Diversity of Plasmids and Genes Encoding Resistance to Extended-Spectrum β-Lactamase in Escherichia coli from Different Animal Sources
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains in Study and Isolation Method
2.2. Typing of E. coli Using BOX AIR-1 PCR
2.3. Phylogroup Identification
2.4. Susceptibilities of Isolates against 18 Antibiotics
2.5. Identification of ESBL E. coli
2.6. Whole Genome Sequencing and Genome Assembly and Analysis
3. Results and Discussion
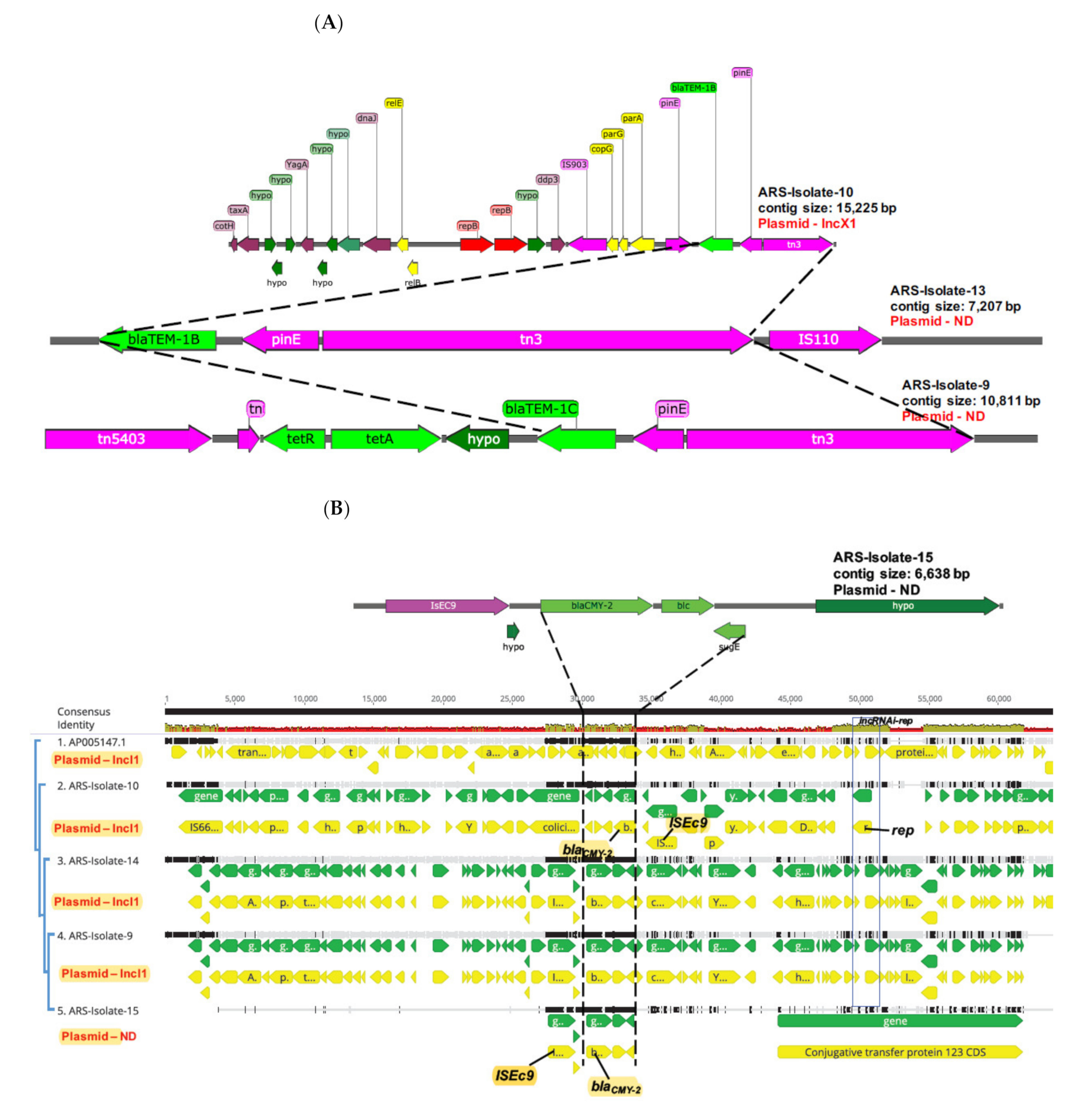
3.1. Whole Genome Sequencing of ESBL Isolates
3.2. Plasmids Carrying β-Lactam Resistance
3.3. Phylogenetic Analysis
3.4. Relative Abundances of ARGs and Virulence Factor (VFs) in ESBL E. coli
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Stephan, R.; Power, K.; Yan, Q.; Hächler, H.; Fanning, S. Nucleotide sequences of 16 transmissible plasmids identified in nine multidrug-resistant Escherichia coli isolates expressing an ESBL phenotype isolated from food-producing animals and healthy humans. J. Antimicrob. Chemother. 2014, 69, 2658–2668. [Google Scholar] [CrossRef]
- Coque, T.M.; Baquero, F.; Cantón, R. Increasing prevalence of ESBL-producing Enterobacteriaceae in Europe. Euro Surveill. 2008, 13, 19044. [Google Scholar] [CrossRef] [PubMed]
- Hawkey, P.M.; Jones, A.M. The changing epidemiology of resistance. J. Antimicrob. Chemother. 2009, 64, i3–i10. [Google Scholar] [CrossRef]
- Pitout, J.D.; Laupland, K.B. Extended-spectrum b-lactamase-producing Enterobacteriaceae: An emerging public-health concern. Lancet Infect. Dis. 2008, 8, 159–166. [Google Scholar] [CrossRef]
- Durso, L.M.; Miller, D.N.; Wienhold, B.J. Distribution and quantification of antibiotic resistant genes and bacteria across agricultural and non-agricultural metagenomes. PLoS ONE 2012, 7, e48325. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef]
- Lima, T.; Domingues, S.; Da Silva, G.J. Manure as a potential hotspot for antibiotic resistance dissemination by horizontal gene transfer events. Vet. Sci. 2020, 7, 110. [Google Scholar] [CrossRef]
- Abraham, S.; Chapman, T.A.; Zhang, R.; Chin, J.; Mabbett, A.N.; Totsika, M.; Schembri, M.A. Molecular characterization of Escherichia coli strains that cause symptomatic and asymptomatic urinary tract infections. J. Clin. Microbiol. 2012, 50, 1027–1030. [Google Scholar] [CrossRef][Green Version]
