Iron in Translation: From the Beginning to the End
Abstract
1. Introduction
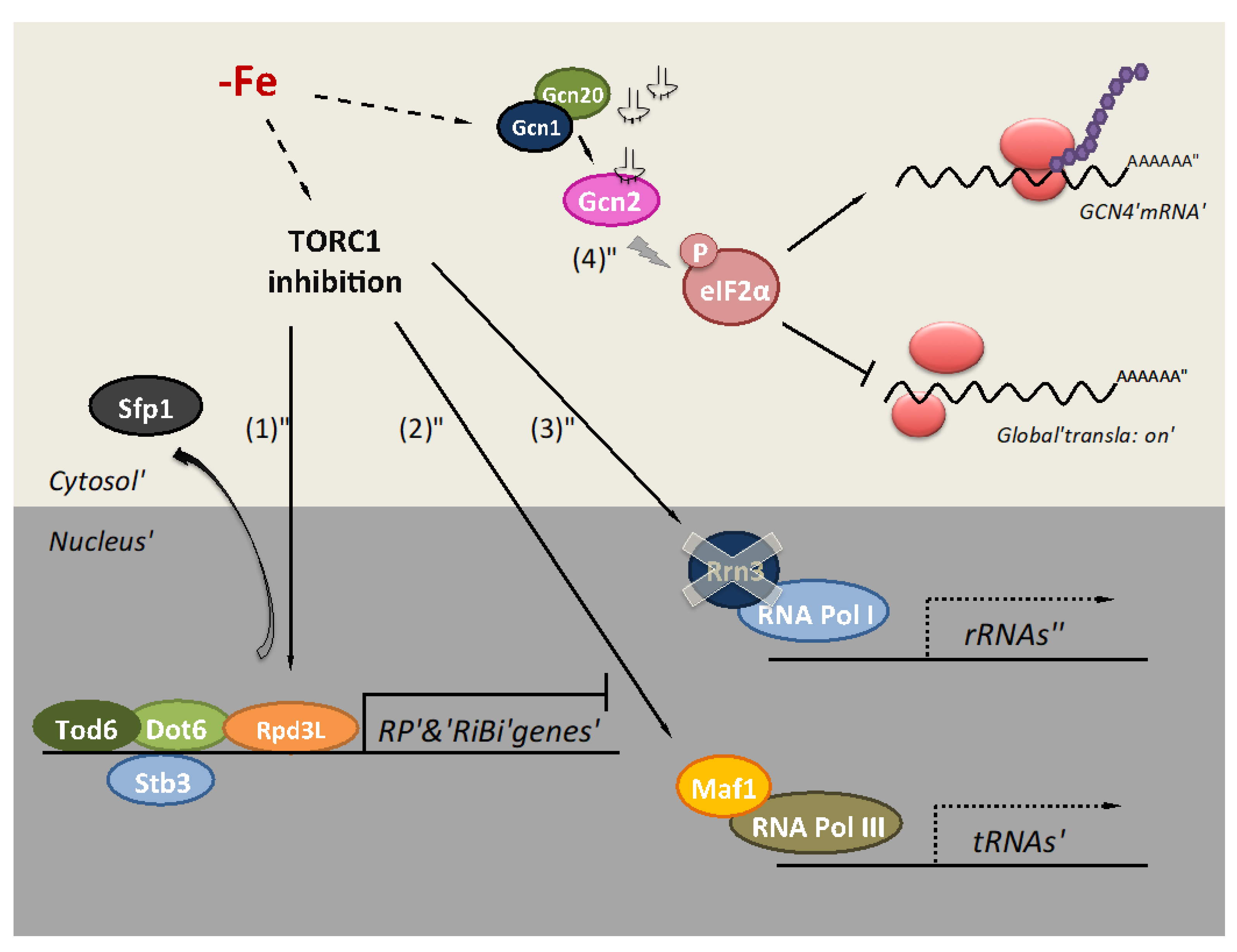
2. Iron Deficiency Impairs Translation at the Initiation Step
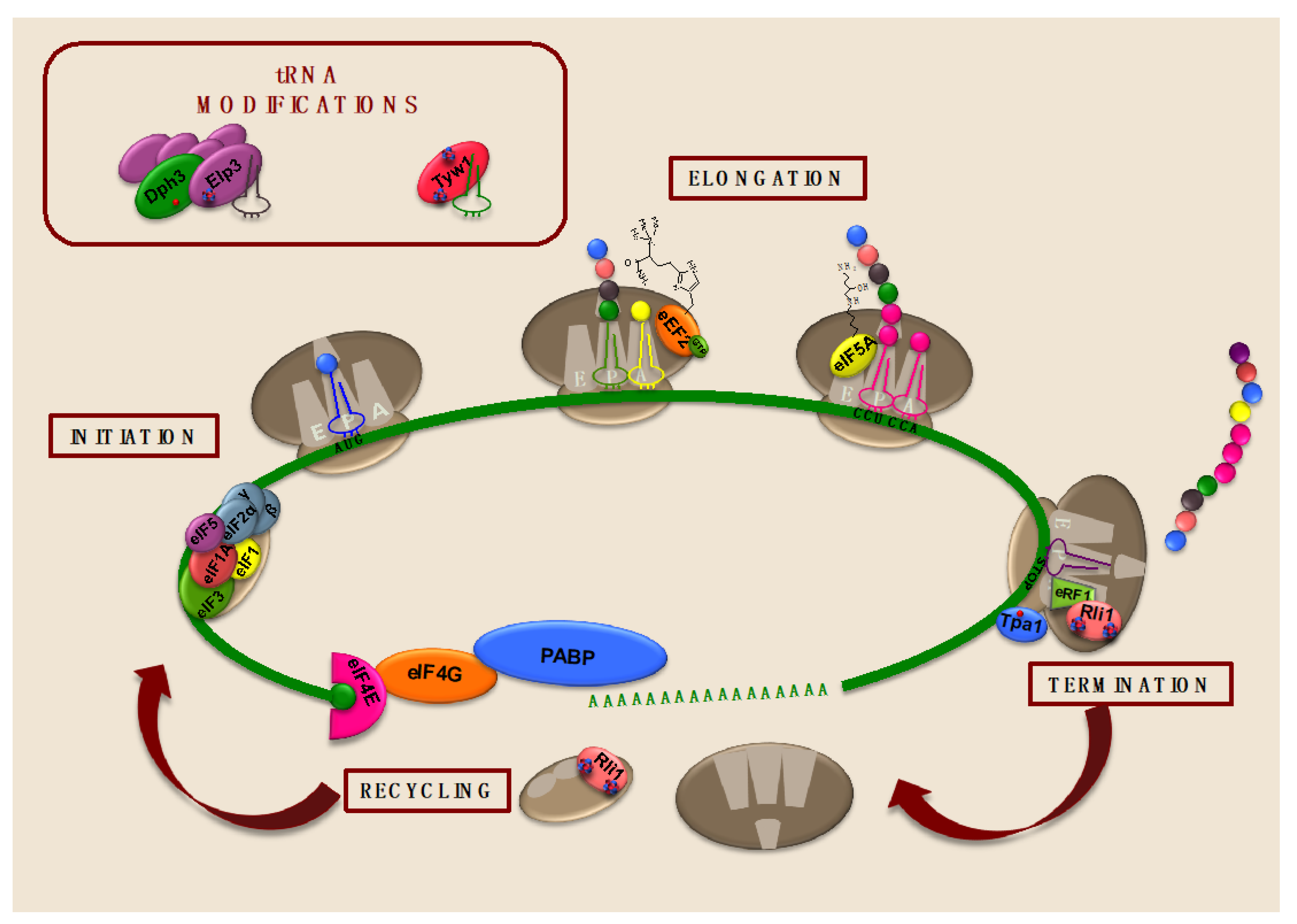
3. The Relevance of Iron in Translation Elongation
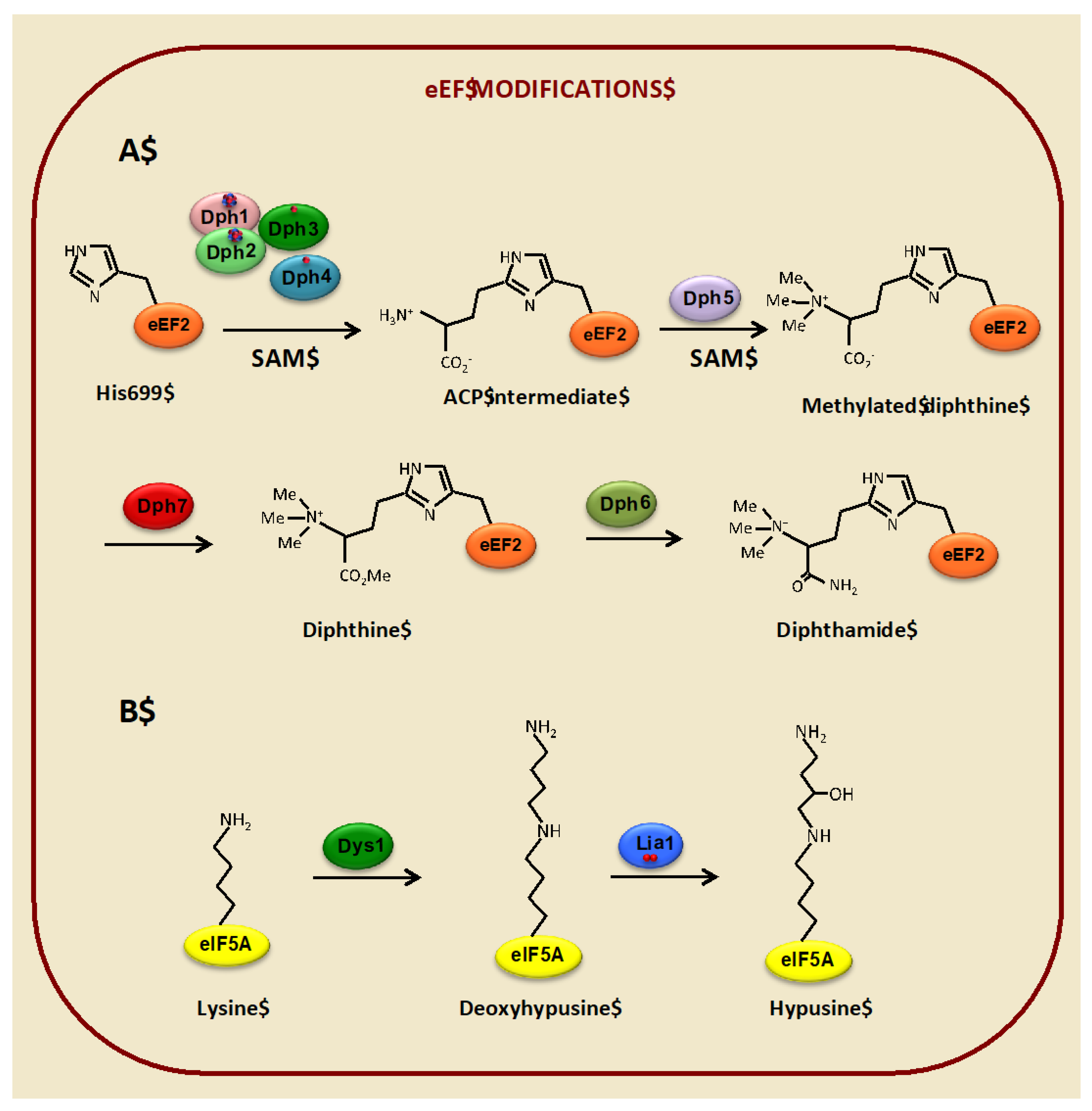
3.1. Diphthamide Modification of the Translational Elongation Factor eEF2 Depends on Iron
3.2. Hypusine Modification of the Translation Elongation Factor eIF5A Depends on Iron
4. Iron Is Required for Translation Termination
4.1. The Conserved Iron-Containing Protein ABCE1/Rli1 Is Essential for Translation Termination and Ribosome Recycling and Reinitiation
4.2. The Iron-Dependent Prolyl Hydroxylase Tpa1 Modulates Translation Termination
5. Iron-Dependent Enzymes Catalyze tRNA Modifications Important for Translation
5.1. The Fe/S Cluster Enzyme Tyw1 Is Important for Wybutosine Modification of tRNAPhe and Protection against High-Iron Conditions
5.2. The Elongator Fe/S Cluster Subunit Elp3 Enhances Translation Efficiency by Catalyzing tRNA Wobble Uridine Modifications
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Zimmermann, M.B.; Hurrell, R.F. Nutritional iron deficiency. Lancet 2007, 370, 511–520. [Google Scholar] [CrossRef]
- Briat, J.F.; Dubos, C.; Gaymard, F. Iron nutrition, biomass production, and plant product quality. Trends Plant Sci. 2015, 20, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Lill, R.; Freibert, S.A. Mechanisms of Mitochondrial Iron-Sulfur Protein Biogenesis. Annu. Rev. Biochem. 2020, 89, 471–499. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Lai, R.; Nielsen, K.; Fekete, C.A.; Qiu, H.; Hinnebusch, A.G. The essential ATP-binding cassette protein RLI1 functions in translation by promoting preinitiation complex assembly. J. Biol. Chem. 2004, 279, 42157–42168. [Google Scholar] [CrossRef]
- Yarunin, A.; Panse, V.G.; Petfalski, E.; Dez, C.; Tollervey, D.; Hurt, E.C. Functional link between ribosome formation and biogenesis of iron-sulfur proteins. EMBO J. 2005, 24, 580–588. [Google Scholar] [CrossRef]
- Kispal, G.; Sipos, K.; Lange, H.; Fekete, Z.; Bedekovics, T.; Janaky, T.; Bassler, J.; Aguilar Netz, D.J.; Balk, J.; Rotte, C.; et al. Biogenesis of cytosolic ribosomes requires the essential iron-sulphur protein Rli1p and mitochondria. EMBO J. 2005, 24, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Netz, D.J.; Stith, C.M.; Stumpfig, M.; Kopf, G.; Vogel, D.; Genau, H.M.; Stodola, J.L.; Lill, R.; Burgers, P.M.; Pierik, A.J. Eukaryotic DNA polymerases require an iron-sulfur cluster for the formation of active complexes. Nat. Chem. Biol. 2011, 8, 125–132. [Google Scholar] [CrossRef]
- Puig, S.; Ramos-Alonso, L.; Romero, A.M.; Martinez-Pastor, M.T. The elemental role of iron in DNA synthesis and repair. Metallomics 2017, 9, 1483–1500. [Google Scholar] [CrossRef]
- Puig, S.; Askeland, E.; Thiele, D.J. Coordinated remodeling of cellular metabolism during iron deficiency through targeted mRNA degradation. Cell 2005, 120, 99–110. [Google Scholar] [CrossRef]
- Puig, S.; Vergara, S.V.; Thiele, D.J. Cooperation of two mRNA-binding proteins drives metabolic adaptation to iron deficiency. Cell Metab. 2008, 7, 555–564. [Google Scholar] [CrossRef]
- Ramos-Alonso, L.; Romero, A.M.; Soler, M.A.; Perea-Garcia, A.; Alepuz, P.; Puig, S.; Martinez-Pastor, M.T. Yeast Cth2 protein represses the translation of ARE-containing mRNAs in response to iron deficiency. PLoS Genet. 2018, 14, e1007476. [Google Scholar] [CrossRef]
- Romero, A.M.; Ramos-Alonso, L.; Montella-Manuel, S.; Garcia-Martinez, J.; de la Torre-Ruiz, M.A.; Perez-Ortin, J.E.; Martinez-Pastor, M.T.; Puig, S. A genome-wide transcriptional study reveals that iron deficiency inhibits the yeast TORC1 pathway. Biochim. Biophys. Acta Gene Regul. Mech. 2019, 1862, 194414. [Google Scholar] [CrossRef]
- Ramos-Alonso, L.; Romero, A.M.; Martinez-Pastor, M.T.; Puig, S. Iron Regulatory Mechanisms in Saccharomyces cerevisiae. Front. Microbiol. 2020, 11, 582830. [Google Scholar] [CrossRef]
- Mancera-Martinez, E.; Brito Querido, J.; Valasek, L.S.; Simonetti, A.; Hashem, Y. ABCE1: A special factor that orchestrates translation at the crossroad between recycling and initiation. RNA Biol. 2017, 14, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Schaffrath, R.; Abdel-Fattah, W.; Klassen, R.; Stark, M.J. The diphthamide modification pathway from Saccharomyces cerevisiae-revisited. Mol. Microbiol. 2014, 94, 1213–1226. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Behringer, R.R. Ovca1 regulates cell proliferation, embryonic development, and tumorigenesis. Genes Dev. 2004, 18, 320–332. [Google Scholar] [CrossRef]
- Dong, M.; Su, X.; Dzikovski, B.; Dando, E.E.; Zhu, X.; Du, J.; Freed, J.H.; Lin, H. Dph3 is an electron donor for Dph1-Dph2 in the first step of eukaryotic diphthamide biosynthesis. J. Am. Chem. Soc. 2014, 136, 1754–1757. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Chitoor, B.; Goswami, A.V.; Pareek, G.; Atreya, H.S.; D’Silva, P. Structure and mechanistic insights into novel iron-mediated moonlighting functions of human J-protein cochaperone, Dph4. J. Biol. Chem. 2012, 287, 13194–13205. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Aravind, L.; Wolff, E.C.; Kaevel, J.; Kim, Y.S.; Park, M.H. Molecular cloning, expression, and structural prediction of deoxyhypusine hydroxylase: A HEAT-repeat-containing metalloenzyme. Proc. Natl. Acad. Sci. USA 2006, 103, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kang, K.R.; Wolff, E.C.; Bell, J.K.; McPhie, P.; Park, M.H. Deoxyhypusine hydroxylase is a Fe(II)-dependent, HEAT-repeat enzyme. Identification of amino acid residues critical for Fe(II) binding and catalysis [corrected. J. Biol. Chem. 2006, 281, 13217–13225. [Google Scholar] [CrossRef] [PubMed]
- Cano, V.S.; Medrano, F.J.; Park, M.H.; Valentini, S.R. Evidence for conformational changes in the yeast deoxyhypusine hydroxylase Lia1 upon iron displacement from its active site. Amino Acids 2010, 38, 479–490. [Google Scholar] [CrossRef]
