissues. Methods Mol. Biol. 2014, 1119, 165–196. [Google Scholar] [CrossRef] [PubMed]
- Harkins, L.E.; Matlaf, L.A.; Soroceanu, L.; Klemm, K.; Britt, W.J.; Wang, W.; Bland, K.I.; Cobbs, C.S. Detection of human cytomegalovirus in normal and neoplastic breast epithelium. Herpesviridae 2010, 1, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, H.; Touma, J.; Davoudi, B.; Benard, M.; Sauer, T.; Geisler, J.; Vetvik, K.; Rahbar, A.; Soderberg-Naucler, C. Human cytomegalovirus infection is correlated with enhanced cyclooxygenase-2 and 5-lipoxygenase protein expression in breast cancer. J. Cancer Res. Clin. Oncol. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahbar, A.; Stragliotto, G.; Orrego, A.; Peredo, I.; Taher, C.; Willems, J.; Söderberg-Naucler, C. Low levels of Human Cytomegalovirus Infection in Glioblastoma multiforme associates with patient survival; -a case-control study. Herpesviridae 2012, 3, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahbar, A.; Orrego, A.; Peredo, I.; Dzabic, M.; Wolmer-Solberg, N.; Straat, K.; Stragliotto, G.; Soderberg-Naucler, C. Human cytomegalovirus infection levels in glioblastoma multiforme are of prognostic value for survival. J. Clin. Virol. 2013, 57, 36–42. [Google Scholar] [CrossRef]
- Prichard, J.W. Overview of automated immunohistochemistry. Arch. Pathol. Lab. Med. 2014, 138, 1578–1582. [Google Scholar] [CrossRef] [Green Version]
- Kaufmann, S.K. Methods in Microbiology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 37. [Google Scholar]
- Dziurzynski, K.; Chang, S.M.; Heimberger, A.B.; Kalejta, R.F.; McGregor Dallas, S.R.; Smit, M.; Soroceanu, L.; Cobbs, C.S. Consensus on the role of human cytomegalovirus in glioblastoma. Neuro Oncol. 