- Brinas, L.; Moreno, M.A.; Zarazaga, M.; Porrero, C.; Saenz, Y.; Garcia, M.; Dominguez, L.; Torres, C. Detection of CMY-2, CTX-M-14, and SHV-12 beta-lactamases in Escherichia coli fecal-sample isolates from healthy chickens. Antimicrob. Agents Chemother. 2003, 47, 2056–2058. [Google Scholar] [CrossRef]
- Haftu, R.; Taddele, H.; Gugsa, G.; Kalayou, S. Prevalence, bacterial causes, and antimicrobial susceptibility profile of mastitis isolates from cows in large-scale dairy farms of Northern Ethiopia. Trop. Anim. Health Prod. 2012, 44, 1765–1771. [Google Scholar] [CrossRef]
- Dahms, C.; Hubner, N.O.; Kossow, A.; Mellmann, A.; Dittmann, K.; Kramer, A. Occurrence of ESBL-producing Escherichia coli in livestock and farm workers in Mecklenburg-Western Pomerania, Germany. PLoS ONE 2015, 10, e0143326. [Google Scholar] [CrossRef] [PubMed]
- Valentin, L.; Sharp, H.; Hille, K.; Seibt, U.; Fischer, J.; Pfeifer, Y.; Michael, G.B.; Nickel, S.; Schmiedel, J.; Falgenhauer, L.; et al. Subgrouping of ESBL-producing Escherichia coli from animal and human sources: An approach to quantify the distribution of ESBL types between different reservoirs. Int. J. Med. Microbiol. 2014, 304, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Gutkind, G.O.; Di Conza, J.; Power, P.; Radice, M. Beta-lactamase mediated resistance: A biochemical, epidemiological and genetic overview. Curr. Pharm. Des. 2013, 19, 164–208. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Wang, L.; Yang, X.; Wang, J.; Gan, X.; Wang, W.; Xu, J.; Chen, Q.; Lan, R.; Fanning, S.; et al. Prevalence and Molecular Characteristics of Extended-Spectrum-Lactamase Genes in Escherichia coli Isolated from Diarrheic Patients in China. Front. Microbiol. 2017, 8, 144. [Google Scholar] [CrossRef]
- Hamprecht, A.; Rohde, A.M.; Behnke, M.; Feihl, S.; Gastmeier, P.; Gebhardt, F.; Kern, W.V.; Knobloch, J.K.; Mischnik, A.; Obsermann, B.; et al. Colonization with third-generation cephalosporin-resistant Enterobacteriaceae on hospital admission: Prevalence and risk factors. J. Antimicrob. Chemother. 2016, 71, 2957–2963. [Google Scholar] [CrossRef] [PubMed][Green Version]
- EUCAST. Guidelines for Detection of Resistance Mechanisms and Specific Resistances of Clinical and/or Epidemiological Importance. 2013. Available online: https://www.eucast.org/organization/ (accessed on 18 November 2020).