- Noma, A.; Kirino, Y.; Ikeuchi, Y.; Suzuki, T. Biosynthesis of wybutosine, a hyper-modified nucleoside in eukaryotic phenylalanine tRNA. EMBO J. 2006, 25, 2142–2154. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jia, X.; Ward, D.M.; Kaplan, J. Yap5 protein-regulated transcription of the TYW1 gene protects yeast from high iron toxicity. J. Biol. Chem. 2011, 286, 38488–38497. [Google Scholar] [CrossRef]
- Keeling, K.M.; Salas-Marco, J.; Osherovich, L.Z.; Bedwell, D.M. Tpa1p is part of an mRNP complex that influences translation termination, mRNA deadenylation, and mRNA turnover in Saccharomyces cerevisiae. Mol. Cell Biol. 2006, 26, 5237–5248. [Google Scholar] [CrossRef]
- Loenarz, C.; Sekirnik, R.; Thalhammer, A.; Ge, W.; Spivakovsky, E.; Mackeen, M.M.; McDonough, M.A.; Cockman, M.E.; Kessler, B.M.; Ratcliffe, P.J.; et al. Hydroxylation of the eukaryotic ribosomal decoding center affects translational accuracy. Proc. Natl. Acad. Sci. USA 2014, 111, 4019–4024. [Google Scholar] [CrossRef]
- Singleton, R.S.; Liu-Yi, P.; Formenti, F.; Ge, W.; Sekirnik, R.; Fischer, R.; Adam, J.; Pollard, P.J.; Wolf, A.; Thalhammer, A.; et al. OGFOD1 catalyzes prolyl hydroxylation of RPS23 and is involved in translation control and stress granule formation. Proc. Natl Acad. Sci. USA 2014, 111, 4031–4036. [Google Scholar] [CrossRef]
- Shivange, G.; Kodipelli, N.; Monisha, M.; Anindya, R. A role for Saccharomyces cerevisiae Tpa1 protein in direct alkylation repair. J. Biol. Chem. 2014, 289, 35939–35952. [Google Scholar] [CrossRef]
- Martinez-Pastor, M.T.; Estruch, F. Sudden depletion of carbon source blocks translation, but not transcription, in the yeast Saccharomyces cerevisiae. FEBS Lett. 1996, 390, 319–322. [Google Scholar] [CrossRef]
- Ashe, M.P.; De Long, S.K.; Sachs, A.B. Glucose depletion rapidly inhibits translation initiation in yeast. Mol. Biol. Cell 2000, 11, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Shenton, D.; Smirnova, J.B.; Selley, J.N.; Carroll, K.; Hubbard, S.J.; Pavitt, G.D.; Ashe, M.P.; Grant, C.M. Global translational responses to oxidative stress impact upon multiple levels of protein synthesis. J. Biol. Chem. 2006, 281, 29011–29021. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Izawa, S. Adaptive response in stress granule formation and bulk translational repression upon a combined stress of mild heat shock and mild ethanol stress in yeast. Genes Cells 2013, 18, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Hughes Hallett, J.E.; Luo, X.; Capaldi, A.P. State transitions in the TORC1 signaling pathway and information processing in Saccharomyces cerevisiae. Genetics 2014, 198, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Huber, A.; Bodenmiller, B.; Uotila, A.; Stahl, M.; Wanka, S.; Gerrits, B.; Aebersold, R.; Loewith, R. Characterization of the rapamycin-sensitive phosphoproteome reveals that Sch9 is a central coordinator of protein synthesis. Genes Dev. 2009, 23, 1929–1943. [Google Scholar] [CrossRef]
- Humphrey, E.L.; Shamji, A.F.; Bernstein, B.E.; Schreiber, S.L. Rpd3p relocation mediates a transcriptional response to rapamycin in yeast. Chem. Biol. 2004, 11, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Marion, R.M.; Regev, A.; Segal, E.; Barash, Y.; Koller, D.; Friedman, N.; O’Shea, E.K. Sfp1 is a stress- and nutrient-sensitive regulator of ribosomal protein gene expression. Proc. Natl. Acad. Sci. USA 2004, 101, 14315–14322. [Google Scholar] [CrossRef]
- Romero, A.M.; Ramos-Alonso, L.; Alepuz, P.; Puig, S.; Martinez-Pastor, M.T. Global translational repression induced by iron deficiency in yeast depends on the Gcn2/eIF2alpha pathway. Sci. Rep. 2020, 10, 233. [Google Scholar] [CrossRef]
- Sonenberg, N.; Hinnebusch, A.G. Regulation of translation initiation in eukaryotes: Mechanisms and biological targets. Cell 2009, 136, 731–745. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.J.; Hellen, C.U.; Pestova, T.V. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat. Rev. Mol. Cell Biol. 2010, 11, 113–127. [Google Scholar] [CrossRef]
- Dong, J.; Qiu, H.; Garcia-Barrio, M.; Anderson, J.; Hinnebusch, A.G. Uncharged tRNA activates GCN2 by displacing the protein kinase moiety from a bipartite tRNA-binding domain. Mol. Cell 2000, 6, 269–279. [Google Scholar] [CrossRef]
- Garcia-Barrio, M.; Dong, J.; Ufano, S.; Hinnebusch, A.G. Association of GCN1-GCN20 regulatory complex with the N-terminus of eIF2alpha kinase GCN2 is required for GCN2 activation. EMBO J. 2000, 19, 1887–1899. [Google Scholar] [CrossRef]
- Pochopien, A.A.; Beckert, B.; Kasvandik, S.; Berninghausen, O.; Beckmann, R.; Tenson, T.; Wilson, D.N. Structure of Gcn1 bound to stalled and colliding 80S ribosomes. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, K.; Meyer, M.R.; Jackson, B.M.; Slade, D.; Roberts, C.; Hinnebusch, A.G.; Marton, M.J. Transcriptional profiling shows that Gcn4p is a master regulator of gene expression during amino acid starvation in yeast. Mol. Cell Biol. 2001, 21, 4347–4368. [Google Scholar] [CrossRef] [PubMed]
- Han, A.P.; Yu, C.; Lu, L.; Fujiwara, Y.; Browne, C.; Chin, G.; Fleming, M.; Leboulch, P.; Orkin, S.H.; Chen, J.J. Heme-regulated eIF2alpha kinase (HRI) is required for translational regulation and survival of erythroid precursors in iron deficiency. EMBO J. 2001, 20, 6909–6918. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Macias-Garcia, A.; Ulirsch, J.C.; Velazquez, J.; Butty, V.L.; Levine, S.S.; Sankaran, V.G.; Chen, J.J. HRI coordinates translation necessary for protein homeostasis and mitochondrial function in erythropoiesis. Elife 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Suragani, R.N.; Zachariah, R.S.; Velazquez, J.G.; Liu, S.; Sun, C.W.; Townes, T.M.; Chen, J.J. Heme-regulated eIF2alpha kinase activated Atf4 signaling pathway in oxidative stress and erythropoiesis. Blood 2012, 119, 5276–5284. [Google Scholar] [CrossRef]
