Abstract
This study pertains to measure differences in bacterial communities along the wastewater pathway, from sewage sources through the environment. Our main focus was on taxa which include pathogenic genera, and genera harboring antibiotic resistance (henceforth referred to as “target taxa”). Our objective was to measure the relative abundance of these taxa in clinical wastewaters compared to non-clinical wastewaters, and to investigate what changes can be detected along the wastewater pathway. The study entailed a monthly sampling campaign along a wastewater pathway, and taxa identification through 16S rRNA amplicon sequencing. Results indicated that clinical and non-clinical wastewaters differed in their overall bacterial composition, but that target taxa were not enriched in clinical wastewater. This suggests that treatment of clinical wastewater before release into the wastewater system would only remove a minor part of the potential total pathogen load in wastewater treatment plants. Additional findings were that the relative abundance of most target taxa was decreased after wastewater treatment, yet all investigated taxa were detected in 68% of the treated effluent samples—meaning that these bacteria are continuously released into the receiving surface water. Temporal variation was only observed for specific taxa in surface water, but not in wastewater samples.
1. Introduction
Antimicrobial resistance (AMR) is recognized as a major threat to public health at a global scale [1]. The ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) play an important role in nosocomial infections, pathogenesis, and AMR spread [2,3]. In 2018 the World Health Organization (WHO) published a global priority list of antimicrobial-resistant bacteria (AMRB) for which research and development of new antibiotics is urgently needed [4].
After consumption of antibiotics, the bacterial composition in the gut can be altered [5,6] and even be enriched in AMRB [7]. Some bacteria are found to thrive after antibiotic treatment and can cause antibiotic-associated diarrhoea (AAD) [8,9,10]. Such, and other, pathogenic bacteria are expected to be more abundant in clinical settings, where the consumption of antibiotics is higher than in the general community [11]. Previous research showed that clinical wastewaters contain a higher level of AMR bacteria, antimicrobial resistance genes and antimicrobial residues than non-clinical wastewaters [12,13,14,15]. Nonetheless, clinical wastewater constitutes a minor proportion of all wastewater and the magnitude of the impact that clinical wastewater has on AMR bacterial load in downstream wastewaters is not yet fully understood. Paulus et al. showed that pre-treatment of hospital wastewater helps to reduce the presence of ARGs in the receiving WWTP [13], yet Buelow et al. did not observe differences in the relative abundance of ARGs in WWTPs that did or did not receive hospital wastewater [14]. More insight into the bacterial composition of wastewater is critical to decide on the benefit of dedicated wastewater treatment at the level of clinical institutions.
Human gut bacteria are released into the wastewater system via feces and can reach the environment via this pathway. Wastewater from both clinical and non-clinical settings converges in the wastewater treatment plant (WWTP). WWTP’s are designed to reduce the biological oxygen demand, nitrogen, phosphorus, and the total suspended solids, but not the removal of pharmaceutically active compounds and bacteria. Earlier studies have shown that most pathogenic bacteria are decreased in their relative abundance in the WWTP, while others (e.g., Mycobacterium spp. and Clostridium spp.) increase [16,17]. Although wastewater and surface waters are different environments from the human gut, several pathogenic bacteria are known to be water-borne pathogens (species that are able to spread via aquatic ecosystems) while others can be classified as water-based pathogens (species that are able to grow and thrive in water systems) [18,19,20,21,22].
This study aimed to investigate the contribution of clinical wastewater on the bacterial composition in wastewater and the environment. We used 16S rRNA to broadly screen the relative abundance and fate of bacterial target taxa that were selected based on pathogenic potential: (i) pathogens of clinical relevance and AMR features (ESKAPE pathogens and WHO priority pathogens) [2,3,4]; (ii) water-based pathogens that can grow and thrive in water systems [18,19,20,21,22]; (iii) antibiotic-associated diarrhea (AAD) bacteria [8,9,10] and; (iv) bacteria that increase after WWTP treatment [16,17].
The results of this study provide insight into the community composition origin in a wastewater chain, the difference between clinical and non-clinical wastewater, and how this affects the bacterial community in the WWTP and the receiving surface water. In doing so, the study sheds light on the likely fate of potentially pathogenic bacteria in this wastewater chain.
2. Materials and Methods
2.1. Sampling Campaign
The sampling campaign was conducted in 2017 across the wastewater network in Sneek (33,855 inhabitants), The Netherlands. While a more detailed description of the sampling campaign can be found in our previous research [12] a brief summary of the study is as follows: wastewater sample locations encompassed: (a) hospital (300 beds), nursing home (220 beds) and domestic (80 households) wastewater sources (all sites selected to exclude the influence of industrial waste and/or rainwater); (b) influent and effluent from the conventional WWTP at Sneek (aerobic treatment, 73,000 p. e.) which receives wastewater from the hospital, nursing home and city district where the domestic wastewater sample was obtained. Wastewater samples were collected as twenty-four-hour samples sampled flow proportionally (WWTP) or time proportionally (domestic, hospital, and nursing home wastewater). Surface water samples were collected as grab samples, taken at ~20 cm depth and one-meter distance from the waterside. Surface water samples were taken from the receiving surface water of the Geeuw canal at two locations, 330 m south-west (N 53°02′15.10″, E 5°63′72.76″) and 388 m north-east (N 53°02′72.15″, E 5°64′28.97″) from the WWTP discharge point (N 53°02′38.85″, E 5°64′03.20″), and from a nature reserve “de Deelen” which has previously been shown to lack human influence [23]. The locations of the sampling points in Sneek are indicated in the map of Sneek in Supplementary Figure S1. Samples collected twice per month for one year, transported cooled and processed within the same day.
