Diversity of Aerobic Anoxygenic Phototrophs and Rhodopsin-Containing Bacteria in the Surface Microlayer, Water Column and Epilithic Biofilms of Lake Baikal
Abstract
1. Introduction
2. Materials and Methods
3. Results
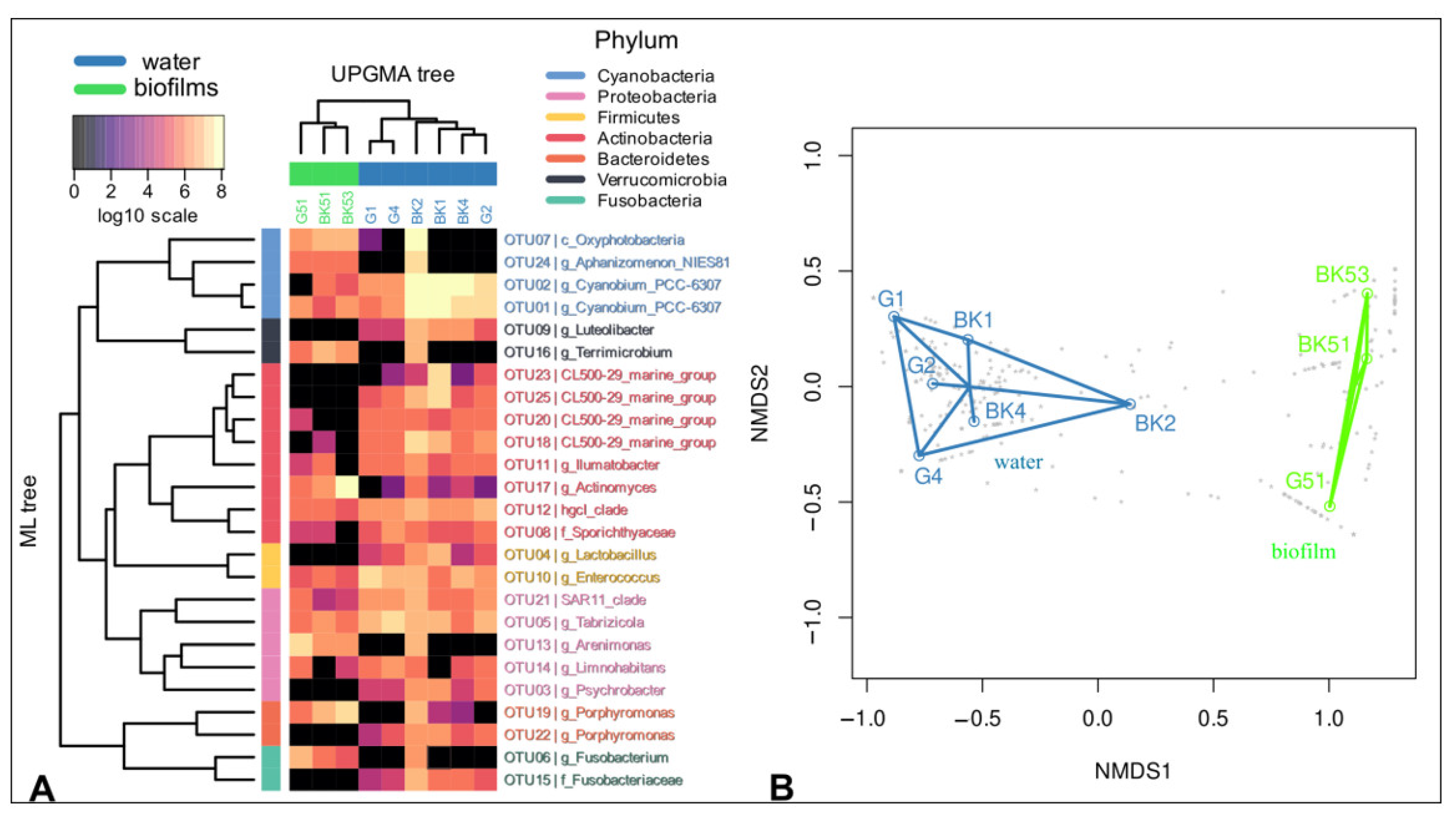
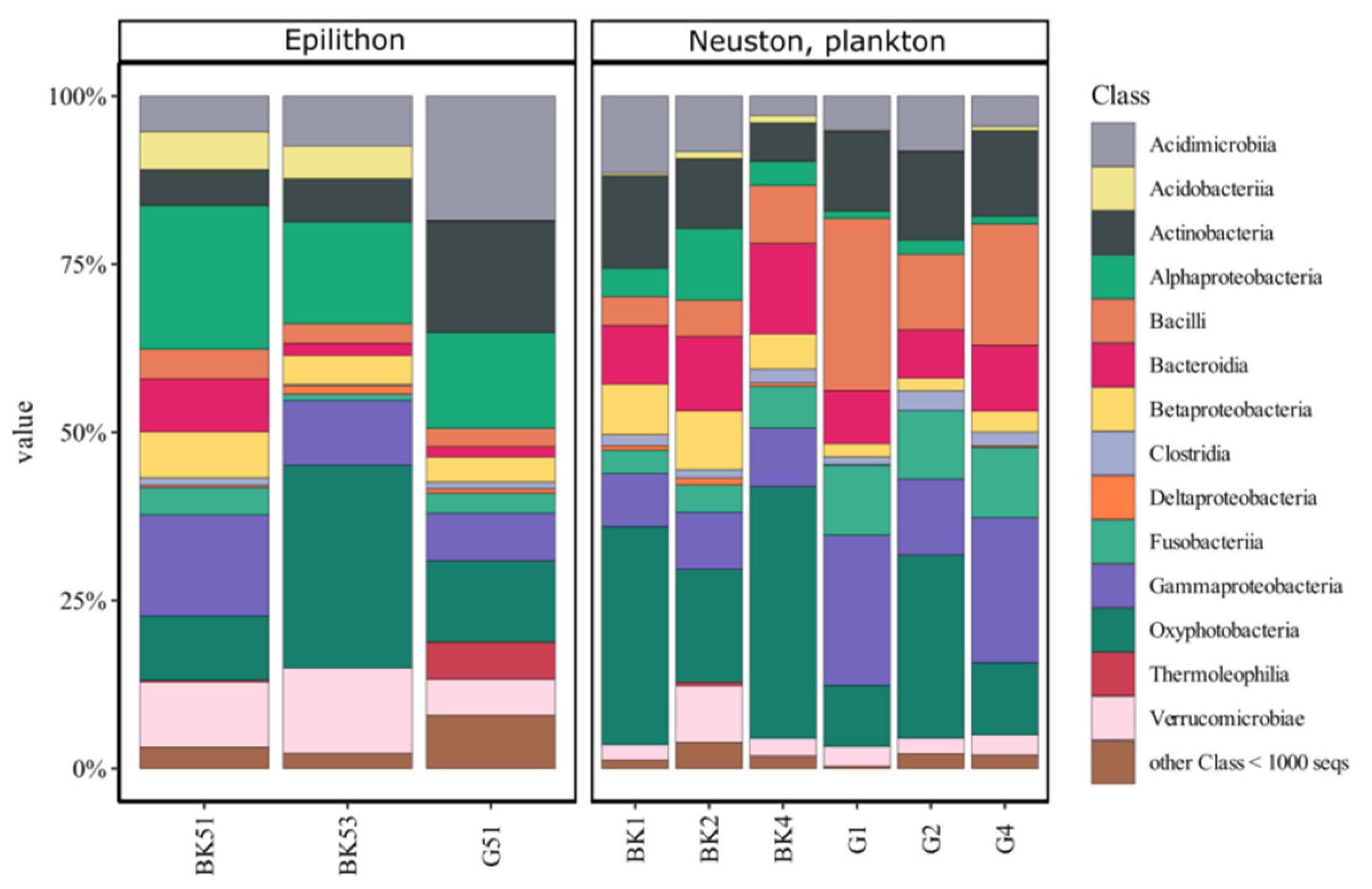
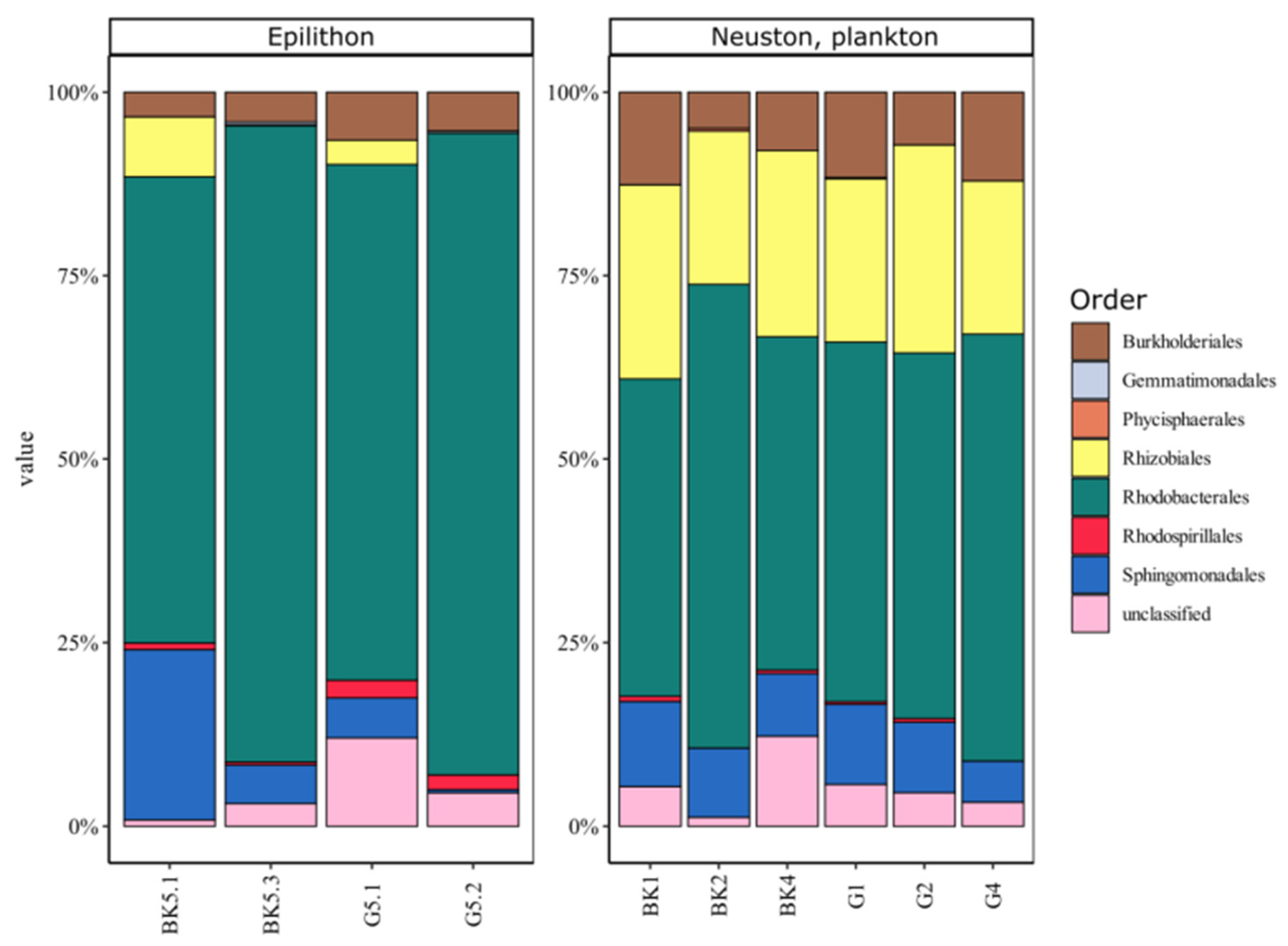
3.1. 16S rRNA
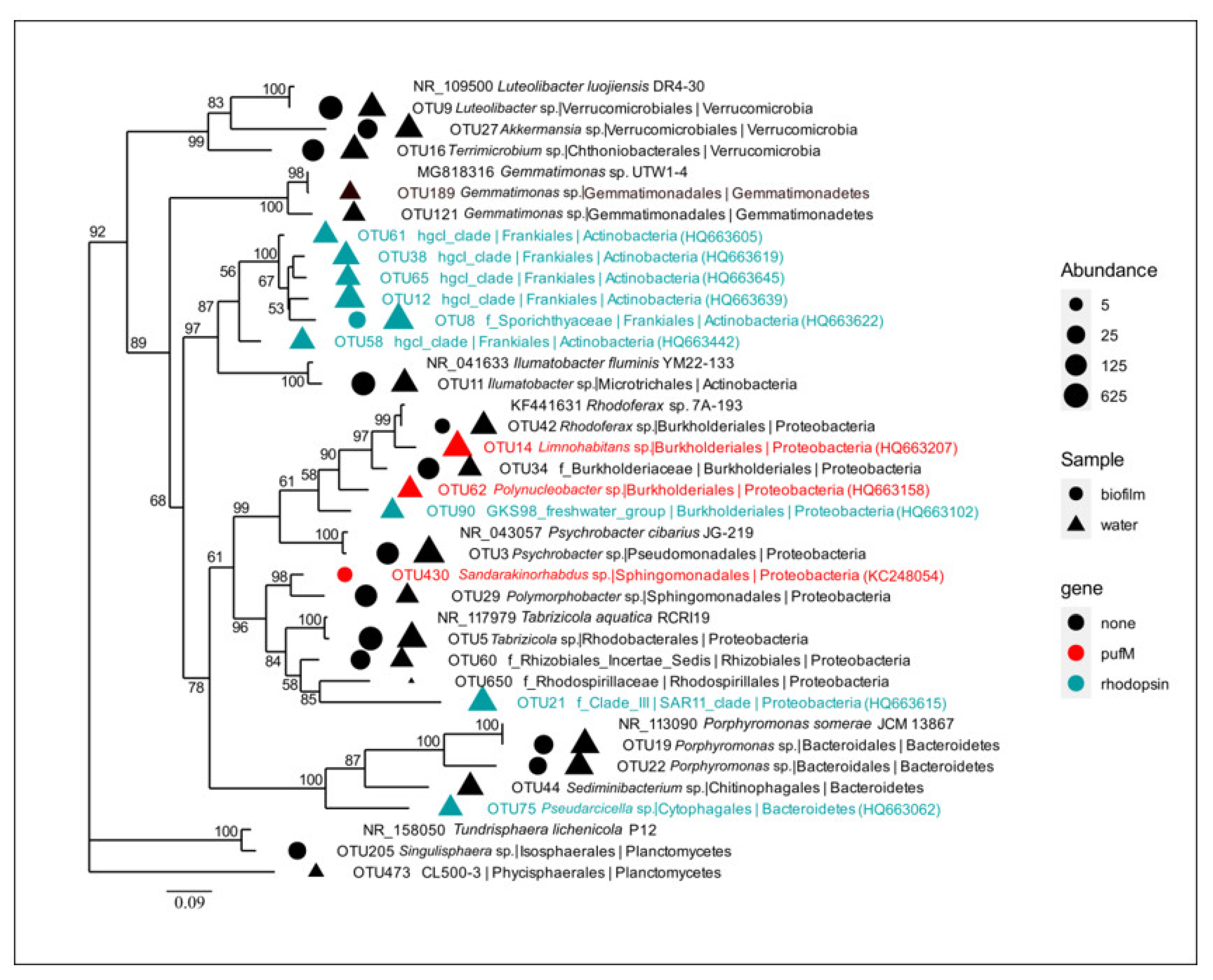
3.2. Aerobic Anoxygenic Phototrophs
3.3. Rhodopsin-Containing Bacteria
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Béjà, O.; Suzuki, M.T.; Heidelberg, J.F.; Nelson, W.C.; Preston, C.M.; Hamada, T.; Eisen, J.A.; Fraser, C.M.; DeLong, E.F. Unsuspected diversity among marine aerobic anoxygenic phototrophs. Nature 2002, 415, 630–633. [Google Scholar] [CrossRef]
- Yurkov, V.V.; Beatty, J.T. Aerobic anoxygenic phototrophic bacteria. Microbiol. Mol. Biol. Rev. 1998, 62, 695–724. [Google Scholar] [CrossRef] [PubMed]
- Yurkov, V.; Csotonyi, J.T. New light on aerobic anoxygenic phototrophs. In The Purple Phototrophic Bacteria. Advances in PhotoSynthesis and Respiration; Hunter, C.N., Daldal, F., Thurnauer, M.C., Beatty, J.T., Eds.; Springer: Dordrecht, The Netherlands, 2009; Volume 28, pp. 31–55. [Google Scholar] [CrossRef]