- Bayeva, M.; Khechaduri, A.; Puig, S.; Chang, H.C.; Patial, S.; Blackshear, P.J.; Ardehali, H. mTOR regulates cellular iron homeostasis through tristetraprolin. Cell Metab. 2012, 16, 645–657. [Google Scholar] [CrossRef]
- Sato, T.; Chang, H.C.; Bayeva, M.; Shapiro, J.S.; Ramos-Alonso, L.; Kouzu, H.; Jiang, X.; Liu, T.; Yar, S.; Sawicki, K.T.; et al. mRNA-binding protein tristetraprolin is essential for cardiac response to iron deficiency by regulating mitochondrial function. Proc. Natl. Acad. Sci. USA 2018, 115, E6291–E6300. [Google Scholar] [CrossRef]
- Jorgensen, R.; Merrill, A.R.; Andersen, G.R. The life and death of translation elongation factor 2. Biochem. Soc. Trans. 2006, 34, 1–6. [Google Scholar] [CrossRef]
- Prabhakar, A.; Choi, J.; Wang, J.; Petrov, A.; Puglisi, J.D. Dynamic basis of fidelity and speed in translation: Coordinated multistep mechanisms of elongation and termination. Protein Sci. 2017, 26, 1352–1362. [Google Scholar] [CrossRef]
- Su, X.; Lin, Z.; Lin, H. The biosynthesis and biological function of diphthamide. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 515–521. [Google Scholar] [CrossRef]
- Botet, J.; Rodriguez-Mateos, M.; Ballesta, J.P.; Revuelta, J.L.; Remacha, M. A chemical genomic screen in Saccharomyces cerevisiae reveals a role for diphthamidation of translation elongation factor 2 in inhibition of protein synthesis by sordarin. Antimicrob. Agents Chemother. 2008, 52, 1623–1629. [Google Scholar] [CrossRef] [PubMed]
- Bar, C.; Zabel, R.; Liu, S.; Stark, M.J.; Schaffrath, R. A versatile partner of eukaryotic protein complexes that is involved in multiple biological processes: Kti11/Dph3. Mol. Microbiol. 2008, 69, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Uthman, S.; Bar, C.; Scheidt, V.; Liu, S.; ten Have, S.; Giorgini, F.; Stark, M.J.; Schaffrath, R. The amidation step of diphthamide biosynthesis in yeast requires DPH6, a gene identified through mining the DPH1-DPH5 interaction network. PLoS Genet. 2013, 9, e1003334. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, X.; Torelli, A.T.; Lee, M.; Dzikovski, B.; Koralewski, R.M.; Wang, E.; Freed, J.; Krebs, C.; Ealick, S.E.; et al. Diphthamide biosynthesis requires an organic radical generated by an iron-sulphur enzyme. Nature 2010, 465, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Zhang, Y.; Lin, H. Noncanonical Radical SAM Enzyme Chemistry Learned from Diphthamide Biosynthesis. Biochemistry 2018, 57, 3454–3459. [Google Scholar] [CrossRef]
- Dong, M.; Dando, E.E.; Kotliar, I.; Su, X.; Dzikovski, B.; Freed, J.H.; Lin, H. The asymmetric function of Dph1-Dph2 heterodimer in diphthamide biosynthesis. J. Biol. Inorg. Chem. 2019, 24, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Dong, M.; Zhang, Y.; Lee, E.A.; Lin, H. Cbr1 is a Dph3 reductase required for the tRNA wobble uridine modification. Nat. Chem. Biol. 2016, 12, 995–997. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Johansson, M.J.; Bystrom, A.S. An early step in wobble uridine tRNA modification requires the Elongator complex. RNA 2005, 11, 424–436. [Google Scholar] [CrossRef]
- Villahermosa, D.; Fleck, O. Elp3 and Dph3 of Schizosaccharomyces pombe mediate cellular stress responses through tRNA(Lys)UUU modifications. Sci. Rep. 2017, 7, 7225–1. [Google Scholar] [CrossRef]
- Su, X.; Lin, Z.; Chen, W.; Jiang, H.; Zhang, S.; Lin, H. Chemogenomic approach identified yeast YLR143W as diphthamide synthetase. Proc. Natl. Acad. Sci. USA 2012, 109, 19983–19987. [Google Scholar] [CrossRef] [PubMed]
- De Crecy-Lagard, V.; Forouhar, F.; Brochier-Armanet, C.; Tong, L.; Hunt, J.F. Comparative genomic analysis of the DUF71/COG2102 family predicts roles in diphthamide biosynthesis and B12 salvage. Biol. Direct 2012, 7, 32. [Google Scholar] [CrossRef]
- Kimata, Y.; Kohno, K. Elongation factor 2 mutants deficient in diphthamide formation show temperature-sensitive cell growth. J. Biol. Chem. 1994, 269, 13497–13501. [Google Scholar] [CrossRef]
- Chen, J.Y.; Bodley, J.W.; Livingston, D.M. Diphtheria toxin-resistant mutants of Saccharomyces cerevisiae. Mol. Cell Biol. 1985, 5, 3357–3360. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, S.; Milne, G.T.; Kuremsky, J.G.; Fink, G.R.; Leppla, S.H. Identification of the proteins required for biosynthesis of diphthamide, the target of bacterial ADP-ribosylating toxins on translation elongation factor 2. Mol. Cell Biol. 2004, 24, 9487–9497. [Google Scholar] [CrossRef] [PubMed]
- Hawer, H.; Utkur, K.; Arend, M.; Mayer, K.; Adrian, L.; Brinkmann, U.; Schaffrath, R. Importance of diphthamide modified EF2 for translational accuracy and competitive cell growth in yeast. PLoS ONE 2018, 13, e0205870. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.; da Silva Mateus Seidl, A.R.; Ducret, A.; Kux van Geijtenbeek, S.; Michel, S.; Racek, T.; Birzele, F.; Haas, A.K.; Rueger, R.; Gerg, M.; et al. Loss of diphthamide pre-activates NF-kappaB and death receptor pathways and renders MCF7 cells hypersensitive to tumor necrosis factor. Proc. Natl. Acad. Sci. USA 2015, 112, 10732–10737. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Bachran, C.; Gupta, P.; Miller-Randolph, S.; Wang, H.; Crown, D.; Zhang, Y.; Wein, A.N.; Singh, R.; Fattah, R.; et al. Diphthamide modification on eukaryotic elongation factor 2 is needed to assure fidelity of mRNA translation and mouse development. Proc. Natl. Acad. Sci. USA 2012, 109, 13817–13822. [Google Scholar] [CrossRef]
- Hawer, H.; Mendelsohn, B.A.; Mayer, K.; Kung, A.; Malhotra, A.; Tuupanen, S.; Schleit, J.; Brinkmann, U.; Schaffrath, R. Diphthamide-deficiency syndrome: A novel human developmental disorder and ribosomopathy. Eur. J. Hum. Genet. 2020, 28, 1497–1508. [Google Scholar] [CrossRef]
- Webb, T.R.; Cross, S.H.; McKie, L.; Edgar, R.; Vizor, L.; Harrison, J.; Peters, J.; Jackson, I.J. Diphthamide modification of eEF2 requires a J-domain protein and is essential for normal development. J. Cell Sci. 2008, 121, 3140–3145. [Google Scholar] [CrossRef]