2012, 14, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Adamson, C.S.; Nevels, M.M. Bright and Early: Inhibiting Human Cytomegalovirus by Targeting Major Immediate-Early Gene Expression or Protein Function. Viruses 2020, 12, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tewary, S.; Arun, I.; Ahmed, R.; Chatterjee, S.; Chakraborty, C. AutoIHC-scoring: A machine learning framework for automated Allred scoring of molecular expression in ER- and PR-stained breast cancer tissue. J. Microsc. 2017, 268, 172–185. [Google Scholar] [CrossRef]
- Howat, W.J.; Blows, F.M.; Provenzano, E.; Brook, M.N.; Morris, L.; Gazinska, P.; Johnson, N.; McDuffus, L.A.; Miller, J.; Sawyer, E.J.; et al. Performance of automated scoring of ER, PR, HER2, CK5/6 and EGFR in breast cancer tissue microarrays in the Breast Cancer Association Consortium. J. Pathol. Clin. Res. 2015, 1, 18–32. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, T.O.; Leung, S.C.Y.; Rimm, D.L.; Dodson, A.; Acs, B.; Badve, S.; Denkert, C.; Ellis, M.J.; Fineberg, S.; Flowers, M.; et al. Assessment of Ki67 in Breast Cancer: Updated Recommendations from the International Ki67 in Breast Cancer Working Group. J. Natl. Cancer Inst. 2020. [Google Scholar] [CrossRef]
- Meermira, D.; Swain, M.; Gowrishankar, S. Study of Ki-67 index in the molecular subtypes of breast cancer: Inter-observer variability and automated scoring. Indian J. Cancer 2020, 57, 289–295. [Google Scholar] [CrossRef]
- Bankhead, P.; Fernandez, J.A.; McArt, D.G.; Boyle, D.P.; Li, G.; Loughrey, M.B.; Irwin, G.W.; Harkin, D.P.; James, J.A.; McQuaid, S.; et al. Integrated tumor identification and automated scoring minimizes pathologist involvement and provides new insights to key biomarkers in breast cancer. Lab. Investig. 2018, 98, 15–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humphries, M.P.; Hynes, S.; Bingham, V.; Cougot, D.; James, J.; Patel-Socha, F.; Parkes, E.E.; Blayney, J.K.; O’Rorke, M.A.; Irwin, G.W.; et al. Automated Tumour Recognition and Digital Pathology Scoring Unravels New Role for PD-L1 in Predicting Good Outcome in ER-/HER2+ Breast Cancer. J. Oncol. 2018, 2018, 2937012. [Google Scholar] [CrossRef] [PubMed]