2.2. DNA Extraction and 16S rRNA Gene Amplicon Sequencing
Water samples were filtered in volumes of 25 mL (wastewaters) or 200 mL (surface water and effluent) using sterile 0.22 µm polyvinylidene difluoride (PVDF)-membrane filters. Filters were stored at −80 °C until DNA extraction was performed. DNA was extracted using the DNeasy Power Water kit (Qiagen, Hilden, Germany) and quantified using the Qubit dsDNA BR (broad range) Assay kit (ThermoFisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. DNA was stored at −20 °C before subsequent analysis. Amplicon sequencing of the V3–V4 regions of the 16S rRNA gene was performed on an Illumina MiSeq (Illumina, San Diego, CA, USA). Libraries were prepared by using the Nextera XT DNA Library Preparation Kit following the 16S Metagenomic Sequencing Library Preparation protocol, according to the manufacturer’s instructions [24]: the V3-V4 regions of the 16S rRNA gene were amplified by the polymerase chain reaction (PCR) using Amplicon primers with overhang adaptors (16S Amplicon PCR Forward Primer = 5′ TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG 16S Amplicon PCR Reverse Primer = 5′ GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC). A second PCR was used for attaching indices and Illumina sequencing adapters using the Nextera® XT Index Kit (Illumina, San Diego, CA, USA). Fragments were cleaned after each PCR using freshly prepared Ampure XP Beads (Beckman Coulter Genomics, Danvers, MA, USA). In total, 171 samples were successfully sequenced, resulting in an average of 50,734 reads per sample (Table 1). The sample with the lowest number of reads was a downstream sample (18,059 reads), and the sample with the highest number of reads came from the hospital (138,846 reads). Sequence data are available in the NCBI sequence read archive (SRA) under project numbers PRJNA668059 and PRJNA668064.

Table 1.
16S rRNA gene amplicon sequencing results. The number of samples obtained per location, as well as the number of reads (average, minimal and maximal) are shown together with the rarefied and non-rarefied amplicon sequence variants (ASV) and the resulting average detection limit at log ratio. Libraries were rarefied for statistical comparison to 18,059 reads per sample.
2.3. Data Processing and Visualization
Adaptor sequences were removed from the FASTQ and reads were filtered by length (200–550 nucleotides) using Qiagen CLC Bio Genomics Workbench 10.1.1. (Qiagen, Germantown, MD, USA). Sequence reads were de-noised using DADA2 (v. 1.11.0) in R Statistical Software (v. 3.5.0). Taxonomy was assigned to representative sequences for amplicon sequence variants (ASV) using the SILVA database (v. 132) [25]. The sample by ASV frequency matrix was combined with taxonomic assignments using the biom-format package. Representative sequences were then imported into QIIME2 (v. 2018.11) [26] for alignment using MAFFT [27]. The alignment was then filtered for gaps and used to build a phylogeny using Fasttree2 [28]. These data were then imported in R as a phyloseq object [29] and filtered to exclude Chloroplasts and Mitochondria and to retain only Bacteria for downstream analyses of microbial diversity and community structure.
ASV counts were normalized to the total number of reads per sample for calculating unweighted and weighted UniFrac distances among samples [30,31]. Principal coordinates analysis (PCoA) visualizations based on the distance matrices were used to assess the phylogenetic composition and structure of bacterial communities at different locations along the wastewater pathway. Global and pairwise PERMANOVA [32,33] were applied to test for differential clustering of sample locations. Additional tests of homogeneity of group dispersion were performed with the betadisper function and a permutation test with 999 permutations.
2.3.1. Selection of Target Taxa for Fate Monitoring through Wastewater Pathway
A total of 24 bacterial genera or species were selected as “target taxa” to study their changes in relative abundance and fate along the studied wastewater pathway (Table 2), based on pathogenicity, antibiotic resistance potential and known or possible association with WWTP. The taxa selection included: (1) the ESKAPE pathogens that are of particular clinical relevance and prone to antibiotic resistance [2,3], (2) the WHO priority pathogens for AMR [4], (3) water-based pathogens [18,19,20,21,22], (4) bacteria which are AAD associated/increased after antibiotic (AB) treatment [8,9,10], and (5) bacteria previously found at increased concentrations after WWTP treatment [16,17]. Some genera include bacteria that match with more than one of the five selection criteria.

Table 2.
Target taxa. Taxa that include pathogenic bacteria of clinical interest monitored from wastewater to environment. If no reference is given in the cell, the bacteria does not fall under this category.
To compare the relative abundances of target taxa among locations, we first rarefied all ASV counts to a sequencing depth corresponding to the sample with the lowest coverage (18,059 reads per sample). A pseudo-count of 1 was added to all counts to enable log transformation of read counts where target taxa were absent in a particular sample or location. The relative abundance of target taxa in a given sample was then calculated as the natural log-ratio between taxon read count and the total sample read count. The log ratio of the limit of detection was determined as ln(2)-ln(total no. of reads). Location differences were tested using the χ2 statistic (Kruskal–Wallis test). Pairwise differences among locations were assessed using Dunn’s test using PMCMR [34] with p-value corrections using the Benjamin–Hochberg procedure (1995) [35]. The correlation between absolute counts of Klebsiella spp. and Aeromonas spp. with their relative abundances were confirmed using Pearson correlation analysis.
Differential abundance testing across all observed taxa, at the ASV level, was performed with DESeq2 [36] with default settings after applying a variance-stabilizing transformation of the raw counts [37]. We first analyzed differential ASV abundances between clinical (i.e., hospital and nursing home) and domestic wastewater samples. Then, among significantly more abundant ASVs in clinical versus domestic wastewater samples (hereafter referred to as clinically enriched taxa), we assessed whether they were more abundant in influent samples than in domestic wastewaters. Effect sizes and test statistics for pairwise contrasts between (groups of) locations were considered significant at false discovery rate (FDR)-corrected q < 0.1.
2.3.2. Indication of Temporal Effects on Phylum Abundance
Temporal changes in bacterial community composition in water are often driven by temperature [38]. Therefore, the ambient temperature was measured on the day of sampling to relate to temporal patterns of the bacterial community composition in the water samples. Acknowledging microbial community compositionality, the QIIME2-plugin for Songbird [39] was used to run multinomial regression for estimating seasonality in bacterial phylum abundances for each location. Phylum Bacteroidetes was chosen as the reference frame standard for this regression analysis, because Bacteroidetes showed stable abundances in all the locations.
3. Results and Discussion
In this study, we analyzed the bacterial compositions along a whole wastewater pathway.
3.1. Bacterial Composition Differs between Sources and along the Studied Wastewater Pathway
The phylogenetic structure of wastewater microbial communities differed significantly between sampling locations. Significant differences among the three source locations, as well as WWTP influent and effluent microbial communities are shown in pairwise PERMANOVA analyses (Supplementary Tables S1 and S2). The assumption of homogeneity of multivariate dispersion was met for all but one comparison for weighted UniFrac, but never for unweighted UniFrac. The strong ordination patterns and the large fraction of explained variation (R2 = 39–69%) suggest that significant differences are unlikely to represent statistical artifacts.