- De Been, M.; Lanza, V.F.; de Toro, M.; Scharringa, J.; Dohmen, W.; Du, Y.; Hu, J.; Lei, Y.; Li, N.; Tooming-Klunderug, A.; et al. Dissemination of cephalosporin resistance genes between Escherichia coli strains from farm animals and humans by specific plasmid lineages. PLoS Genet. 2014, 10, e1004776. [Google Scholar] [CrossRef]
- Tenaillon, O.; Skurnik, D.; Picard, B.; Denamur, E. The population genetics of commensal Escherichia coli. Nat. Rev. Microbiol. 2010, 8, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Savageau, G. Escherichia coli habitats, cell types, and molecular mechanisms of gene control. Am. Nat. 1983, 122, 732–744. [Google Scholar] [CrossRef]
- Seiffert, S.N.; Carattoli, A.; Schwendener, S.; Collaud, A.; Endimiani, A.; Perreten, V. Plasmids carrying blaCMY-2/4 in Escherichia coli from poultry, poultry meat, and humans belong to a novel IncK Subgroup designated IncK2. Front. Microbiol. 2017, 8, 407. [Google Scholar] [CrossRef] [PubMed]
- Den Bakker, H.C.; Allard, M.W.; Bopp, D.; Brown, E.W.; Fontana, J.; Iqbal, Z.; Kinney, A.; Limberger, R.; Musser, K.A.; Shudt, M.; et al. Rapid whole-genome sequencing for surveillance of salmonella enterica serovar enteritidis. Emerg. Infect. Dis. 2014, 20, 1306–1314. [Google Scholar] [CrossRef]
- Koser, C.U.; Holden, M.T.G.; Ellington, M.J.; Cartwright, E.J.P.; Brown, N.M.; Ogilvy-Stuart, A.L.; Hsu, L.Y.; Chewapreecha, C.; Croucher, N.J.; Harris, S.R.; et al. Rapid whole-genome sequencing for investigation of a neonatal MRSA outbreak. N. Eng. J. Med. 2012, 366, 2267–2275. [Google Scholar] [CrossRef] [PubMed]
- Roetzer, A.; Diel, R.; Kohl, T.A.; Ruckert, C.; Nubel, U.; Blom, J.; Wirth, T.; Jaenicke, S.; Schuback, S.; Rusch-Gerdes, S.; et al. Whole genome sequencing versus traditional genotyping for investigation of a Mycobacterium tuberculosis outbreak: A longitudinal molecular epidemiological study. PLoS Med. 2013, 10, e1001387. [Google Scholar] [CrossRef] [PubMed]
- Ducey, T.F.; Durso, L.M.; Ibekwe, A.M.; Dungan, R.S.; Jackson, C.R.; Frye, J.G.; Castleberry, B.L.; Rashash, D.M.C.; Rothrock, M.J.; Boykin, D.; et al. A newly developed Escherichia coli isolate panel from a cross section of U.S. animal production systems reveals geographic and commodity-based differences in antibiotic resistance gene carriage. J. Hazard. Mater. 2020, 382, 120991. [Google Scholar] [CrossRef] [PubMed]
- Ibekwe, A.M.; Murinda, S.E.; Graves, A.K. Genetic diversity and antimicrobial resistance of Escherichia coli from human and animal sources uncovers multiple resistances from human sources. PLoS ONE 2011, 6, e20819. [Google Scholar] [CrossRef] [PubMed]
- Eaton, A.D.; Clesceri, L.S.; Greenberg, A.E.; Franson, M.A.H. Standard Method for Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Dombek, P.E.; Johnson, L.K.; Zimmerley, S.T.; Sadowsky, M.J. Use of repetitive DNA sequences and the PCR to differentiate Escherichia coli isolates from human and animal sources. Appl. Environ. Microbiol. 2000, 66, 2572–2577. [Google Scholar] [CrossRef]
- Goto, K.; Yan, T. Genotypic diversity of Escherichia coli in the water and soil of tropical watersheds in Hawaii. Appl. Environ. Microbiol. 2011, 77, 3988–3997. [Google Scholar] [CrossRef]
- Lyautey, E.; Lu, Z.; Lapen, D.A.; Wilkes, G.; Scott, A.; Berkers, T.; Edge, T.A.; Topp, E. Distribution and diversity of Escherichia coli populations in the South Nation river drainage basin, eastern Ontario, Canada. Appl. Environ. Microbiol. 2010, 76, 1486–1496. [Google Scholar] [CrossRef]
- Rademaker, J.L.W.; de Bruijn, F.J. Characterization and classification of microbes by rep-PCR genomic fingerprinting and computer-assisted pattern analysis. In DNA Markers: Protocols, Applications, and Overviews; Caetano-Anollés, G., Gresshoff, P.M., Eds.; Wiley and Sons: New York, NY, USA, 1997; pp. 151–171. [Google Scholar]
- Stern, M.J.; Ames, G.F.-L.; Smith, N.H.; Robinson, E.C.; Higgins, C.F. Repetitive extragenic palindromic sequences: A major component of the bacterial genome. Cell 1984, 37, 1015–1026. [Google Scholar] [CrossRef]
- Clermont, O.; Christenson, J.K.; Denamur, E.; Gordon, D.M. The Clermont Escherichiacoli phylo-typing method revisited: Improvement of specificity and detection of new phylo-groups. Environ. Microbiol. Rep. 2013, 5, 58–65. [Google Scholar] [CrossRef]
- Clermont, O.; Bonacorsi, S.; Bingen, E. Rapid and simple determination of Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 2000, 66, 4555–4558. [Google Scholar] [CrossRef]
- CLSI M100. Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; Available online: https://clsi.org/about/press-releases/clsi-to-publish-29th-edition-of-annually-updated-ast-supplement-m100/ (accessed on 18 November 2020).