- Moussawi, F.A.; Kumar, A.; Pasquereau, S.; Tripathy, M.K.; Karam, W.; Diab-Assaf, M.; Herbein, G. The transcriptome of human mammary epithelial cells infected with the HCMV-DB strain displays oncogenic traits. Sci. Rep. 2018, 8, 12574. [Google Scholar] [CrossRef]
- Yang, Z.; Tang, X.; Meng, G.; Benesch, M.G.K.; Mackova, M.; Belon, A.P.; Serrano-Lomelin, J.; Goping, I.S.; Brindley, D.N.; Hemmings, D.G. Latent Cytomegalovirus Infection in Female Mice Increases Breast Cancer Metastasis. Cancers 2019, 11, 447. [Google Scholar] [CrossRef] [Green Version]
- Nehme, Z.; Pasquereau, S.; Haidar Ahmad, S.; Coaquette, A.; Molimard, C.; Monnien, F.; Algros, M.P.; Adotevi, O.; Diab Assaf, M.; Feugeas, J.P.; et al. Polyploid giant cancer cells, stemness and epithelial-mesenchymal plasticity elicited by human cytomegalovirus. Oncogene 2021, 40, 3030–3046. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiyrizadeh, S.; Hosseini, S.Y.; Yaghobi, R.; Safaei, A.; Sarvari, J. Almost Complete Lack of Human Cytomegalovirus and Human papillomaviruses Genome in Benign and Malignant Breast Lesions in Shiraz, Southwest of Iran. Asian Pac. J. Cancer Prev. 2017, 18, 3319–3324. [Google Scholar] [CrossRef]
- Lau, S.K.; Chen, Y.-Y.; Chen, W.-G.; Diamond, D.J.; Mamelak, A.N.; Zaia, J.A.; Weiss, L.M. Lack of association of cytomegalovirus with human brain tumors. Mod. Pathol. 2005, 18, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martinez, A.; Alenda, C.; Irles, E.; Ochoa, E.; Quintanar, T.; Rodriguez-Lescure, A.; Soto, J.L.; Barbera, V.M. Lack of cytomegalovirus detection in human glioma. Virol. J. 2017, 14, 216. [Google Scholar] [CrossRef] [Green Version]
- Holdhoff, M.; Guner, G.; Rodriguez, F.J.; Hicks, J.L.; Zheng, Q.; Forman, M.S.; Ye, X.; Grossman, S.A.; Meeker, A.K.; Heaphy, C.M.; et al. Absence of Cytomegalovirus in Glioblastoma and Other High-grade Gliomas by Real-time PCR, Immunohistochemistry, and In Situ Hybridization. Clin. Cancer Res. 2017, 23, 3150–3157. [Google Scholar] [CrossRef] [PubMed] [Green Version]