The differences observed between wastewater sources might be caused by the higher use of antibiotics and other drugs in the clinical settings [12]. Only one other study reported a direct comparison between hospital wastewater, domestic wastewater and influent [40], and showed that these three waters were contained in the same cluster, with domestic wastewater more similar to hospital wastewater than to influent.
In contrast, in our study, domestic wastewater was more similar to influent than to hospital wastewater (Figure 1). Since in Sneek influent is mainly sourced by domestic wastewater from different neighborhoods, it was expected that the bacterial community structure (weighted UniFrac PCoA) of influent was most similar to domestic wastewater. However, the phylogenetic composition (unweighted UniFrac PCoA) of influent resembled nursing home wastewater more than municipal wastewater (Figure 1B), this could be related to the distance of the sampling points to the WWTP, with the nursing home more closely located to the WWTP than the municipal wastewater location (Supplementary Figure S1). Differences between raw wastewater and influent could result from passage through the sewer pipes from the source to the WWTP, which alters bacterial composition by shifting dominance from obligate anaerobes to facultative anaerobes [41], and probably is determined by the length of the sewer network. Larger group dispersions in weighted versus unweighted UniFrac suggest variation in relative abundances among bacterial lineage groups among samples at each location. Further investigation is necessary to determine whether such variations may be caused by the influence of other wastewater sources and rain events, which fluctuate throughout the year.
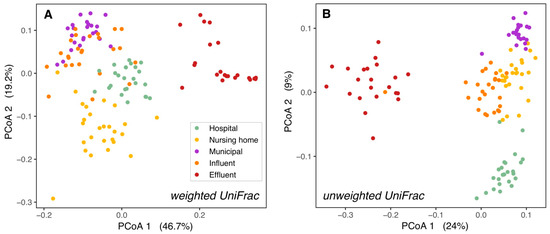
Figure 1.
Bacterial ß-diversity along the wastewater pathway. Principal coordinates ordination of different wastewaters based on weighted UniFrac distances (A) and unweighted UniFrac distances (B). 66% of the diversity in bacterial composition and 39% of the diversity in bacterial membership could be explained by the locations. The percentage of variation explained by each axis is shown between parentheses. H = hospital, N = nursing home, M = municipal, I = influent, E = effluent.
While communities from the hospital, nursing home, and domestic wastewater formed separate clusters, they clustered more closely to WWTP influent than to effluent or surface water. This held true both for the relative abundances of taxonomic groups (weighted UniFrac PCoAs, Figure 1A and Supplementary Figure S2A) and their presence/absence (unweighted UniFrac PCoAs, Figure 1B and Supplementary Figure S2B). This is as expected as raw wastewater bacterial communities are formed mainly by human gut bacteria [16,42], while effluent bacterial communities are a mixture of both wastewater and activated sludge bacteria from the WWTP [16,40], and surface water in the WWTP vicinity contains mostly environmental bacteria to which effluent bacteria are added [43,44,45].
3.2. Microbial Community Differences between Clinical and Non-Clinical Wastewaters
In order to investigate differences between the raw wastewater sources in more detail, the relative abundance of single bacterial taxa was compared between clinical and domestic wastewater. Significant differences were observed in the relative abundance of 335 bacterial taxa collected from clinical wastewaters (i.e., hospital and nursing home sources) and domestic wastewater.
The differences between bacterial compositions of clinical and non-clinical wastewaters were examined in just a few studies [14,40,46], and differential community composition of hospital and domestic wastewater did not exhibit a general pattern across studies or locations. Quintela-Baluja et al. [40] reported that hospital wastewater was best characterized by Lactobacillales and Enterobacteriales, while domestic wastewater was best characterized by Clostridiales and Erysipelotrichales. In contrast, our results indicated that a large part of the clinically enriched taxa belonged to Clostridiales (25%, n = 51, Supplementary Table S3). Our data also showed that only eight of the clinically enriched taxa belonged to Lactobacillales or Enterobacteriales (Supplementary Table S3). A likely explanation for the differences in observations resides in the difference in geographical locations at which the studies were conducted, because the human microbiome is country-specific [47]. Other likely explanations are the type of medication used in the different clinical settings [48], and also the methodology of obtaining and processing data - which can all affect the final results. More studies investigating the differences between clinical and non-clinical wastewater microbiomes are therefore necessary to establish which bacteria are typically clinically enriched.
Of the 335 taxa that were found to differ significantly in relative abundances between clinical and non-clinical wastewater, 62% (n = 207) were enriched in both hospital and nursing home wastewater (and termed “clinically enriched taxa”, Figure 2). Only 15% of the clinically enriched taxa (n = 30) belonged to the target bacteria of relevance listed in Table 2; most of them belonged to the genus Bacteroides (n = 16) (Supplementary Table S3). Five other taxa listed in Table 2—Aeromonas, Enterobacter, Klebsiella, Pseudomonas, and Streptococcus spp.—were also found between the clinically enriched taxa, but only at very low relative abundance.
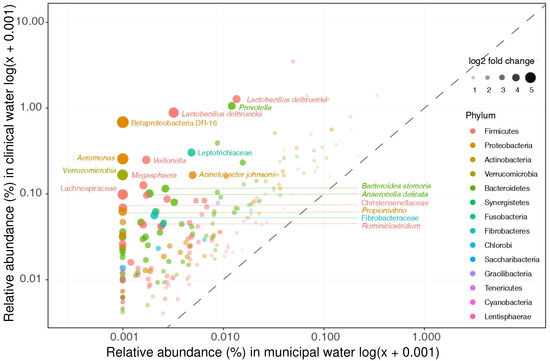
Figure 2.
Clinical enriched taxa. Clinical enriched taxa were defined as taxa that were significantly more abundant in both hospital and nursing home wastewater as compared to municipal wastewater. The relative abundances of these taxa in hospital and municipal wastewaters are depicted. The clinical most enriched taxa are not members of our selected target taxa (i.e., Lactobacillus delbrueckii and Prevotella).