- Korzeniewska, E.; Korzeniewska, A.; Harnisz, M. Antibiotic resistant Escherichia coli in hospital and municipal sewage and their emission to the environment. Ecot. Environ. Saf. 2013, 91, 96–102. [Google Scholar] [CrossRef]
- Dierikx, C.M.; van Duijkeren, E.; Schoormans, A.H.W.; van Essen-Zandbergen, A.; Veldman, K.; Kant, A.; Huijsdens, X.W.; van der Zwaluw, K.; Wagenaar, J.A.; Mevius, D.J. Occurrence and characteristics of extended-spectrum-β-lactamaseand AmpC-producing clinical isolates derived from companion animals and horses. J. Antimicrob. Chemother. 2020, 67, 1368–1374. [Google Scholar] [CrossRef]
- Wang, J.; Gibbons, J.F.; McGrath, K.; Bai, L.; Li, F.; Leonard, F.C.; Stephan, R.; Fanning, S. Molecular characterization of blaESBL-producing Escherichia coli cultured from pig farms in Ireland. J. Antimicrob. Chemother. 2016, 71, 3062–3065. [Google Scholar] [CrossRef]
- Ceccarelli, D.; Kant, A.; van Essen-Zandbergen, A.; Dierikx, C.; Hordijk, J.; Wit, B.; Mevius, D.J.; Veldman, K.T. Diversity of plasmids and genes encoding resistance to extended spectrum cephalosporins in commensal Escherichia coli from dutch livestock in 2007–2017. Front. Microbiol. 2019, 10, 76. [Google Scholar] [CrossRef]
- Kamaruzzaman, E.A.; Aziz, S.A.; Bitrus, A.A.; Zakaria, Z.; Hassan, L. Occurrence and characteristics of Extended-Spectrum β-Lactamase-producing Escherichia coli from dairy cattle, milk, and farm environment in Peninsular Malaysia. Pathogens 2020, 9, 1009. [Google Scholar] [CrossRef]
- Watson, E.; Jeckel, S.; Snow, L.; Stubbs, R.; Teale, C.; Wearing, H.; Coldham, N. Epidemiology of extended spectrum beta-lactamase E. coli (CTX-M-15) on a commercial dairy farm. Vet. Microbiol. 2012, 154, 339–346. [Google Scholar] [CrossRef]
- Reist, M.; Geser, N.; Hächler, H.; Schärrer, S.; Stephan, R. ESBL-producing Enterobacteriaceae: Occurrence, risk factors for faecal carriage and strain traits in the Swiss slaughter cattle population younger than 2 years sampled at abattoir level. PLoS ONE 2013, 8, e71725. [Google Scholar] [CrossRef]
- Hourigan, S.K.; Subramanian, P.; Hasan, N.A.; Ta, A.; Klein, E.; Chettout, N.; Huddleston, K.; Deopujari, V.; Levy, S.; Baveja, R.; et al. Comparison of infant gut and skin microbiota, resistome and virulome between neonatal intensive care unit (NICU) environments. Front. Microbiol. 2018, 9, 1361. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.A.; Young, B.A.; Minard-Smith, A.T.; Saeed, K.; Li, H.; Heizer, E.M.; McMillan, N.J.; Isom, R.; Abdullah, A.S.; Bornman, D.M.; et al. Microbial community profiling of human saliva using shotgun metagenomic sequencing. PLoS ONE 2014, 9, e97699. [Google Scholar] [CrossRef]
- Hasan, N.A.; Choi, S.Y.; Eppinger, M.; Clark, P.W.; Chen, A.; Alam, M.; Haley, B.J.; Taviani, E.; Hine, E.; Su, Q.; et al. Genomic diversity of 2010 Haitian cholera outbreak strains. Proc. Natl. Acad. Sci. USA 2012, 109, E2010–E2017. [Google Scholar] [CrossRef]
- Lax, S.; Smith, D.P.; Marcell, J.H.; Owens, S.; Handley, K.M.; Scott, N.M.; Gibbons, S.M.; Larsen, P.; Shogan, B.D.; Weiss, S.; et al. Longitudinal analysis of microbial interaction between humans and the indoor environment. Science 2014, 345, 1048–1052. [Google Scholar] [CrossRef]
- Ponnusamy, D.; Kozlova, E.V.; Sha, J.; Erova, T.E.; Azar, S.R.; Fitts, E.C.; Kirtley, M.L.; Tiner, B.L.; Andersson, J.A.; Grim, C.J.; et al. Cross-talk among flesh-eating Aeromonas hydrophila strains in mixed infection leading to necrotizing fasciitis. Proc. Natl. Acad. Sci. USA 2016, 113, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Antipov, D.; Hartwick, N.; Shen, M.; Raiko, M.; Lapidus, A.; Pevzner, P.A. plasmidSPAdes: Assembling plasmids from whole genome sequencing data. Bioinformatics 2016, 32, 3380–3387. [Google Scholar] [CrossRef] [PubMed]