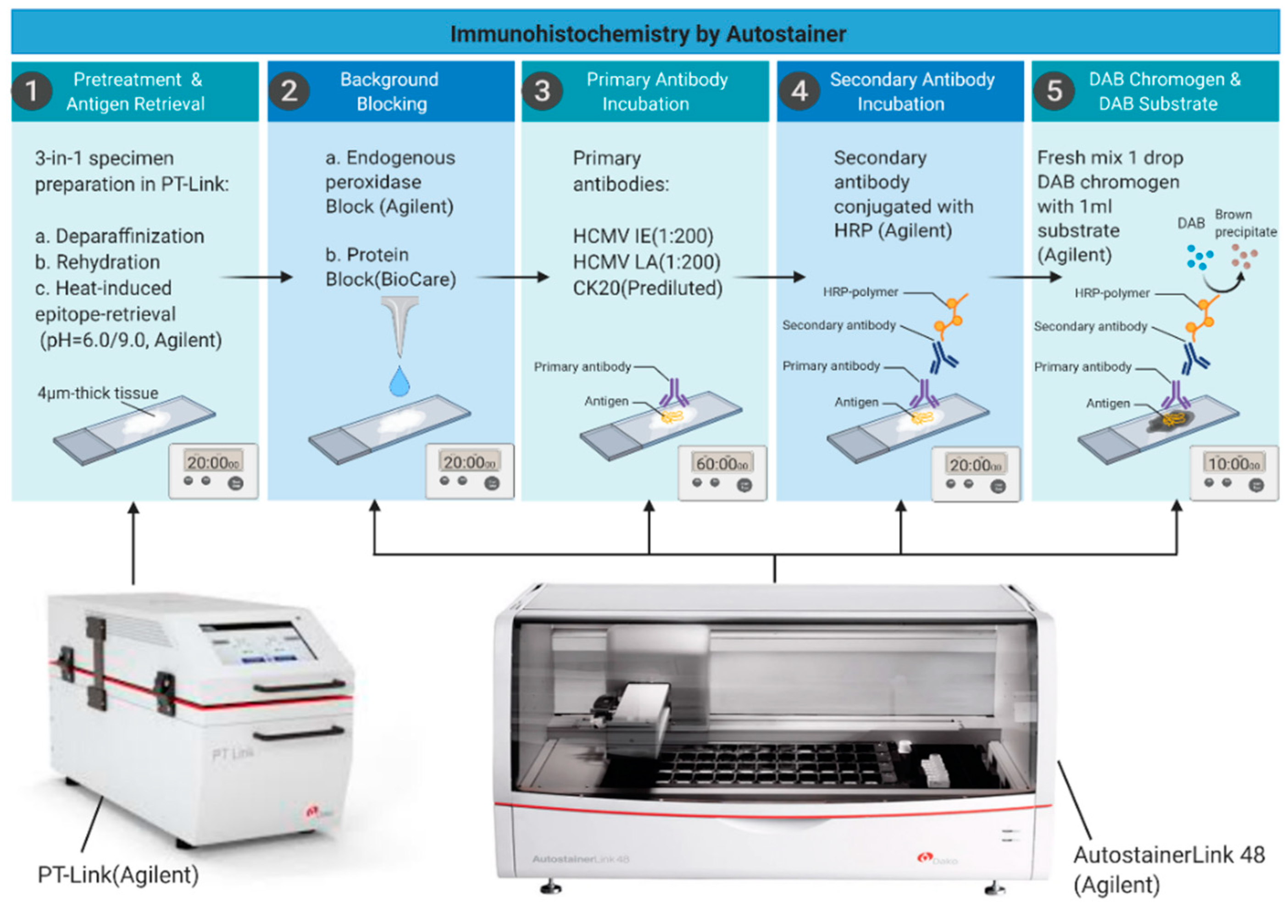

| Method | Specimens | Comments |
|---|---|---|
| Serology | Blood | The presence of IgG determines that a patient has had HCMV infection in the past and is considered a carrier of latent virus. Detection of IgM without detectable IgG indicates acute primary infection, while detection of both IgM and IgG indicates reactivated HCMV infection. |
| Antigenemia | Blood | Use of monoclonal antibodies to detect the presence of HCMV pp65 in neutrophils during the early period of the virus replication cycle. Reported as number of pp65-positive cells per number of neutrophils counted. Sensitive but limited by the lack of automation, assay standardization and subjective interpretation. |
| Cell culture | Blood, Urine, Saliva | Conventional approach where clinical specimens are inoculated onto human fibroblasts, incubated and observed over time. Takes 2–21 days for reporting a result based on a morphological analysis. It is possible to obtain a faster detection of HCMV in culture already 24 h post-infection by using staining with anti-IE antibodies. Highly specific but low sensitivity for HCMV infection. |
| Polymerase Chain Reaction | Blood, Urine, Saliva, Tissue | Rapid and sensitive method based on amplification of nucleic acids. Targets even low levels of immediate early and late genes or transcripts. Quantitative nucleic acid amplification guides preemptive strategies, monitors response to therapy and is the preferred method for diagnosis of HCMV infection. |
| Immunohistochemical Staining | Blood, Urine, Saliva, Tissue | Monoclonal or polyclonal antibodies are applied against various HCMV proteins and visualized by dye or fluorescently labelled antibodies or enzyme/polymer labelled secondary antibodies, allowing morphological identification of HCMV in the specimen. It is a highly sensitive and very specific technique. Considered goldstandard for diagnosis in HCMV end-organ diseases. |