- Kalapis, D.; Bezerra, A.R.; Farkas, Z.; Horvath, P.; Bodi, Z.; Daraba, A.; Szamecz, B.; Gut, I.; Bayes, M.; Santos, M.A.; et al. Evolution of Robustness to Protein Mistranslation by Accelerated Protein Turnover. PLoS Biol. 2015, 13, e1002291. [Google Scholar] [CrossRef]
- Kapur, M.; Ackerman, S.L. mRNA Translation Gone Awry: Translation Fidelity and Neurological Disease. Trends Genet. 2018, 34, 218–231. [Google Scholar] [CrossRef]
- Ortiz, P.A.; Ulloque, R.; Kihara, G.K.; Zheng, H.; Kinzy, T.G. Translation elongation factor 2 anticodon mimicry domain mutants affect fidelity and diphtheria toxin resistance. J. Biol. Chem. 2006, 281, 32639–32648. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.; Mundigl, O.; Kettenberger, H.; Birzele, F.; Stahl, S.; Pastan, I.; Brinkmann, U. Diphthamide affects selenoprotein expression: Diphthamide deficiency reduces selenocysteine incorporation, decreases selenite sensitivity and pre-disposes to oxidative stress. Redox Biol. 2019, 20, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Tsuda-Sakurai, K.; Miura, M. The hidden nature of protein translational control by diphthamide: The secrets under the leather. J. Biochem. 2019, 165, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dever, T.E.; Gutierrez, E.; Shin, B.S. The hypusine-containing translation factor eIF5A. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 413–425. [Google Scholar] [CrossRef]
- Mathews, M.B.; Hershey, J.W. The translation factor eIF5A and human cancer. Biochim. Biophys. Acta 2015, 1849, 836–844. [Google Scholar] [CrossRef]
- Schnier, J.; Schwelberger, H.G.; Smit-McBride, Z.; Kang, H.A.; Hershey, J.W. Translation initiation factor 5A and its hypusine modification are essential for cell viability in the yeast Saccharomyces cerevisiae. Mol. Cell Biol. 1991, 11, 3105–3114. [Google Scholar] [CrossRef]
- Schwelberger, H.G.; Kang, H.A.; Hershey, J.W. Translation initiation factor eIF-5A expressed from either of two yeast genes or from human cDNA. Functional identity under aerobic and anaerobic conditions. J. Biol. Chem. 1993, 268, 14018–14025. [Google Scholar] [CrossRef]
- Park, M.H.; Nishimura, K.; Zanelli, C.F.; Valentini, S.R. Functional significance of eIF5A and its hypusine modification in eukaryotes. Amino Acids 2010, 38, 491–500. [Google Scholar] [CrossRef]
- Kang, H.A.; Hershey, J.W. Effect of initiation factor eIF-5A depletion on protein synthesis and proliferation of Saccharomyces cerevisiae. J. Biol. Chem. 1994, 269, 3934–3940. [Google Scholar] [CrossRef]
- Gregio, A.P.; Cano, V.P.; Avaca, J.S.; Valentini, S.R.; Zanelli, C.F. eIF5A has a function in the elongation step of translation in yeast. Biochem. Biophys. Res. Commun. 2009, 380, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Saini, P.; Eyler, D.E.; Green, R.; Dever, T.E. Hypusine-containing protein eIF5A promotes translation elongation. Nature 2009, 459, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Doerfel, L.K.; Wohlgemuth, I.; Kothe, C.; Peske, F.; Urlaub, H.; Rodnina, M.V. EF-P is essential for rapid synthesis of proteins containing consecutive proline residues. Science 2013, 339, 85–88. [Google Scholar] [CrossRef]
- Ude, S.; Lassak, J.; Starosta, A.L.; Kraxenberger, T.; Wilson, D.N.; Jung, K. Translation elongation factor EF-P alleviates ribosome stalling at polyproline stretches. Science 2013, 339, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Peil, L.; Starosta, A.L.; Lassak, J.; Atkinson, G.C.; Virumae, K.; Spitzer, M.; Tenson, T.; Jung, K.; Remme, J.; Wilson, D.N. Distinct XPPX sequence motifs induce ribosome stalling, which is rescued by the translation elongation factor EF-P. Proc. Natl. Acad. Sci. USA 2013, 110, 15265–15270. [Google Scholar] [CrossRef]
- Gutierrez, E.; Shin, B.; Woolstenhulme, C.; Kim, J.; Saini, P.; Buskirk, A.; Dever, T. eIF5A Promotes Translation of Polyproline Motifs. Mol. Cell 2013, 51, 35–45. [Google Scholar] [CrossRef]
- Li, T.; Belda-Palazon, B.; Ferrando, A.; Alepuz, P. Fertility and polarized cell growth depends on eIF5A for translation of polyproline-rich formins in Saccharomyces cerevisiae. Genetics 2014, 197, 1191–1200. [Google Scholar] [CrossRef]
- Dias, C.A.; Garcia, W.; Zanelli, C.F.; Valentini, S.R. eIF5A dimerizes not only in vitro but also in vivo and its molecular envelope is similar to the EF-P monomer. Amino Acids 2013, 44, 631–644. [Google Scholar] [CrossRef]
- Melnikov, S.; Mailliot, J.; Shin, B.S.; Rigger, L.; Yusupova, G.; Micura, R.; Dever, T.E.; Yusupov, M. Crystal Structure of Hypusine-Containing Translation Factor eIF5A Bound to a Rotated Eukaryotic Ribosome. J. Mol. Biol. 2016, 428, 3570–3576. [Google Scholar] [CrossRef]
- Schmidt, C.; Becker, T.; Heuer, A.; Braunger, K.; Shanmuganathan, V.; Pech, M.; Berninghausen, O.; Wilson, D.N.; Beckmann, R. Structure of the hypusinylated eukaryotic translation factor eIF-5A bound to the ribosome. Nucleic Acids Res. 2016, 44, 1944–1951. [Google Scholar] [CrossRef]
- Pelechano, V.; Alepuz, P. eIF5A facilitates translation termination globally and promotes the elongation of many non polyproline-specific tripeptide sequences. Nucleic Acids Res. 2017, 45, 7326–7338. [Google Scholar] [CrossRef] [PubMed]
- Schuller, A.P.; Wu, C.C.; Dever, T.E.; Buskirk, A.R.; Green, R. eIF5A Functions Globally in Translation Elongation and Termination. Mol. Cell 2017, 66, 194–205.e5. [Google Scholar] [CrossRef] [PubMed]
- Manjunath, H.; Zhang, H.; Rehfeld, F.; Han, J.; Chang, T.C.; Mendell, J.T. Suppression of Ribosomal Pausing by eIF5A Is Necessary to Maintain the Fidelity of Start Codon Selection. Cell Rep. 2019, 29, 3134–3146.e6. [Google Scholar] [CrossRef]
- Bassani, F.; Zink, I.A.; Pribasnig, T.; Wolfinger, M.T.; Romagnoli, A.; Resch, A.; Schleper, C.; Blasi, U.; La Teana, A. Indications for a moonlighting function of translation factor aIF5A in the crenarchaeum Sulfolobus solfataricus. RNA Biol. 2019, 16, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Rosorius, O.; Reichart, B.; Kratzer, F.; Heger, P.; Dabauvalle, M.C.; Hauber, J. Nuclear pore localization and nucleocytoplasmic transport of eIF-5A: Evidence for direct interaction with the export receptor CRM1. J. Cell Sci. 1999, 112, 2369–2380. [Google Scholar] [CrossRef]