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Larsen, M.V. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Raphenya, A.R.; Alcock, B.; Waglechner, N.; Guo, P.; Tsang, K.K.; Lago, B.A.; Dave, B.M.; Pereira, S.; Sharma, A.N.; et al. CARD: Expansion and modelcentric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res. 2017, 45, D566–D573. [Google Scholar] [CrossRef]
- Carattoli, A.; Zankari, E.; Garcia-Fernandez, A.; Voldby Larsen, M.; Lund, O.; Villa, L.; Aarestrup, F.M.; Hasman, H. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef]
- Oladeinde, A.; Cook, K.; Lakin, S.M.; Woyda, R.; Abdo, Z.; Looft, T.; Herrington, K.; Zock, G.; Lawrence, J.P.; Thomas, J.C.; et al. Horizontal gene transfer and acquired antibiotic resistance in Salmonella enterica serovar Heidelberg following in vitro incubation in broiler ceca. Appl. Environ. Microbiol. 2019, 85, e01903–e01919. [Google Scholar] [CrossRef]
- Treangen, T.J.; Ondov, B.D.; Koren, S.; Phillippy, A.M. The Harvest suite for rapid coregenome alignment and visualization of thousands of intraspecific microbial genomes. Genome Biol. 2014, 15, 524. [Google Scholar] [CrossRef]
- Page, A.J.; Taylor, B.; Keane, J.A. Multilocus sequence typing by blast from de novo assemblies against PubMLST. J. Open Source Softw. 2016. [Google Scholar] [CrossRef]
- Ramos, S.; Silva, V.; de Lurdes Enes Dapkevicius, M.; Canica, M.; Tejedor-Junco, M.T.; Igrejas, G.; Poeta, P. Escherichia coli as commensal and pathogenic bacteria among food-producing animals: Health implications of extended spectrum β-lactamase (ESBL) production. Animals 2020, 10, 2239. [Google Scholar] [CrossRef]
- Roer, L.; Overballe-Petersen, S.; Hansen, F.; Schønning, K.; Wang, M.; Røder, B.L.; Hansen, D.S.; Justesen, U.S.; Andersen, L.P.; Fulgsang-Dumgaard, D.; et al. Escherichia coli sequence type 410 is causing new international high-risk clones. mSphere 2018, 3, e00337-18. [Google Scholar] [CrossRef]
- Arredondo-Alonso, S.; Willems, R.J.; Van Schaik, W.; Schürch, A.C. On the (im)possibility of reconstructing plasmids from whole-genome short-read sequencing data. Microb. Genom. 2017, 3, e000128. [Google Scholar] [CrossRef]
- Berbers, B.; Saltykova, A.; Garcia-Graells, C.; Philipp, P.; Arella, F.; Marchal, K.; Winand, R.; Vanneste, K.; Roosens, N.H.C.; De Keersmaecker, S.C.J. Combining short and long read sequencing to characterize antimicrobial resistance genes on plasmids applied to an unauthorized genetically modified Bacillus. Sci. Rep. 2020, 10, 4310. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Brettin, T.; Davis, J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Olsen, G.J.; Olson, R.; Overbeek, R.; Parrello, B.; Pusch, G.D.; et al. RASTtk: A modular and extensible implementation of the RAST algorithm for building custom annotation pipelines and annotating batches of genomes. Sci. Rep. 2015, 8365. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, D.; Jin, Q.; Chen, L.; Yang, J. VFDB 2019: A comparative pathogenomic platform with an interactive web interface. Nucleic Acids Res. 2019, 47, D687–D692. [Google Scholar] [CrossRef]
- Stamatakis, A.; Hoover, P.; Rougemont, J. A rapid bootstrap algorithm for the RAxML web servers. Syst. Biol. 2000, 57, 758–771. [Google Scholar] [CrossRef] [PubMed]
- Zurfluh, K.; Wang, J.; Klumpp, J.; Nüesch-Inderbinen, M.; Fanning, S.; Stephan, R. Vertical transmission of highly similar blaCTX-M-1-harboring IncI1 plasmids in Escherichia coli with different MLST types in the poultry production pyramid. Front. Microbiol. 2014, 5, 519. [Google Scholar] [CrossRef]
- Wang, Y.; Song, C.; Duan, G.; Zhu, J.; Yang, H.; Xi, Y.; Fan, Q. Transposition of ISEcp1 modulates blaCTX-M-55-mediated Shigella flexneri resistance to cefalothin. Int. J. Antimicrob. Agents 2013, 42, 507–512. [Google Scholar] [CrossRef]