| HCMV-IE | HCMV-LA | |
|---|---|---|
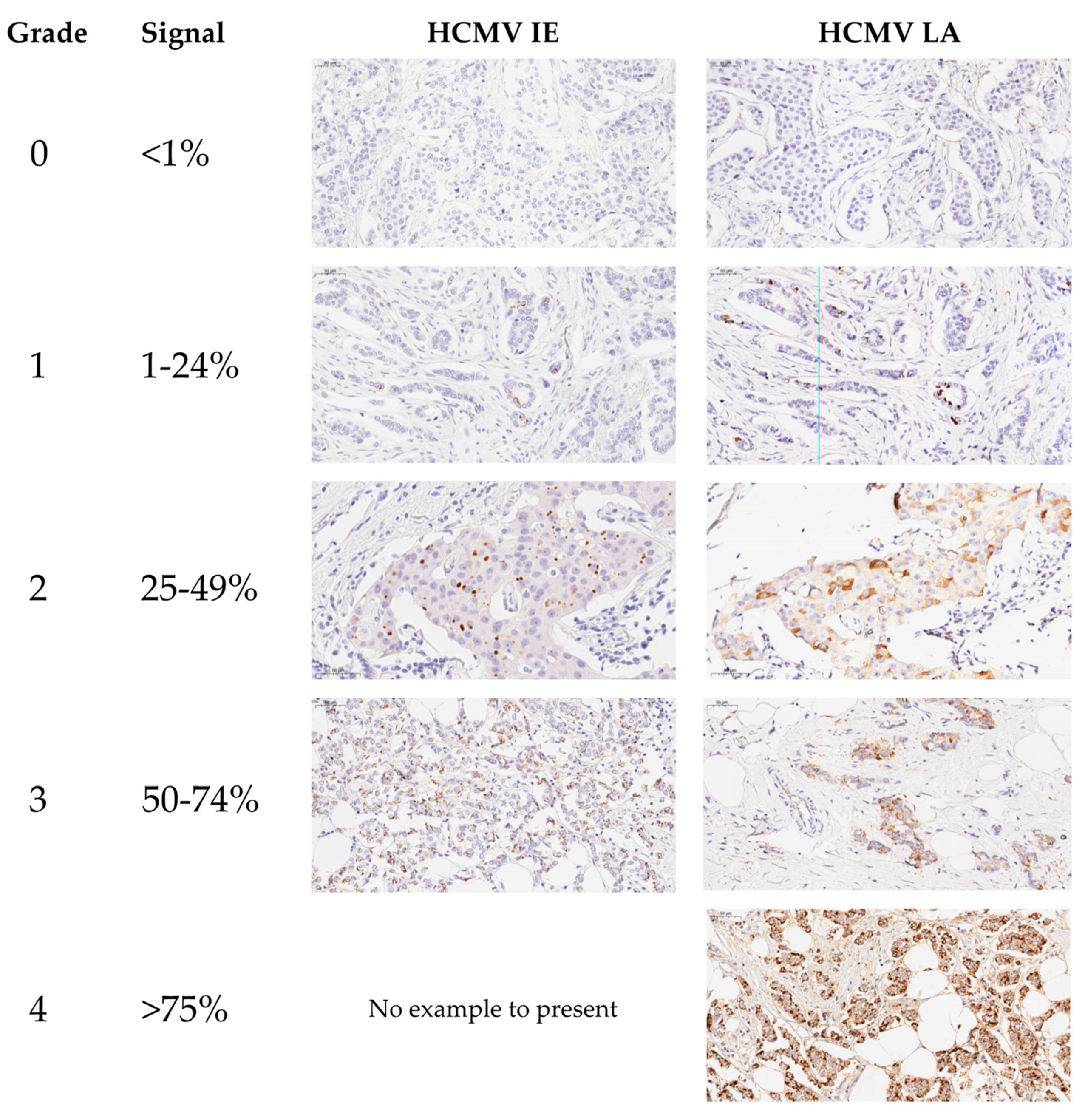
| Grade 0 | 102 (91.9%) | 28 (25.2%) |
| Grade 1 | 4 (3.6%) | 27 (24.3%) |
| Grade 2 | 4 (3.6%) | 22 (19.8%) |
| Grade 3 | 1 (0.9%) | 17 (15.3%) |
| Grade 4 | 0 | 17 (15.3%) |
| Positive (Total) | 9 (8.1%) | 83 (74.8%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Touma, J.; Liu, Y.; Rahbar, A.; Pantalone, M.R.; Almazan, N.M.; Vetvik, K.; Söderberg-Nauclér, C.; Geisler, J.; Sauer, T. Detection of Human Cytomegalovirus Proteins in Paraffin-Embedded Breast Cancer Tissue Specimens—A Novel, Automated Immunohistochemical Staining Protocol. Microorganisms 2021, 9, 1059. https://doi.org/10.3390/microorganisms9051059
Touma J, Liu Y, Rahbar A, Pantalone MR, Almazan NM, Vetvik K, Söderberg-Nauclér C, Geisler J, Sauer T. Detection of Human Cytomegalovirus Proteins in Paraffin-Embedded Breast Cancer Tissue Specimens—A Novel, Automated Immunohistochemical Staining Protocol. Microorganisms. 2021; 9(5):1059. https://doi.org/10.3390/microorganisms9051059
Chicago/Turabian StyleTouma, Joel, Yan Liu, Afsar Rahbar, Mattia Russel Pantalone, Nerea Martin Almazan, Katja Vetvik, Cecilia Söderberg-Nauclér, Jürgen Geisler, and Torill Sauer. 2021. "Detection of Human Cytomegalovirus Proteins in Paraffin-Embedded Breast Cancer Tissue Specimens—A Novel, Automated Immunohistochemical Staining Protocol" Microorganisms 9, no. 5: 1059. https://doi.org/10.3390/microorganisms9051059
APA StyleTouma, J., Liu, Y., Rahbar, A., Pantalone, M. R., Almazan, N. M., Vetvik, K., Söderberg-Nauclér, C., Geisler, J., & Sauer, T. (2021). Detection of Human Cytomegalovirus Proteins in Paraffin-Embedded Breast Cancer Tissue Specimens—A Novel, Automated Immunohistochemical Staining Protocol. Microorganisms, 9(5), 1059. https://doi.org/10.3390/microorganisms9051059