- Yurkov, V.V.; Hughes, E. Chapter eleven—Genes associated with the peculiar phenotypes of the aerobic anoxygenic phototrophs. Adv. Bot. Res. 2013, 66, 327–358. [Google Scholar] [CrossRef]
- Yurkov, V.; Hughes, E. Aerobic anoxygenic phototrophs: Four decades of mystery. In Modern Topics in the Phototrophic Prokaryotes; Hallenbeck, P., Ed.; Springer: Cham, Switzerland, 2017; pp. 193–214. [Google Scholar] [CrossRef]
- Béjà, O.; Aravind, L.; Koonin, E.V.; Suzuki, M.T.; Hadd, A.; Nguyen, L.P.; Jovanovich, S.B.; Gates, C.M.; Feldman, R.A.; Spudich, J.L.; et al. Bacterial rhodopsin: Evidence for a new type of phototrophy in the sea. Science 2000, 289, 1902–1906. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Consarnau, L.; Akram, N.; Lindell, K.; Pedersen, A.; Neutze, R.; Milton, D.L.; González, J.M.; Pinhassi, J. Proteorhodopsin phototrophy promotes survival of marine bacteria during starvation. PLoS Biol. 2010, 8. [Google Scholar] [CrossRef] [PubMed]
- Pinhassi, J.; DeLong, E.F.; Béjà, O.; González, J.M.; Pedrós-Alió, C. Marine bacterial and archaeal ion-pumping rhodopsins: Genetic diversity, physiology, and ecology. Microbiol. Mol. Biol. Rev. 2016, 80, 929–954. [Google Scholar] [CrossRef]