- Ewers, C.; Bethe, A.; Semmler, T.; Guenther, S.; Wieler, L.H. Extended-spectrum-β–lactamase-producing and AmpC-producing Escherichia coli from livestock and companion animals, and their putative impact on public health: A global perspective. Clin. Microbiol. Infect. 2012, 18, 646–655. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, X.; Li, J.; Lv, N.; Liu, F.; Wu, J.; Lin, I.Y.C.; Wu, N.; Weimer, B.C.; Gao, G.F.; et al. The bacterial mobile resistome transfer network connecting the animal and human microbiomes. Appl. Environ. Microbiol. 2016, 82, 6672–6681. [Google Scholar] [CrossRef]
- Matsumura, Y.; Johnson, J.R.; Yamamoto, M.; Nagao, M.; Tanaka, M.; Takakura, S.; Ichiyama, S. CTX-M-27- and CTX-M-14-producing, ciprofloxacin-resistant Escherichia coli of the H30 subclonal group within ST131 drive a Japanese regional ESBL epidemic. J. Antimicrob. Chemoth. 2015, 70, 1639–1649. [Google Scholar]
- Kudinha, T.; Johnson, J.R.; Andrew, S.D.; Kong, F.; Anderson, P.; Gilbert, G.L. Distribution of phylogenetic groups, sequence type ST131, and virulence associated traits among Escherichia coli isolates from men with pyelonephritis or cystitis and healthy controls. Clin. Microbiol. Infect. 2013, 19, E173–E180. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.; Carvalho, I.; Currie, C.; Sousa, M.; Igrejas, G.; Poeta, P. Extended-spectrum-β-lactamase and carbapenemase-producing Enterobacteriaceae in food-producing animals in Europe. Antibiot. Drug Resist. 2010, 12, 261–273. [Google Scholar]
- Moreno, E.; Johnson, J.R.; P’erez, T.; Prats, G.; Kuskowski, M.A.; Andreu, A. Structure and urovirulence characteristics of the fecal Escherichia coli population among healthy women. Microbes Infect. 2009, 11, 274–280. [Google Scholar] [CrossRef]
- Moreno, E.; Andreu, A.; Pigrau, C.; Kuskowski, M.A.; Johnson, J.R.; Prats, G. Relationship between Escherichia coli strains causing acute cystitis in women and the fecal E. coli population of the host. J. Clin. Microbiol. 2008, 46, 2529–2534. [Google Scholar] [CrossRef]
- Rendon, M.A.; Saldana, Z.; Erdemet, A.L.; Monteiro-Neto, V.; Vazquez, A.; Kaper, J.B.; Puente, J.L.; Giro, J.A. Commensal and pathogenic Escherichia coli use a common pilus adherence factor for epithelial cell colonization. Proc. Natl. Acad. Sci. USA 2007, 104, 10637–10642. [Google Scholar] [CrossRef]
- Johnson, J.R.; Stell, A.L. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J. Infect. Dis. 2000, 181, 261–272. [Google Scholar] [CrossRef]
- Pompilio, A.; Crocetta, V.; Savini, V.; Petrelli, D.; Di Nicola, M.; Bucco, S.; Amoroso, L.; Bonomini, M.; Di Bonaventura, G. Phylogenetic relationships, biofilm formation, motility, antibiotic resistance and extended virulence genotypes among Escherichia coli strains from women with community-onset primitive acute pyelonephritis. PLoS ONE 2018, 13, e0196260. [Google Scholar] [CrossRef]
- Ibekwe, A.M.; Murinda, S.E.; DebRoy, C.; Reddy, G.B. Potential pathogens, antimicrobial patterns, and genotypic diversity of Escherichia coli isolates in constructed wetlands treating swine wastewater. FEMS Microbiol. Ecol. 2016. [Google Scholar] [CrossRef]
- Normark, B.H.; Normark, S. Evolution and spread of antibiotic resistance. J. Intern. Med. 2002, 252, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Agga, G.E.; Arthur, T.M.; Durso, L.M.; Harhay, D.M.; Schmidt, J.W. Antimicrobial-Resistant Bacterial Populations and Antimicrobial Resistance Genes Obtained from Environments Impacted by Livestock and Municipal Waste. PLoS ONE 2015, 10, e0132586. [Google Scholar] [CrossRef]
- Agga, G.E.; Scott, H.M.; Amachawadi, R.G.; Nagaraja, T.G.; Vinasco, J.; Bai, J.; Norby, B.; Renter, D.G.; Dritz, S.S.; Nelssen, J.L.; et al. Effects of chlortetracycline and copper supplementation on antimicrobial resistance of fecal Escherichia coli from weaned pigs. Prev. Vet. Med. 2014, 114, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Brichta-Harhay, D.M.; Arthur, T.M.; Bosilevac, J.M.; Kalchayanand, N.; Shackelford, S.D.; Wheeler, T.L.; Koohmaraie, M. Diversity of multidrug-resistant Salmonella enterica strains associated with cattle at harvest in the United States. Appl. Environ. Microbiol. 2011, 77, 1783–1796. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.P.; Adeli, A.; McLaughlin, M.R. Microbial ecology, bacterial pathogens, and antibiotic resistant genes in swine manure wastewater as influenced by three swine management systems. Water Res. 2014, 57, 96–103. [Google Scholar] [CrossRef]