- Schrader, R.; Young, C.; Kozian, D.; Hoffmann, R.; Lottspeich, F. Temperature-sensitive eIF5A mutant accumulates transcripts targeted to the nonsense-mediated decay pathway. J. Biol. Chem. 2006, 281, 35336–35346. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.; Park, J.Y.; Chang, Y.J.; Luchessi, A.D.; Cambiaghi, T.D.; Shamanna, R.; Hanauske-Abel, H.M.; Holland, B.; Pe’ery, T.; Tian, B.; et al. Regulation of gene expression by translation factor eIF5A: Hypusine-modified eIF5A enhances nonsense-mediated mRNA decay in human cells. Translation 2017, 5, e1366294. [Google Scholar] [CrossRef]
- Quintas-Granados, L.I.; Carvajal Gamez, B.I.; Villalpando, J.L.; Ortega-Lopez, J.; Arroyo, R.; Azuara-Liceaga, E.; Alvarez-Sanchez, M.E. Bifunctional activity of deoxyhypusine synthase/hydroxylase from Trichomonas vaginalis. Biochimie 2016, 123, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Bassani, F.; Romagnoli, A.; Cacciamani, T.; Amici, A.; Benelli, D.; Londei, P.; Martens, B.; Blasi, U.; La Teana, A. Modification of translation factor aIF5A from Sulfolobus solfataricus. Extremophiles 2018, 22, 769–780. [Google Scholar] [CrossRef]
- Park, J.H.; Wolff, E.C.; Folk, J.E.; Park, M.H. Reversal of the deoxyhypusine synthesis reaction. Generation of spermidine or homospermidine from deoxyhypusine by deoxyhypusine synthase. J. Biol. Chem. 2003, 278, 32683–32691. [Google Scholar] [CrossRef]
- Lassak, J.; Wilson, D.N.; Jung, K. Stall no more at polyproline stretches with the translation elongation factors EF-P and IF-5A. Mol. Microbiol. 2016, 99, 219–235. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Wolff, E.C.; Smit-McBride, Z.; Hershey, J.W.; Folk, J.E. Comparison of the activities of variant forms of eIF-4D. The requirement for hypusine or deoxyhypusine. J. Biol. Chem. 1991, 266, 7988–7994. [Google Scholar] [CrossRef]
- Park, J.H.; Dias, C.A.; Lee, S.B.; Valentini, S.R.; Sokabe, M.; Fraser, C.S.; Park, M.H. Production of active recombinant eIF5A: Reconstitution in E. coli of eukaryotic hypusine modification of eIF5A by its coexpression with modifying enzymes. Protein Eng. Des. Sel. 2011, 24, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Barba-Aliaga, M.; Villarroel-Vicente, C.; Stanciu, A.; Corman, A.; Martinez-Pastor, M.T.; Alepuz, P. Yeast Translation Elongation Factor eIF5A Expression Is Regulated by Nutrient Availability through Different Signalling Pathways. Int. J. Mol. Sci. 2020, 22, 219. [Google Scholar] [CrossRef] [PubMed]
- Lowry, C.V.; Zitomer, R.S. Oxygen regulation of anaerobic and aerobic genes mediated by a common factor in yeast. Proc. Natl. Acad. Sci. USA 1984, 81, 6129–6133. [Google Scholar] [CrossRef]
- Lowry, C.V.; Lieber, R.H. Negative regulation of the Saccharomyces cerevisiae ANB1 gene by heme, as mediated by the ROX1 gene product. Mol. Cell Biol. 1986, 6, 4145–4148. [Google Scholar] [CrossRef]
- Bisschops, M.M.; Vos, T.; Martinez-Moreno, R.; Cortes, P.T.; Pronk, J.T.; Daran-Lapujade, P. Oxygen availability strongly affects chronological lifespan and thermotolerance in batch cultures of Saccharomyces cerevisiae. Microb. Cell 2015, 2, 429–444. [Google Scholar] [CrossRef]
- Devaux, F.; Thiebaut, A. The regulation of iron homeostasis in the fungal human pathogen Candida glabrata. Microbiology 2019, 165, 1041–1060. [Google Scholar] [CrossRef]
- Denecker, T.; Zhou Li, Y.; Fairhead, C.; Budin, K.; Camadro, J.M.; Bolotin-Fukuhara, M.; Angoulvant, A.; Lelandais, G. Functional networks of co-expressed genes to explore iron homeostasis processes in the pathogenic yeast Candida glabrata. NAR Genom. Bioinform. 2020, 2. [Google Scholar] [CrossRef]
- Vasudevan, S.; Peltz, S.W. Regulated ARE-mediated mRNA decay in Saccharomyces cerevisiae. Mol. Cell 2001, 7, 1191–1200. [Google Scholar] [CrossRef]
- Clement, P.M.; Hanauske-Abel, H.M.; Wolff, E.C.; Kleinman, H.K.; Park, M.H. The antifungal drug ciclopirox inhibits deoxyhypusine and proline hydroxylation, endothelial cell growth and angiogenesis in vitro. Int. J. Cancer 2002, 100, 491–498. [Google Scholar] [CrossRef]
- Eberhard, Y.; McDermott, S.P.; Wang, X.; Gronda, M.; Venugopal, A.; Wood, T.E.; Hurren, R.; Datti, A.; Batey, R.A.; Wrana, J.; et al. Chelation of intracellular iron with the antifungal agent ciclopirox olamine induces cell death in leukemia and myeloma cells. Blood 2009, 114, 3064–3073. [Google Scholar] [CrossRef]
- Luo, Y.; Zhou, H.; Liu, L.; Shen, T.; Chen, W.; Xu, B.; Han, X.; Zhang, F.; Scott, R.S.; Alexander, J.S.; et al. The fungicide ciclopirox inhibits lymphatic endothelial cell tube formation by suppressing VEGFR-3-mediated ERK signaling pathway. Oncogene 2011, 30, 2098–2107. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Shen, T.; Luo, Y.; Liu, L.; Chen, W.; Xu, B.; Han, X.; Pang, J.; Rivera, C.A.; Huang, S. The antitumor activity of the fungicide ciclopirox. Int. J. Cancer 2010, 127, 2467–2477. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Schmidt, M.; Endo, T.; Lu, D.; Carson, D.; Schmidt-Wolf, I.G. Targeting the Wnt/beta-catenin pathway with the antifungal agent ciclopirox olamine in a murine myeloma model. In Vivo 2011, 25, 887–893. [Google Scholar] [PubMed]
- Melis, N.; Rubera, I.; Cougnon, M.; Giraud, S.; Mograbi, B.; Belaid, A.; Pisani, D.F.; Huber, S.M.; Lacas-Gervais, S.; Fragaki, K.; et al. Targeting eIF5A Hypusination Prevents Anoxic Cell Death through Mitochondrial Silencing and Improves Kidney Transplant Outcome. J. Am. Soc. Nephrol. 2017, 28, 811–822. [Google Scholar] [CrossRef]
- Giraud, S.; Kerforne, T.; Zely, J.; Ameteau, V.; Couturier, P.; Tauc, M.; Hauet, T. The inhibition of eIF5A hypusination by GC7, a preconditioning protocol to prevent brain death-induced renal injuries in a preclinical porcine kidney transplantation model. Am. J. Transpl. 2020, 20, 3326–3340. [Google Scholar] [CrossRef]