- Kirchman, D.L.; Hanson, T.E. Bioenergetics of photoheterotrophic bacteria in the oceans. Environ. Microbiol. Rep. 2013, 5, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Koblížek, M. Ecology of aerobic anoxygenic phototrophs in aquatic environments. FEMS Microbiol. Rev. 2015, 39, 854–870. [Google Scholar] [CrossRef] [PubMed]
- Harashima, K.; Shiba, T.; Murata, N. Aerobic Photosynthetic Bacteria; Scientific Societies Press: Tokyo, Japan, 1989. [Google Scholar]
- Yurkov, V.V.; Gorlenko, V.M. Erythrobacter sibiricus sp. nov., a new freshwater aerobic species containing bacteriochlorophyll a. Mikrobiologiya 1990, 59, 120–126. (In English) [Google Scholar]
- Yurkov, V.V.; Gorlenko, V.M. New species of aerobic bacteria from the genus Erythromicrobium containing bacteriochlorophyll a. Mikrobiologiya 1992, 61, 248–255. (In English) [Google Scholar]
- Salka, I.; Cŭperová, Z.; Mašín, M.; Koblížek, M.; Grossart, H.P. Rhodoferax-related pufM gene cluster dominates the aerobic anoxygenic phototrophic communities in German freshwater lakes. Environ. Microbiol. 2011, 13, 2865–2875. [Google Scholar] [CrossRef] [PubMed]
- Cŭperová, Z.; Holzer, E.; Salka, I.; Sommaruga, R.; Koblízek, M. Temporal changes and altitudinal distribution of aerobic anoxygenic phototrophs in mountain lakes. Appl. Environ. Microbiol. 2013, 79, 6439–6446. [Google Scholar] [CrossRef]