3.3. Clinical Wastewater Does Not Impact the Overall Bacterial Composition of Influent
In order to investigate whether the clinically enriched taxa still showed increased abundance in WWTP influent (representing a mix of clinical and domestic wastewater), the abundance of clinically enriched taxa was compared between raw domestic wastewater and WWTP influent. Most of the taxa that have a higher relative abundance in clinical wastewater do not impact the overall bacterial community in influent (Figure 2 and Supplementary Table S3). Only 10 out of the 207 clinically enriched taxa were significantly more abundant in influent than in domestic wastewater (Figure 3, Supplementary Table S4). In addition, influent clustered more closely to domestic wastewater than to the clinical wastewaters in the weighted UniFrac PCoA ordination (Figure 1A). Seeing as that the overall bacterial composition of influent is most comparable with that of domestic wastewater, our results indicate that clinical wastewater has a limited impact on the abundance of target taxa in WWTP influent for the locations investigated in this study.
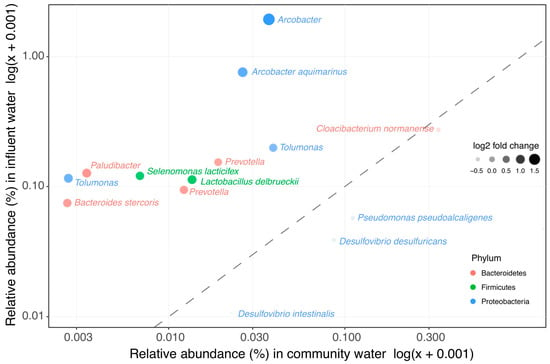
Figure 3.
Clinical enriched taxa in influent vs. wastewater collected from households. The 207 taxa found to be enriched in clinical wastewaters were compared in their relative abundance in influent and in the wastewater collected from households. In total, 10 taxa were significantly more abundant in influent than in wastewater collected from households.
The low impact of clinical wastewater on the influent can be explained by the low volume of both hospital and nursing home wastewaters in this study (they constitute less than 1% of the total influent). In two other studies, the bacterial composition of influent that received hospital wastewater was more similar to influent that did not receive hospital wastewater than to the hospital wastewater [14,46], which also indicates that hospital wastewater is too much diluted by other wastewater sources to be traced back in the receiving influent. In our previous study, the impact of hospital wastewater on culturable AMR bacteria in influent was also low [12]. Indeed, considering the dilution factor of the clinical wastewater in influent (1:100), the abundance of bacteria in clinical wastewater has to be 100 times greater than in non-clinical wastewater to affect influent. This was not the case for any of the clinically enriched taxa in this study (Supplementary Table S3).
Among the ten clinically enriched taxa, one species (Bacteroides stercoris, which can cause abdominal infections [49]), belongs to the selected target taxa that contain pathogenic bacteria. Bacteroides spp. was the only target taxa present in a high relative abundance in both hospital and nursing home wastewater (Supplementary Figure S3). Bacteroides spp. are found to be present in fecal samples taken from AAD patients [8], and are also shown to increase after treatment with fluoroquinolones and β-lactams [9]. In addition, the genus Arcobacter also contains pathogenic species [50], and some species of the genus Prevotella are associated with rheumatic diseases [51]. Thus, although clinical wastewater does not impact the overall bacterial composition in influent, it can be a source for some of the pathogenic species found in influent.
3.4. Decrease of Relative Abundance in Most Wastewater Taxa during WWTP Treatment
We observed a decrease of relative abundance in most target taxa (Table 2) after WWTP treatment by at least factor 10 (one log ratio) (Supplementary Figure S3). Likewise, genera including clinically enriched taxa found in influent were reduced by at least one log-ratio in the WWTP (Supplementary Figure S4). The decrease in the relative abundance could be due to bacterial removal in the WWTP, or due to the “dilution” of wastewater bacteria by activated sludge bacteria in the effluent. Indeed, the bacterial composition of effluent had the highest diversity among all locations (Supplementary Figure S5). Thus, one cannot infer a reduction in absolute concentrations from changes in relative abundance. Figure 4 shows the results of the absolute counts of Klebsiella spp. and Aeromonas spp. from our previous study [12] next to their relative abundances. A similar pattern is observed for both results over the locations, and the correlation is confirmed by Pearson correlation analysis (R = 0.85 and 0.87; Figure 4). In our previous study we showed that E. coli, Klebsiella spp. and Aeromonas spp. were significantly reduced (<99%) in the WWTP [12], therefore, it is most likely that the other genera are also reduced in the WWTP. Although most genera decreased in their relative abundance in the WWTP, all of them were still present in at least 68% of the effluent samples. The number of pathogens surviving could be important from a human health risk assessment standpoint.
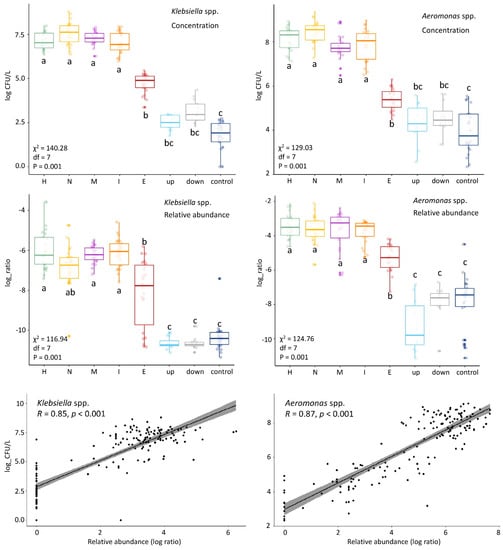
Figure 4.
Comparison of colony forming units (CFU) counts and relative abundances in the different locations of Klebsiella spp. (left) and Aeromonas spp. (right). The CFU counts (log(CFU/L) and relative abundance (log ratio) show similar patterns along the locations. This was confirmed by Pearson correlation analysis (R) shown in the linear regression curves. Kruskal-Wallis statistics of the CFU counts and relative abundances are shown on the left side of each panel: chi-squared (χ2), df and p-value). Group differences were assessed by the Dunn’s test with the p-value adjustment method: BH. The locations are shown on the horizontal axis. H = hospital, N = nursing home, C = community (municipal wastewater), I = influent, E = effluent, up = upstream surface water, down = downstream surface water, and control = surface water collected at nature reserve “de Deelen”.