- Bourourou, M.; Gouix, E.; Melis, N.; Friard, J.; Heurteaux, C.; Tauc, M.; Blondeau, N. Inhibition of eIF5A hypusination pathway as a new pharmacological target for stroke therapy. J. Cereb. Blood Flow Metab. 2021, 41, 1080–1090. [Google Scholar] [CrossRef] [PubMed]
- Bisbal, C.; Martinand, C.; Silhol, M.; Lebleu, B.; Salehzada, T. Cloning and characterization of a RNAse L inhibitor. A new component of the interferon-regulated 2-5A pathway. J. Biol. Chem. 1995, 270, 13308–13317. [Google Scholar] [CrossRef] [PubMed]
- Dean, M.; Annilo, T. Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Annu. Rev. Genomics Hum. Genet. 2005, 6, 123–142. [Google Scholar] [CrossRef]
- Barthelme, D.; Scheele, U.; Dinkelaker, S.; Janoschka, A.; Macmillan, F.; Albers, S.V.; Driessen, A.J.; Stagni, M.S.; Bill, E.; Meyer-Klaucke, W.; et al. Structural organization of essential iron-sulfur clusters in the evolutionarily highly conserved ATP-binding cassette protein ABCE1. J. Biol. Chem. 2007, 282, 14598–14607. [Google Scholar] [CrossRef]
- Barthelme, D.; Dinkelaker, S.; Albers, S.V.; Londei, P.; Ermler, U.; Tampe, R. Ribosome recycling depends on a mechanistic link between the FeS cluster domain and a conformational switch of the twin-ATPase ABCE1. Proc. Natl. Acad. Sci. USA 2011, 108, 3228–3233. [Google Scholar] [CrossRef]
- Becker, T.; Franckenberg, S.; Wickles, S.; Shoemaker, C.J.; Anger, A.M.; Armache, J.P.; Sieber, H.; Ungewickell, C.; Berninghausen, O.; Daberkow, I.; et al. Structural basis of highly conserved ribosome recycling in eukaryotes and archaea. Nature 2012, 482, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Franckenberg, S.; Becker, T.; Beckmann, R. Structural view on recycling of archaeal and eukaryotic ribosomes after canonical termination and ribosome rescue. Curr. Opin. Struct. Biol. 2012, 22, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Estevez, A.M.; Haile, S.; Steihel, M.; Quijada, L.; Clayton, C. Effects of depletion and overexpression of the Trypanosoma brucei ribonuclease L inhibitor homologue. Mol. Biochem. Parasitol. 2004, 133, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Fang, L.L.; Johnsen, R.; Baillie, D.L. ATP-binding cassette protein E is involved in gene transcription and translation in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 2004, 323, 104–111. [Google Scholar] [CrossRef]
- Zhao, Z.; Sheps, J.A.; Ling, V.; Fang, L.L.; Baillie, D.L. Expression analysis of ABC transporters reveals differential functions of tandemly duplicated genes in Caenorhabditis elegans. J. Mol. Biol. 2004, 344, 409–417. [Google Scholar] [CrossRef]
- Andersen, D.S.; Leevers, S.J. The essential Drosophila ATP-binding cassette domain protein, pixie, binds the 40 S ribosome in an ATP-dependent manner and is required for translation initiation. J. Biol. Chem. 2007, 282, 14752–14760. [Google Scholar] [CrossRef]
- Khoshnevis, S.; Gross, T.; Rotte, C.; Baierlein, C.; Ficner, R.; Krebber, H. The iron-sulphur protein RNase L inhibitor functions in translation termination. EMBO Rep. 2010, 11, 214–219. [Google Scholar] [CrossRef]
- Pisarev, A.V.; Skabkin, M.A.; Pisareva, V.P.; Skabkina, O.V.; Rakotondrafara, A.M.; Hentze, M.W.; Hellen, C.U.; Pestova, T.V. The role of ABCE1 in eukaryotic posttermination ribosomal recycling. Mol. Cell 2010, 37, 196–210. [Google Scholar] [CrossRef]
- Shoemaker, C.J.; Green, R. Kinetic analysis reveals the ordered coupling of translation termination and ribosome recycling in yeast. Proc. Natl. Acad. Sci. USA 2011, 108, 1392. [Google Scholar] [CrossRef] [PubMed]
- Pisareva, V.P.; Skabkin, M.A.; Hellen, C.U.; Pestova, T.V.; Pisarev, A.V. Dissociation by Pelota, Hbs1 and ABCE1 of mammalian vacant 80S ribosomes and stalled elongation complexes. EMBO J. 2011, 30, 1804–1817. [Google Scholar] [CrossRef]
- Gerovac, M.; Tampe, R. Control of mRNA Translation by Versatile ATP-Driven Machines. Trends Biochem. Sci. 2019, 44, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Buskirk, A.R.; Green, R. Ribosome pausing, arrest and rescue in bacteria and eukaryotes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372. [Google Scholar] [CrossRef] [PubMed]
- Young, D.J.; Guydosh, N.R.; Zhang, F.; Hinnebusch, A.G.; Green, R. Rli1/ABCE1 Recycles Terminating Ribosomes and Controls Translation Reinitiation in 3’UTRs In Vivo. Cell 2015, 162, 872–884. [Google Scholar] [CrossRef]
- Guydosh, N.R.; Green, R. Dom34 rescues ribosomes in 3′ untranslated regions. Cell 2014, 156, 950–962. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, H.; Mendell, J.T. Ribosome Recycling by ABCE1 Links Lysosomal Function and Iron Homeostasis to 3′UTR-Directed Regulation and Nonsense-Mediated Decay. Cell Rep. 2020, 32, 107895. [Google Scholar] [CrossRef]
- Kiosze-Becker, K.; Ori, A.; Gerovac, M.; Heuer, A.; Nurenberg-Goloub, E.; Rashid, U.J.; Becker, T.; Beckmann, R.; Beck, M.; Tampe, R. Structure of the ribosome post-recycling complex probed by chemical cross-linking and mass spectrometry. Nat. Commun. 2016, 7, 13248. [Google Scholar] [CrossRef]
- Heuer, A.; Gerovac, M.; Schmidt, C.; Trowitzsch, S.; Preis, A.; Kotter, P.; Berninghausen, O.; Becker, T.; Beckmann, R.; Tampe, R. Structure of the 40S-ABCE1 post-splitting complex in ribosome recycling and translation initiation. Nat. Struct. Mol. Biol. 2017, 24, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Hellen, C.U.T. Translation Termination and Ribosome Recycling in Eukaryotes. Cold Spring Harb. Perspect. Biol. 2018, 10. [Google Scholar] [CrossRef]
- Nurenberg-Goloub, E.; Heinemann, H.; Gerovac, M.; Tampe, R. Ribosome recycling is coordinated by processive events in two asymmetric ATP sites of ABCE1. Life Sci. Alliance 2018, 1. [Google Scholar] [CrossRef] [PubMed]