- Caliz, J.; Casamayor, E.O. Environmental controls and composition of anoxygenic photoheterotrophs in ultraoligotrophic high-altitude lakes (Central Pyrenees). Environ. Microbiol. Rep. 2014, 6, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Hirose, S.; Matsuura, K.; Haruta, S. Phylogenetically diverse aerobic anoxygenic phototrophic bacteria isolated from epilithic biofilms in Tama River, Japan. Microbes Environ. 2016, 31, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wu, X.; Zhou, Q.; Donde, O.O.; Tian, C.; Wang, C.; Feng, B.; Xiao, B. Distribution of aerobic anoxygenic phototrophs in freshwater plateau lakes. Pol. J. Environ. Stud. 2018, 27, 1–9. [Google Scholar] [CrossRef]
- Kolesár Fecskeová, L.; Piwosz, K.; Hanusová, M.; Nedoma, J.; Znachor, P.; Koblížek, M. Diel changes and diversity of pufM expression in freshwater communities of anoxygenic phototrophic bacteria. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Tarhriz, V.; Hirose, S.; Fukushima, S.I.; Hejazi, M.A.; Imhoff, J.F.; Thiel, V.; Hejazi, M.S. Emended description of the genus Tabrizicola and the species Tabrizicola aquatica as aerobic anoxygenic phototrophic bacteria. Antonie Van Leeuwenhoek 2019, 112, 1169–1175. [Google Scholar] [CrossRef]