Legionella, Mycobacterium, Clostridium and Leptospira spp. represent four genera that include pathogenic species, and that show an increased relative abundance from influent to effluent (Supplementary Figure S3). Other studies have also reported an increase of these four bacteria in the WWTP, as shown in [16,17]. Clostridium spp. are common inhabitants of the human gut, and they play an important role in the maintenance of gut homeostasis [52]. Since activated sludge can be a reservoir of Clostridium spp. [53], this might explain the relatively high abundance of Clostridium spp. found in effluent. Among Mycobacterium spp., M. tuberculosis complex is an important pathogen. Many environmental nontuberculous mycobacteria can also be pathogenic [54]. Mycobacterium also represents a foaming bacterium in activated sludge [55], which could be a reason for the observed increase in the WWTP. Legionella spp. are commonly found in moist soil and water, and some Legionella species can cause community and hospital-acquired pneumonia [56]. In the past years, some industrial WWTPs in the Netherlands have shown to be a source for pneumonia caused by Legionella bacteria [57]. Leptospira spp., causing leptospirosis, which is associated with rainfall and flooding, can persist for several months in the environment without a host [58], which might explain why it persists in the WWTP as well. Pathogenic species belonging to these four genera might be problematic for public health if released into surface water in sufficient quantities. However, it should be mentioned that these findings are based on the genus level, and various non-pathogenic species belong to these four genera as well. Further research, i.e., by using whole genome sequencing techniques can provide more insight about the exact species included in these genera.
3.5. The Bacterial Composition Throughout the Year Only Differs in Surface Waters
Changes in bacterial phylum composition potentially caused by temperature changes were limited to surface water samples (Figure 5). For both the human gut phylum of Firmicutes and the environmental bacteria Cyanobacteria, the relative abundance did not significantly change in wastewater and effluent upon fluctuations in temperature, which is in concordance with a recent similar study [46]. The wastewater sources and influent showed a stable bacterial community structure. These waters are mainly dominated by human gut bacteria [16], which in healthy individuals is a stable community [59]. The stable bacterial community structure of effluent can be explained as effluent is mostly sourced by influent and activated sludge bacteria [16,40], which consists of a large part of core bacteria which are present all year round [60].
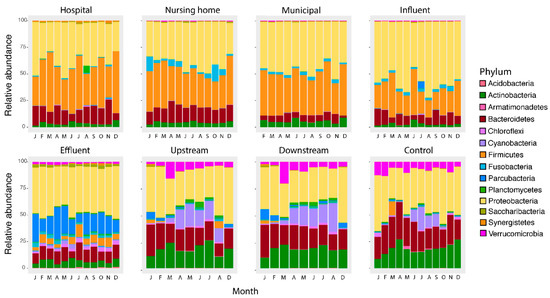
Figure 5.
Relative abundance at the Phylum level per location per month. The main groups in wastewaters (hospital, nursing home, municipal wastewater and influent) consist of Proteobacteria, Firmicutes, Bacteroidetes and Actinobacter, which are significantly changed after the wastewater treatment with Proteobacteria and Parcubacteria as main groups in the effluent. Surface waters are similar to each other but form a distinct group dominated by Proteobacteria, followed by Actinobacteria and Bacteroidetes as the main Phyla.
In contrast, temperature affected abundances in surface water. Still, this effect is limited to Cyanobacteria (up-, downstream, and control surface water, p < 0.001) and Planctomycetes (up- and downstream surface water, p < 0.001), in agreement with the role of temperature- and light intensity driven cyanobacterial growth [61]. In another study, temperature also had no substantial impact on the composition at the Phylum level in river water [62]. The reason why the influence of temperature was limited to Cyanobacteria and Planctomycetes can be explained as Cyanobacteria are photoautotrophic and therefore have a benefit to other bacteria. Higher densities of Planctomycetes are reported after cyanobacterial blooms, suggesting a possible association of this phylum with Cyanobacteria [63].
3.6. Culturing More Sensitive Than Sequencing to Detect Impact of This WWTP on Surface Water
In our previous work on the same water samples, an increase in the concentrations of E. coli was found in the surface water downstream from the WWTP in comparison to the WWTP upstream location and the control location, as determined by culture [12]. 16S rRNA gene amplicon sequencing was not able to detect any impact of the studied WWTP on the receiving surface water. Although most of the investigated genera had a decreased relative abundance in the WWTP, they were still present in at least 68% of the effluent samples, which are then released into the receiving surface water. However, their relative abundances did not differ between the upstream, downstream, and control surface water samples (Figure 4 and Supplementary Figures S3 and S4). In addition, up- and downstream samples clustered together in the UniFrac plots and did not significantly differ (Supplementary Figure S2 and Table S2a,b).
In the receiving surface water, effluent bacteria are mixed with environmental bacteria. Therefore, the relative abundance of effluent bacteria is reduced, making it more difficult to detect differences in effluent bacteria between surface waters up- and downstream from the WWTP. In a similar study, the difference in bacterial community structure between up and downstream water was also not significant [46]. In another comparable study, a difference between up- and downstream water bacterial composition was observed. However, in this study, the sampling was performed in summer when dilution of effluent in the receiving water was minimized [40]. Overall, the influence of WWTP effluent may differ per WWTP and depend on its degree of dilution with surface water. Methods other than 16S rRNA gene amplicon sequencing, such as bacterial enumeration of taxa present predominantly in effluent by culture, may be necessary to detect WWTP effects in situations when effluent is highly diluted.
This study provides insight in the microbial community structure in wastewaters obtained from different sources. It is the first study to highlight the differences in community structure between clinical and non-clinical wastewaters. Nevertheless, some limitations should be noted. The 16S rRNA gene amplicon sequencing does not allow for the strain-level resolution needed to specifically detect pathogenic (sub-) species. Thus, this study was primarily directed at generating hypotheses about pathogens that could potentially be enriched in clinical wastewaters. Follow-up studies applying different methods than 16S rRNA profiling are therefore needed to verify whether the pathogenic or potentially target taxa are indeed not enriched in clinical wastewaters. The 16S, furthermore, only provides information about relative abundances instead of absolute concentrations. However, comparisons with our previous study from which we obtained absolute counts of Klebsiella spp., and Aeromonas spp., showed a similar pattern of absolute concentrations and relative abundances along the wastewater pathway. Still, detection by absolute means (i.e., culturing or quantitative PCR (qPCR)) would be needed to confirm the decline observed in the WWTP treatment. Furthermore, the results of this study are limited to the particular characteristics of the sampled locations, i.e., the volume of wastewater originating from the sampled hospital and nursing home relative to the total community, the degree of use of medicines in the hospital and nursing home, the properties of the WWTP, and the dilution factor of WWTP effluent in the receiving surface water. Future comparisons between multiple studies will help elucidate the range that the impact of single healthcare institutions on the overall municipal wastewater can have.