- Dungan, R.S.; Klein, M.; Leytem, A.B. Quantification of bacterial indicators and zoonotic pathogens in dairy wastewater ponds. Appl. Environ. Microbiol. 2012, 78, 8089–8095. [Google Scholar] [CrossRef]
- Frye, J.G.; Jackson, C.R. Genetic mechanisms of antimicrobial resistance identified in Salmonella enterica, Escherichia coli, and Enteroccocus spp. isolated from U.S. food animals. Front. Microbiol. 2013, 4, 135. [Google Scholar] [CrossRef]
- Haley, C.A.; Dargatz, D.A.; Bush, E.J.; Erdman, M.M.; Fedorka-Cray, P.J. Salmonella prevalence and antimicrobial susceptibility from the National Animal Health Monitoring System Swine 2000 and 2006 studies. J. Food Prot. 2012, 75, 428–436. [Google Scholar] [CrossRef]
- Berendonk, T.U.; Manaia, C.M.; Merlin, C.; Fatta-Kassinos, D.; Cytryn, E.; Walsh, F.; Bürgmann, H.; Sørum, H.; Norström, M.; Pons, M.N.; et al. Tackling antibiotic resistance: The environmental framework. Nat. Rev. Microbiol. 2015, 13, 310–317. [Google Scholar] [CrossRef]
- McKinney, C.W.; Loftin, K.A.; Davis, J.G.; Meyer, M.T.; Pruden, A. tet and sul antibiotic resistance genes in livestock lagoons of various operation type, configuration, and antibiotic occurrence. Environ. Sci. Technol. 2012, 44, 6102–6109. [Google Scholar] [CrossRef]
- Pruden, A.; Pei, R.; Storteboom, H.N.; Carlson, K.H. Antibiotic resistance genes as emerging contaminants: Studies in northern Colorado. Environ. Sci. Technol. 2006, 40, 7445–7450. [Google Scholar] [CrossRef]
- Pruden, A.; Arabi, M.; Storteboom, H.N. Correlation between upstream human activities and riverine antibiotic resistance Genes. Environ. Sci. Technol. 2012, 46, 6102–6109. [Google Scholar] [CrossRef]
- Storteboom, H.N.; Arabi, M.; Davis, J.G.; Crimi, B.; Pruden, A. Tracking antibiotic resistance genes in the South Platte River basin using molecular signatures of urban, agricultural, and pristine sources. Environ. Sci. Technol. 2010, 44, 7397–7404. [Google Scholar] [CrossRef]
- Storteboom, H.N.; Arabi, M.; Davis, J.G.; Crimi, B.; Pruden, A. Identification of antibiotic resistance gene molecular signatures suitable as tracers of pristine river, urban, and agricultural sources. Environ. Sci. Technol. 2010, 44, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Aarestrup, F.M.; Kruse, H.; Tast, E.; Hammerum, A.M.; Jensen, L.B. Associations between the use of antimicrobial agents for growth promotion and the occurrence of resistance among Enterococcus faecium from broilers and pigs in Denmark, Finland, and Norway. Microbial. Drug Resist. 2000, 6, 63–70. [Google Scholar] [CrossRef]
- Murinda, S.E.; Ebner, P.D.; Nguyen, L.T.; Mathew, A.G.; Oliver, S.P. Antimicrobial resistance and class 1 integrons in pathogenic Escherichia coli from dairy farms. Foodborne Path. Dis. 2005, 2, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Baquero, F.; Martınez, J.L.; Canton, R. Antibiotics and antibiotic resistance in water environments. Curr. Opin. Biotech. 2008, 9, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, A.R.; Curriero, F.C.; Gibson, K.E.; Schwab, K.J. Antibiotic-resistant enterococci and fecal indicators in surface water and groundwater impacted by a concentrated swine feeding operation. Environ. Health Perspect. 2007, 115, 1040–1045. [Google Scholar] [CrossRef] [PubMed]


| Isolate ID | Source | Phylogroup | ST-Type | Plasmids Identified * | Resistance Phenotype a | Acquired ARG b,* | Point Mutation |
|---|---|---|---|---|---|---|---|
| Isolate_15-ESBL+ | swine | C | 6913 | IncFII, IncI1, ColRNAI | AMC, AM, AZM, S, TE | aac(3)-IV, aadA1, aadA2, blaCMY-28*, cml, sul3, tetA, dfrA15 | None |