- Kasalický, V.; Zeng, Y.; Piwosz, K.; Šimek, K.; Kratochvilová, H.; Koblížek, M. Aerobic anoxygenic photosynthesis is commonly present within the genus Limnohabitans. Appl. Environ. Microbiol. 2018, 84. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, M.; Swan, B.K.; Poulton, N.J.; Gomez, M.L.; Masland, D.; Sieracki, M.E.; Stepanauskas, R. High-throughput single-cell sequencing identifies photoheterotrophs and chemoautotrophs in freshwater bacterioplankton. ISME J. 2012, 6, 113–123. [Google Scholar] [CrossRef]
- Zeng, A.; Koblížek, M. Phototrophic Gemmatimonadetes: A new “purple” branch of the bacterial tree of life. In Modern Topics in the Phototrophic Prokaryotes: Environmental and Applied Aspects; Hallenbeck, P.C., Ed.; Springer: Cham, Switzerland, 2017; pp. 163–192. [Google Scholar] [CrossRef]
- Fauteux, L.; Cottrell, M.T.; Kirchman, D.L.; Borrego, C.M.; Garcia-Chaves, M.C.; del Giorgio, P.A. Patterns in abundance, cell size and pigment content of aerobic anoxygenic phototrophic bacteria along environmental gradients in northern lakes. PLoS ONE 2015, 10, e0124035. [Google Scholar] [CrossRef]