4. Conclusions
In summary, this study provides new insights into shifts in bacterial community composition from wastewater sources to the environment. We found that clinical and non-clinical wastewaters significantly differ in their composition, but this difference was mainly caused by genera not included within the target taxa.
Clinical wastewater had little impact on the bacterial composition found in influent. From the 207 clinically enriched taxa, only 10 were more abundant in influent than in domestic wastewater; however, a part of these consisted of genera also included pathogenic genera. Most of the bacterial genera investigated in this study decreased in their relative abundance in the WWTP, except for Clostridium spp., Mycobacterium spp., Legionella spp., and Leptospira spp. Still, all taxa studied were detected in the majority of the effluent samples. For an assessment of the impact on public health, absolute concentrations of confirmed pathogens would be needed.
In this study, 16S rRNA gene amplicon sequencing was not sensitive enough to demonstrate the impact of the WWTP on the surface water, in contrast to previous culture-based analyses. Finally, in this study, temperature had a limited impact on the bacterial composition in surface water, and the impact on the bacterial composition in wastewater and effluent was negligible. Therefore, sampling campaigns studying microbial communities in wastewater might not necessarily have to cover all seasons. In conclusion, our results suggest a limited role of clinical wastewaters on the bacterial community in the receiving treatment plant. Furthermore, both culture- and DNA-based analyses should be combined to better elucidate the impact of WWTP effluents on the environment.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/microorganisms9040718/s1, Figure S1: Locations of the sampling points in Sneek, Table S1a: Statistical analysis of weighted UniFrac distances along the wastewater pathway, Table S1b: Statistical analysis of unweighted UniFrac distances along the wastewater pathway, Figure S2: Bacterial beta diversity off all the locations, Table S2a: Statistical analysis of weighted UniFrac distances along all the locations, Table S2b: Statistical analysis of unweighted UniFrac distances along all the locations, Figure S3: Target genera based on pathogenic potential, Table S3: Clinical enriched bacteria, Table S4: Clinical indicator bacteria in influent, Figure S4: Pathway of genera including clinical enriched genera, and Figure S5: ASV richness and Shannon diversity across all sites.
Author Contributions
Conceptualization, H.S., L.H.L., S.G.-C., K.W., A.W.F., H.P.J.v.V., J.W.A.R. and I.V.; methodology, H.S., S.G.-C., H.P.J.v.V. and I.V.; software, H.S., H.P.J.v.V. and I.V.; validation, I.V., H.S., S.G.-C. and H.P.J.v.V.; formal analysis, I.V., H.S., L.H.L., S.G.-C. and K.W.; investigation, I.V.; data curation, I.V. and H.P.J.v.V.; writing—original draft preparation, I.V.; writing—review and editing, I.V., H.S., L.H.L., K.W., S.G.-C., J.W.A.R. and A.W.F.; visualization, H.P.J.v.V., I.V., H.S., L.H.L. and S.G.-C.; supervision, J.W.A.R., H.S., L.H.L., S.G.-C. and K.W.; project administration, I.V. and L.H.L.; funding acquisition, L.H.L. and A.W.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was performed in the cooperation framework of Wetsus, European Centre of Excellence for Sustainable Water Technology (www.wetsus.nl, accessed on 1 March 2021). Wetsus is co-funded by the Dutch Ministry of Economic Affairs and Ministry of Infrastructure and Environment, the European Union Regional Development Fund, the Province of Fryslân and the Northern Netherlands Provinces. This work was partly supported by the INTERREG VA (202085) funded project EurHealth−1 Health, part of a Dutch–German cross-border network supported by the European Commission, the Dutch Ministry of Health, Welfare and Sport (VWS), the Ministry of Economy, Innovation, Digitalisation, and Energy of the German Federal State of North Rhine-Westphalia and the German Federal State of Lower Saxony.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Sequence data is available in the NCBI sequence read archive (SRA) under project numbers PRJNA668059 and PRJNA668064.
Acknowledgments
The authors would like to thank the participants of the research theme Source Sanitated Separation for the fruitful discussions and their financial support. Thanks to Wetterskip Fryslân, Gemeente Súdwest-Fryslân, Ielânen and the St. Antonius hospital for their cooperation and permissions for the sampling collection at their sites. Special thanks to Maarten van Putten for the practical support during the sequencing work.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- World Health Organization (WHO). Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014; Available online: https://scholar.google.com/scholar_lookup?title=Antimicrobial+Resistance+Global+Report+on+Surveillance&publication_year=2014& (accessed on 21 February 2018).
- Rice, L.B. Federal Funding for the Study of Antimicrobial Resistance in Nosocomial Pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef]
- Pendleton, J.N.; Gorman, S.P.; Gilmore, B.F. Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti-Infect. Ther. 2013, 11, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Raymond, F.; Ouameur, A.A.; Déraspe, M.; Iqbal, N.; Gingras, H.; Dridi, B.; Leprohon, P.; Plante, P.-L.; Giroux, R.; Bérubé, È.; et al. The initial state of the human gut microbiome determines its reshaping by antibiotics. ISME J. 2016, 10, 707–720. [Google Scholar] [CrossRef]
- Hocquet, D.; Muller, A.; Bertrand, X. What happens in hospitals does not stay in hospitals: Antibiotic-resistant bacteria in hospital wastewater systems. J. Hosp. Infect. 2016, 93, 395–402. [Google Scholar] [CrossRef]
- Sandegren, L. Selection of antibiotic resistance at very low antibiotic concentrations. Upsala J. Med. Sci. 2014, 119, 103–107. [Google Scholar] [CrossRef]
- Pituch, H.; Obuch-Woszczatyński, P.; Łuczak, M.; Meisel-Mikołajczyk, F. Clostridium difficile and enterotoxigenic Bacteroides fragilis strains isolated from patients with antibiotic associated diarrhoea. Anaerobe 2003, 9, 161–163. [Google Scholar] [CrossRef]
- Panda, S.; El Khader, I.; Casellas, F.; Vivancos, J.L.; Cors, M.G.; Santiago, A.; Cuenca, S.; Guarner, F.; Manichanh, C. Short-Term Effect of Antibiotics on Human Gut Microbiota. PLoS ONE 2014, 9, e95476. [Google Scholar] [CrossRef] [PubMed]
- Larcombe, S.; Hutton, M.L.; Lyras, D. Involvement of Bacteria Other Than Clostridium difficile in Antibiotic-Associated Diar-rhoea. Trends Microbiol. 2016, 24, 463–476. [Google Scholar] [CrossRef]
- SWAB (Dutch Working Party on Antibiotic Policy). Nethmap 2018—Consumption on Antimicrobial Agents and Antimicrobial Resistance among Medically Important Bacteria in The Netherlands; RIVM: Bilthoven, The Netherlands, 2018.