- Nurenberg-Goloub, E.; Kratzat, H.; Heinemann, H.; Heuer, A.; Kotter, P.; Berninghausen, O.; Becker, T.; Tampe, R.; Beckmann, R. Molecular analysis of the ribosome recycling factor ABCE1 bound to the 30S post-splitting complex. EMBO J. 2020, 39, e103788. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, A.; Brito Querido, J.; Myasnikov, A.G.; Mancera-Martinez, E.; Renaud, A.; Kuhn, L.; Hashem, Y. eIF3 Peripheral Subunits Rearrangement after mRNA Binding and Start-Codon Recognition. Mol. Cell 2016, 63, 206–217. [Google Scholar] [CrossRef]
- Pisarev, A.V.; Hellen, C.U.; Pestova, T.V. Recycling of eukaryotic posttermination ribosomal complexes. Cell 2007, 131, 286–299. [Google Scholar] [CrossRef] [PubMed]
- Beznoskova, P.; Cuchalova, L.; Wagner, S.; Shoemaker, C.J.; Gunisova, S.; von der Haar, T.; Valasek, L.S. Translation initiation factors eIF3 and HCR1 control translation termination and stop codon read-through in yeast cells. PLoS Genet. 2013, 9, e1003962. [Google Scholar] [CrossRef]
- Strunk, B.S.; Novak, M.N.; Young, C.L.; Karbstein, K. A translation-like cycle is a quality control checkpoint for maturing 40S ribosome subunits. Cell 2012, 150, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Alhebshi, A.; Sideri, T.C.; Holland, S.L.; Avery, S.V. The essential iron-sulfur protein Rli1 is an important target accounting for inhibition of cell growth by reactive oxygen species. Mol. Biol. Cell 2012, 23, 3582–3590. [Google Scholar] [CrossRef]
- Benchouaia, M.; Ripoche, H.; Sissoko, M.; Thiebaut, A.; Merhej, J.; Delaveau, T.; Fasseu, L.; Benaissa, S.; Lorieux, G.; Jourdren, L.; et al. Comparative Transcriptomics Highlights New Features of the Iron Starvation Response in the Human Pathogen Candida glabrata. Front. Microbiol. 2018, 9, 2689. [Google Scholar] [CrossRef]
- Mills, E.W.; Wangen, J.; Green, R.; Ingolia, N.T. Dynamic Regulation of a Ribosome Rescue Pathway in Erythroid Cells and Platelets. Cell Rep. 2016, 17, 1–10. [Google Scholar] [CrossRef]
- Guengerich, F.P. Introduction: Metals in Biology: Alpha-Ketoglutarate/Iron-Dependent Dioxygenases. J. Biol. Chem. 2015, 290, 20700–20701. [Google Scholar] [CrossRef]
- Salminen, A.; Kauppinen, A.; Kaarniranta, K. 2-Oxoglutarate-dependent dioxygenases are sensors of energy metabolism, oxygen availability, and iron homeostasis: Potential role in the regulation of aging process. Cell Mol. Life Sci. 2015, 72, 3897–3914. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.J.; Acevedo, J.M.; Loenarz, C.; Galagovsky, D.; Liu-Yi, P.; Perez-Pepe, M.; Thalhammer, A.; Sekirnik, R.; Ge, W.; Melani, M.; et al. Sudestada1, a Drosophila ribosomal prolyl-hydroxylase required for mRNA translation, cell homeostasis, and organ growth. Proc. Natl. Acad. Sci. USA 2014, 111, 4025–4030. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, H.L.; Kim, K.H.; Kim, D.J.; Lee, S.J.; Yoon, J.Y.; Yoon, H.J.; Lee, H.Y.; Park, S.B.; Kim, S.J.; et al. Crystal structure of Tpa1 from Saccharomyces cerevisiae, a component of the messenger ribonucleoprotein complex. Nucleic Acids Res. 2010, 38, 2099–2110. [Google Scholar] [CrossRef] [PubMed]
- Henri, J.; Rispal, D.; Bayart, E.; van Tilbeurgh, H.; Seraphin, B.; Graille, M. Structural and functional insights into Saccharomyces cerevisiae Tpa1, a putative prolylhydroxylase influencing translation termination and transcription. J. Biol. Chem. 2010, 285, 30767–30778. [Google Scholar] [CrossRef] [PubMed]
- Horita, S.; Scotti, J.S.; Thinnes, C.; Mottaghi-Taromsari, Y.S.; Thalhammer, A.; Ge, W.; Aik, W.; Loenarz, C.; Schofield, C.J.; McDonough, M.A. Structure of the ribosomal oxygenase OGFOD1 provides insights into the regio- and stereoselectivity of prolyl hydroxylases. Structure 2015, 23, 639–652. [Google Scholar] [CrossRef]
- Richter, U.; Evans, M.E.; Clark, W.C.; Marttinen, P.; Shoubridge, E.A.; Suomalainen, A.; Wredenberg, A.; Wedell, A.; Pan, T.; Battersby, B.J. RNA modification landscape of the human mitochondrial tRNA(Lys) regulates protein synthesis. Nat. Commun. 2018, 9, 3966. [Google Scholar] [CrossRef]
- Delaunay, S.; Frye, M. RNA modifications regulating cell fate in cancer. Nat. Cell Biol. 2019, 21, 552–559. [Google Scholar] [CrossRef]
- Waas, W.F.; de Crecy-Lagard, V.; Schimmel, P. Discovery of a gene family critical to wyosine base formation in a subset of phenylalanine-specific transfer RNAs. J. Biol. Chem. 2005, 280, 37616–37622. [Google Scholar] [CrossRef] [PubMed]
- Perche-Letuvee, P.; Molle, T.; Forouhar, F.; Mulliez, E.; Atta, M. Wybutosine biosynthesis: Structural and mechanistic overview. RNA Biol. 2014, 11, 1508–1518. [Google Scholar] [CrossRef]
- Suzuki, Y.; Noma, A.; Suzuki, T.; Senda, M.; Senda, T.; Ishitani, R.; Nureki, O. Crystal structure of the radical SAM enzyme catalyzing tricyclic modified base formation in tRNA. J. Mol. Biol. 2007, 372, 1204–1214. [Google Scholar] [CrossRef]
- Goto-Ito, S.; Ishii, R.; Ito, T.; Shibata, R.; Fusatomi, E.; Sekine, S.I.; Bessho, Y.; Yokoyama, S. Structure of an archaeal TYW1, the enzyme catalyzing the second step of wye-base biosynthesis. Acta Crystallogr. D Biol. Crystallogr. 2007, 63, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Perche-Letuvee, P.; Kathirvelu, V.; Berggren, G.; Clemancey, M.; Latour, J.M.; Maurel, V.; Douki, T.; Armengaud, J.; Mulliez, E.; Fontecave, M.; et al. 4-Demethylwyosine synthase from Pyrococcus abyssi is a radical-S-adenosyl-L-methionine enzyme with an additional [4Fe-4S](+2) cluster that interacts with the pyruvate co-substrate. J. Biol. Chem. 2012, 287, 41174–41185. [Google Scholar] [CrossRef]
- Grell, T.A.J.; Young, A.P.; Drennan, C.L.; Bandarian, V. Biochemical and Structural Characterization of a Schiff Base in the Radical-Mediated Biosynthesis of 4-Demethylwyosine by TYW1. J. Am. Chem. Soc. 2018, 140, 6842–6852. [Google Scholar] [CrossRef]
- Otero, G.; Fellows, J.; Li, Y.; de Bizemont, T.; Dirac, A.M.; Gustafsson, C.M.; Erdjument-Bromage, H.; Tempst, P.; Svejstrup, J.Q. Elongator, a multisubunit component of a novel RNA polymerase II holoenzyme for transcriptional elongation. Mol. Cell 1999, 3, 109–118. [Google Scholar] [CrossRef]