| Isolate_13-ESBL+ | swine | D | 100 | IncFIB, IncFII, IncFIC, IncI1, IncA/C2, Col440I | AM, C, GM, K, NA, S, TE, TIC | aph(3′)-Ia, aph(3′’)-Ib, aph(6)-Id, aadA24, aac(3)-Via, blaCMY-28*, blaTEM-1B, floR, sul1, sul2, tetA, strA | parC p.S80I, gyrA p.S83L |
| Isolate_12-ESBL+ | swine | C | 1771 | IncFIB, IncX1, IncX4, IncI1, p0111, ColRNAI, Col (MG828) | AM, S, TE | aph(6)-Id, aph(3′’)-Ib, strA | None |
| Isolate_01 | beef | B1 | 327 | IncFIB, IncFII, IncX1, IncY, ColRNAI | C, S, G, TE | aph(6)-Id, aph(3′’)-Ib, floR, sul2, tetA, strA | None |
| Isolate_03 | beef | A | 1101 | IncFIB, IncFII | S, G, TE | aph(6)-Id, aph(3′’)-Ib, sul2, tetB, strB | None |
| Isolate_11-ESBL+ | swine | C | 410 | IncFIA(B), IncFII, IncX1, IncX4, IncQ1, CollRNAI, Col440I | AM, AMZ, CRO, CF, C, CIP, K, NA, S, G, TE, TIC | aph(6)-Id, aph(3′’)-Ib, aadA5, aph(3′)-Ia, blaCTX-M-15, blaTEM-1B, mphA, ermB, catA1, sul1, sul2, tetB, dfrA17 | parE p.S458A, parC p.S80I, gyrA p.S83L, gyrA p.D87N |
| Isolate_10-ESBL+ | swine | D | 48 | IncX4, IncX1, IncI1 | AM, AZM, CRO, CF, TE, TIC | aph(6)-Id, aph(3′’)-Ib, ermB, lnuG, tetB, blaTEM-1B, blaCMY-2*8, | None |
| Isolate_09-ESBL+ | swine | B1 | 711 | IncFIA(B), IncX1, IncI1, IncI2, ColRNAI, Col(MG828), Col156 | AM, CRO, CF, TE, TIC | BlaCMY-28*, blaTEM-1C, tetA | None |
| Isolate_14-ESBL+ | swine | B1 | 101 | IncX4, IncI1, IncHI2A | AMC, AM, AZM, FOX, TE | BlaCMY-28*, ermB, tetB | None |
| Isolate_08 | dairy | B1 | 710 | IncFIA(B) | CF, G | None | None |
| Isolate_02 | beef | B1 | 43 | IncFIA(B), IncFII | G | None | None |
| Isolate_04 | dairy | A | 1300 | IncFIA(B), IncFII, IncX1, ColRNAI | CF | None | None |
| Isolate_18 | horse | A | 10 | IncFIB, IncFII | S, G | None | None |
| Isolate_20 | sediment | B1 | 154 | IncFIB, IncFII | AZM, G | None | None |
| AgEc_17 | poultry | E | 155 | IncFIB, IncI1, ColRNAI | AM, AMZ, S, G | strB, mef(B) | None |
| Isolate_16 | poultry | D | 10 | ND | ND | ND | ND |
| Isolate_5-ESBL+ | dairy | A | 2 | IncFIB, IncFII | CF, G | strB | parC p.S57T |
| Isolate_6-ESBL+ | dairy | D | 685 | IncI1, IncY | CF, G, TIC | None | None |
| Isolate_07 | dairy | D | 154 | IncFIB, IncFII, IncX1,IncI1, ColRNAI | CF, G, TIC | tetA | None |
| Isolate_19 | lamb | E | 6060 | IncFII, IncX1, IncI1, ColRNAI | TE | tetC | None |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibekwe, A.; Durso, L.; Ducey, T.F.; Oladeinde, A.; Jackson, C.R.; Frye, J.G.; Dungan, R.; Moorman, T.; Brooks, J.P.; Obayiuwana, A.; et al. Diversity of Plasmids and Genes Encoding Resistance to Extended-Spectrum β-Lactamase in Escherichia coli from Different Animal Sources. Microorganisms 2021, 9, 1057. https://doi.org/10.3390/microorganisms9051057
Ibekwe A, Durso L, Ducey TF, Oladeinde A, Jackson CR, Frye JG, Dungan R, Moorman T, Brooks JP, Obayiuwana A, et al. Diversity of Plasmids and Genes Encoding Resistance to Extended-Spectrum β-Lactamase in Escherichia coli from Different Animal Sources. Microorganisms. 2021; 9(5):1057. https://doi.org/10.3390/microorganisms9051057
Chicago/Turabian StyleIbekwe, Abasiofiok, Lisa Durso, Thomas F. Ducey, Adelumola Oladeinde, Charlene R. Jackson, Jonathan G. Frye, Robert Dungan, Tom Moorman, John P. Brooks, Amarachukwu Obayiuwana, and et al. 2021. "Diversity of Plasmids and Genes Encoding Resistance to Extended-Spectrum β-Lactamase in Escherichia coli from Different Animal Sources" Microorganisms 9, no. 5: 1057. https://doi.org/10.3390/microorganisms9051057
APA StyleIbekwe, A., Durso, L., Ducey, T. F., Oladeinde, A., Jackson, C. R., Frye, J. G., Dungan, R., Moorman, T., Brooks, J. P., Obayiuwana, A., Karathia, H., Fanelli, B., & Hasan, N. (2021). Diversity of Plasmids and Genes Encoding Resistance to Extended-Spectrum β-Lactamase in Escherichia coli from Different Animal Sources. Microorganisms, 9(5), 1057. https://doi.org/10.3390/microorganisms9051057