- Verburg, I.; García-Cobos, S.; Leal, L.H.; Waar, K.; Friedrich, A.W.; Schmitt, H. Abundance and Antimicrobial Resistance of Three Bacterial Species along a Complete Wastewater Pathway. Microorganisms 2019, 7, 312. [Google Scholar] [CrossRef] [PubMed]
- Paulus, G.K.; Hornstra, L.M.; Alygizakis, N.; Slobodnik, J.; Thomaidis, N.; Medema, G. The impact of on-site hospital wastewater treatment on the downstream communal wastewater system in terms of antibiotics and antibiotic resistance genes. Int. J. Hyg. Environ. Health 2019, 222, 635–644. [Google Scholar] [CrossRef]
- Buelow, E.; Bayjanov, J.R.; Majoor, E.; Willems, R.J.L.; Bonten, M.J.M.; Schmitt, H.; Van Schaik, W. Limited influence of hospital wastewater on the microbiome and resistome of wastewater in a community sewerage system. FEMS Microbiol. Ecol. 2018, 94. [Google Scholar] [CrossRef]
- Amador, P.P.; Fernadez, R.M.; Prudêncio, M.C.; Barreto, M.P.; Duarte, I.M. Antibiotic resistance in wastewater: Occurrence and fate of Enterobacteriaceae producers of class A and class C beta-lactamases. J. Environ. Sci. Health A Tox Hazard. Subst. Environ. Eng. 2015, 50, 26–39. [Google Scholar] [CrossRef]
- Cai, L.; Ju, F.; Zhang, T. Tracking human sewage microbiome in a municipal wastewater treatment plant. Appl. Microbiol. Biotechnol. 2013, 98, 3317–3326. [Google Scholar] [CrossRef]
- Numberger, D.; Ganzert, L.; Zoccarato, L.; Mühldorfer, K.; Sauer, S.; Grossart, H.-P.; Greenwood, A.D. Characterization of bacterial communities in wastewater with enhanced taxonomic resolution by full-length 16S rRNA sequencing. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Pruden, A.; Ashbolt, N.; Miller, J. Overview of issues for water bacterial pathogens. In Water and Sanitation for the 21st Century: Health and Microbiological Aspects of Excreta and Wastewater Management (Global Water Pathogen Project); Michigan State University Press: East Lansing, MI, USA, 2019. [Google Scholar]
- Li, T.; Abebe, L.S.; Cronk, R.; Bartram, J. A systematic review of waterborne infections from nontuberculous mycobacteria in health care facility water systems. Int. J. Hyg. Environ. Health 2017, 220, 611–620. [Google Scholar] [CrossRef] [PubMed]
- O’Dwyer, J.; Dowling, A.; Adley, C. The Impact of Climate Change on the Incidence of Infectious Waterborne Disease. Urban Water Reuse Handbook; CRC Press: Boca Raton, FL, USA, 2016; pp. 1053–1062. [Google Scholar]
- Falkinham, J.O.; Pruden, A.; Edwards, M. Opportunistic Premise Plumbing Pathogens: Increasingly Important Pathogens in Drinking Water. Pathogens 2015, 4, 373–386. [Google Scholar] [CrossRef]
- Cann, K.F.; Thomas, D.R.; Salmon, R.L.; Wyn-Jones, A.P.; Kay, D. Extreme water-related weather events and waterborne disease. Epidemiol. Infect. 2012, 141, 671–686. [Google Scholar] [CrossRef] [PubMed]
- Veeningen, R. and F. van der Meer, Geneesmiddelen in het oppervlaktewater: Standaarden bij de monitoring en prioritering van ’ hotspots’: [thema] nieuwe verontreinigingen. Bodem Kwart. Inf. Uitwisseling Discuss. Bodem-Bescherm. Bodemsaner. 2015, 25, 31–34. [Google Scholar]
- Illumina, 16S Metagenomic Sequencing Library Preparation; Preparing 16S Ribosomal RNA Gene Amplicons for the Illumina MiSeq System. 2013. Available online: https://www.illumina.com/content/dam/illumina-support/documents/documentation/chemistry_documentation/16s/16s-metagenomic-library-prep-guide-15044223-b.pdf (accessed on 21 February 2018).
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2—Approximately Maximum-Likelihood Trees for Large Alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.; Knight, R. UniFrac: A New Phylogenetic Method for Comparing Microbial Communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Hamady, M.; Kelley, S.T.; Knight, R. Quantitative and qualitative beta diversity measures lead to different insights into factors that structure microbial communities. Appl. Environ. Microbiol. 2007, 73, 1576–1585. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.4-2. 2017. Available online: https://cran.r-project.org/package=vegan (accessed on 30 March 2021).
- Hervé, M. RVAideMemoire: Testing and Plotting Procedures for Biostatistics. R Package Version 0.9-73. 2019. Available online: https://CRAN.R-project.org/package=RVAideMemoire (accessed on 23 January 2020).
- Pohlert, T. The Pairwise Multiple Comparison of Mean Ranks Package (PMCMR). R Package. 2014. Available online: http://CRAN.R-project.org/package=PMCMR (accessed on 23 January 2020).