- Wittschieben, B.O.; Otero, G.; de Bizemont, T.; Fellows, J.; Erdjument-Bromage, H.; Ohba, R.; Li, Y.; Allis, C.D.; Tempst, P.; Svejstrup, J.Q. A novel histone acetyltransferase is an integral subunit of elongating RNA polymerase II holoenzyme. Mol. Cell 1999, 4, 123–128. [Google Scholar] [CrossRef]
- Winkler, G.S.; Kristjuhan, A.; Erdjument-Bromage, H.; Tempst, P.; Svejstrup, J.Q. Elongator is a histone H3 and H4 acetyltransferase important for normal histone acetylation levels in vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 3517–3522. [Google Scholar] [CrossRef]
- Paraskevopoulou, C.; Fairhurst, S.A.; Lowe, D.J.; Brick, P.; Onesti, S. The Elongator subunit Elp3 contains a Fe4S4 cluster and binds S-adenosylmethionine. Mol. Microbiol. 2006, 59, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Glatt, S.; Zabel, R.; Kolaj-Robin, O.; Onuma, O.F.; Baudin, F.; Graziadei, A.; Taverniti, V.; Lin, T.Y.; Baymann, F.; Seraphin, B.; et al. Structural basis for tRNA modification by Elp3 from Dehalococcoides mccartyi. Nat. Struct. Mol. Biol. 2016, 23, 794–802. [Google Scholar] [CrossRef]
- Esberg, A.; Huang, B.; Johansson, M.J.; Bystrom, A.S. Elevated levels of two tRNA species bypass the requirement for elongator complex in transcription and exocytosis. Mol. Cell 2006, 24, 139–148. [Google Scholar] [CrossRef]
- Krogan, N.J.; Greenblatt, J.F. Characterization of a six-subunit holo-elongator complex required for the regulated expression of a group of genes in Saccharomyces cerevisiae. Mol. Cell Biol. 2001, 21, 8203–8212. [Google Scholar] [CrossRef] [PubMed]
- Glatt, S.; Muller, C.W. Structural insights into Elongator function. Curr. Opin. Struct. Biol. 2013, 23, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Setiaputra, D.T.; Cheng, D.T.; Lu, S.; Hansen, J.M.; Dalwadi, U.; Lam, C.H.; To, J.L.; Dong, M.Q.; Yip, C.K. Molecular architecture of the yeast Elongator complex reveals an unexpected asymmetric subunit arrangement. EMBO Rep. 2017, 18, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Dauden, M.I.; Kosinski, J.; Kolaj-Robin, O.; Desfosses, A.; Ori, A.; Faux, C.; Hoffmann, N.A.; Onuma, O.F.; Breunig, K.D.; Beck, M.; et al. Architecture of the yeast Elongator complex. EMBO Rep. 2017, 18, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Abbassi, N.E.H.; Zakrzewski, K.; Chramiec-Glabik, A.; Jemiola-Rzeminska, M.; Rozycki, J.; Glatt, S. The Elongator subunit Elp3 is a non-canonical tRNA acetyltransferase. Nat. Commun. 2019, 10, 625–2. [Google Scholar] [CrossRef] [PubMed]
- Selvadurai, K.; Wang, P.; Seimetz, J.; Huang, R.H. Archaeal Elp3 catalyzes tRNA wobble uridine modification at C5 via a radical mechanism. Nat. Chem. Biol. 2014, 10, 810–812. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, C.; Selth, L.A.; Dirac-Svejstrup, A.B.; Svejstrup, J.Q. An iron-sulfur cluster domain in Elp3 important for the structural integrity of elongator. J. Biol. Chem. 2009, 284, 141–149. [Google Scholar] [CrossRef]
- Tigano, M.; Ruotolo, R.; Dallabona, C.; Fontanesi, F.; Barrientos, A.; Donnini, C.; Ottonello, S. Elongator-dependent modification of cytoplasmic tRNALysUUU is required for mitochondrial function under stress conditions. Nucleic Acids Res. 2015, 43, 8368–8380. [Google Scholar] [CrossRef]
- Fernandez-Vazquez, J.; Vargas-Perez, I.; Sanso, M.; Buhne, K.; Carmona, M.; Paulo, E.; Hermand, D.; Rodriguez-Gabriel, M.; Ayte, J.; Leidel, S.; et al. Modification of tRNA(Lys) UUU by elongator is essential for efficient translation of stress mRNAs. PLoS Genet. 2013, 9, e1003647. [Google Scholar] [CrossRef]
- Bauer, F.; Matsuyama, A.; Candiracci, J.; Dieu, M.; Scheliga, J.; Wolf, D.A.; Yoshida, M.; Hermand, D. Translational control of cell division by Elongator. Cell Rep. 2012, 1, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.L.; Lemmens, R.; Miskiewicz, K.; Broom, W.J.; Hansen, V.K.; van Vught, P.W.; Landers, J.E.; Sapp, P.; Van Den Bosch, L.; Knight, J.; et al. Variants of the elongator protein 3 (ELP3) gene are associated with motor neuron degeneration. Hum. Mol. Genet. 2009, 18, 472–481. [Google Scholar] [CrossRef]
- Ladang, A.; Rapino, F.; Heukamp, L.C.; Tharun, L.; Shostak, K.; Hermand, D.; Delaunay, S.; Klevernic, I.; Jiang, Z.; Jacques, N.; et al. Elp3 drives Wnt-dependent tumor initiation and regeneration in the intestine. J. Exp. Med. 2015, 212, 2057–2075. [Google Scholar] [CrossRef] [PubMed]
| Yeast | Human | Function | References |
|---|---|---|---|
| Rli1 | ABCE1 | Ribosome biogenesis and recycling/ Translation initiation and termination | [6,14] |
| Dph1 | DPH1 | Diphthamide biosynthesis | [15] |
| Dph2 | DPH2L1/OVCA1 | Diphthamide biosynthesis | [15,16] |
| Dph3/Kti11 | DPH3 | Diphthamide biosynthesis/ Subunit of Elongator complex | [15,17] |
| Dph4 | DPH4 | Diphthamide biosynthesis | [15,17,18] |
| Lia1 | DOHH | Deoxyhypusine hydroxylase | [19,20,21] |
| Tyw1 | TYW1A/RSAFD1 | Synthesis of wybutosine in tRNAPhe/Translation accuracy/Protection against high iron conditions | [22,23] |
| Tpa1 | OGFOD1 | Prolyl hydroxylation of Rps23/Translation termination/DNA alkylation repair | [24,25,26,27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero, A.M.; Martínez-Pastor, M.T.; Puig, S. Iron in Translation: From the Beginning to the End. Microorganisms 2021, 9, 1058. https://doi.org/10.3390/microorganisms9051058
Romero AM, Martínez-Pastor MT, Puig S. Iron in Translation: From the Beginning to the End. Microorganisms. 2021; 9(5):1058. https://doi.org/10.3390/microorganisms9051058
Chicago/Turabian StyleRomero, Antonia María, María Teresa Martínez-Pastor, and Sergi Puig. 2021. "Iron in Translation: From the Beginning to the End" Microorganisms 9, no. 5: 1058. https://doi.org/10.3390/microorganisms9051058
APA StyleRomero, A. M., Martínez-Pastor, M. T., & Puig, S. (2021). Iron in Translation: From the Beginning to the End. Microorganisms, 9(5), 1058. https://doi.org/10.3390/microorganisms9051058