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate—A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. Waste Not, Want Not: Why Rarefying Microbiome Data Is Inadmissible. PLoS Comput. Biol. 2014, 10, e1003531. [Google Scholar] [CrossRef]
- Zhang, W.; Bougouffa, S.; Wang, Y.; Lee, O.O.; Yang, J.; Chan, C.; Song, X.; Qian, P.-Y. Toward Understanding the Dynamics of Microbial Communities in an Estuarine System. PLoS ONE 2014, 9, e94449. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.T.; Marotz, C.; Washburne, A.; Silverman, J.; Zaramela, L.S.; Edlund, A.; Zengler, K.; Knight, R. Establishing microbial composition measurement standards with reference frames. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Quintela-Baluja, M.; Abouelnaga, M.; Romalde, J.; Su, J.-Q.; Yu, Y.; Gomez-Lopez, M.; Smets, B.; Zhu, Y.-G.; Graham, D.W. Spatial ecology of a wastewater network defines the antibiotic resistance genes in downstream receiving waters. Water Res. 2019, 162, 347–357. [Google Scholar] [CrossRef] [PubMed]
- McLellan, S.L.; Roguet, A. The unexpected habitat in sewer pipes for the propagation of microbial communities and their imprint on urban waters. Curr. Opin. Biotechnol. 2019, 57, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Newton, R.J.; McLellan, S.L.; Dila, D.K.; Vineis, J.H.; Morrison, H.G.; Eren, A.M.; Sogin, M.L. Sewage Reflects the Microbiomes of Human Populations. mBio 2015, 6, e02574. [Google Scholar] [CrossRef]
- Zwart, G.; Crump, B.C.; Agterveld, M.P.K.-V.; Hagen, F.; Han, S.-K. Typical freshwater bacteria: An analysis of available 16S rRNA gene sequences from plankton of lakes and rivers. Aquat. Microb. Ecol. 2002, 28, 141–155. [Google Scholar] [CrossRef]
- Wei, Y.-M.; Wang, J.-Q.; Liu, T.-T.; Kong, W.-W.; Chen, N.; He, X.-Q.; Jin, Y. Bacterial communities of Beijing surface waters as revealed by 454 pyrosequencing of the 16S rRNA gene. Environ. Sci. Pollut. Res. 2015, 22, 12605–12614. [Google Scholar] [CrossRef]
- Jin, D.; Kong, X.; Cui, B.; Jin, S.; Xie, Y.; Wang, X.; Deng, Y. Bacterial communities and potential waterborne pathogens within the typical urban surface waters. Sci. Rep. 2018, 8, 13368. [Google Scholar] [CrossRef]
- Buelow, E.; Rico, A.; Gaschet, M.; Lourenco, J.; Kennedy, S.P.; Wiest, L.; Ploy, M.; Dagot, C. Classification of hospital and urban wastewater resistome and microbiota over time and their relationship to the eco-exposome. bioXriv 2019. biorxiv:10.1101/697433v2. [Google Scholar]
- Gupta, V.K.; Paul, S.; Dutta, C. Geography, Ethnicity or Subsistence-Specific Variations in Human Microbiome Composition and Diversity. Front. Microbiol. 2017, 8, 1162. [Google Scholar] [CrossRef]
- Vila, A.V.; Collij, V.; Sanna, S.; Sinha, T.; Imhann, F.; Bourgonje, A.R.; Mujagic, Z.; Jonkers, D.M.A.E.; Masclee, A.A.M.; Fu, J.; et al. Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Otte, E.; Nielsen, H.L.; Hasman, H.; Fuglsang-Damgaard, D. First report of metronidazole resistant, nimD-positive, Bacteroides stercoris isolated from an abdominal abscess in a 70-year-old woman. Anaerobe 2017, 43, 91–93. [Google Scholar] [CrossRef]
- Collado, L.; Figueras, M.J. Taxonomy, Epidemiology, and Clinical Relevance of the Genus Arcobacter. Clin. Microbiol. Rev. 2011, 24, 174–192. [Google Scholar] [CrossRef]
- Tong, Y.; Marion, T.; Schett, G.; Luo, Y.; Liu, Y. Microbiota and metabolites in rheumatic diseases. Autoimmun. Rev. 2020, 19, 102530. [Google Scholar] [CrossRef]
- Lopetuso, L.R.; Scaldaferri, F.; Petito, V.; Gasbarrini, A. Commensal clostridia: Leading players in the maintenance of gut homeostasis. Gut Pathog. 2013, 5, 1–23. [Google Scholar] [CrossRef]
- Pillai, S.D.; Widmer, K.W.; Dowd, S.E.; Ricke, S.C. Occurrence of airborne bacteria and pathogen indicators during land application of sewage sludge. Appl. Environ. Microbiol. 1996, 62, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Pfyffer, G.E. Mycobacterium: General Characteristics, Laboratory Detection, and Staining Procedures; 15.6 Packing and Shipping Infectious Substances; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 536–569. [Google Scholar]
- Guo, F.; Zhang, T. Profiling bulking and foaming bacteria in activated sludge by high throughput sequencing. Water Res. 2012, 46, 2772–2782. [Google Scholar] [CrossRef]
- Diederen, B. Legionella spp. and Legionnaires’ disease. J. Infect. 2008, 56, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lodder, W.J.; van den Berg, H.H.J.L.; van Leerdam, R.C.; de Roda Husman, A.M. Potentiële maatregelen tegen verspreiding van Legionella uit afvalwaterzuiveringsinstallaties. Rijksinst. Volksgezond. Milieu (RIVM) 2019. [Google Scholar] [CrossRef]
- Levett, P.N. Leptospirosis. Clin. Microbiol. Rev. 2001, 14, 296–326. [Google Scholar] [CrossRef] [PubMed]
- Sommer, F.; Anderson, J.M.; Bharti, R.; Raes, J.; Rosenstiel, P. The resilience of the intestinal microbiota influences health and disease. Nat. Rev. Microbiol. 2017, 15, 630–638. [Google Scholar] [CrossRef]
- Johnston, J.; LaPara, T.; Behrens, S. Composition and Dynamics of the Activated Sludge Microbiome during Seasonal Nitrifica-tion Failure. Sci. Rep. 2019, 9, 4565. [Google Scholar] [CrossRef] [PubMed]
- Battchikova, N.; Angeleri, M.; Aro, E.-M. Proteomic approaches in research of cyanobacterial photosynthesis. Photosynth. Res. 2014, 126, 47–70. [Google Scholar] [CrossRef] [PubMed]
- Kaevska, M.; Videnska, P.; Sedlar, K.; Slana, I. Seasonal changes in microbial community composition in river water studied using 454-pyrosequencing. SpringerPlus 2016, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Eiler, A.; Bertilsson, S. Composition of freshwater bacterial communities associated with cyanobacterial blooms in four Swedish lakes. Environ. Microbiol. 2014, 6, 1228–1243. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).