- Hojerová, E.; Mašín, M.; Brunet, C.; Ferrera, I.; Gasol, J.M.; Koblížek, M. Distribution and growth of aerobic anoxygenic phototrophs in the Mediterranean Sea. Environ. Microbiol. 2011, 13, 2717–2725. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, M.T.; Mannino, A.; Kirchman, D.L. Aerobic anoxygenic phototrophic bacteria in the Mid-Atlantic Bight and the North Pacific Gyre. Appl. Environ. Microbiol. 2006, 72, 557–564. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oesterhelt, D.; Stoeckenius, W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat. New Biol. 1971, 233, 149–152. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, J.R.; Christianson, L.M.; Béjà, O.; Suzuki, M.T.; Karl, D.M.; Heidelberg, J.; DeLong, E.F. Proteorhodopsin genes are distributed among divergent marine bacterial taxa. Proc. Natl. Acad. Sci. USA 2003, 100, 12830–12835. [Google Scholar] [CrossRef]
- Giovannoni, S.J.; Bibbs, L.; Cho, J.-C.; Stapels, M.D.; Desiderio, R.; Vergin, K.L.; Rappé, M.S.; Laney, S.; Wilhelm, L.J.; Tripp, H.J.; et al. Proteorhodopsin in the ubiquitous marine bacterium SAR11. Nature 2005, 438, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Atamna-Ismaeel, N.; Sabehi, G.; Sharon, I.; Witzel, K.P.; Labrenz, M.; Jürgens, K.; Barkay, T.; Stomp, M.; Huisman, J.; Béjà, O. Widespread distribution of proteorhodopsins in freshwater and brackish ecosystems. ISME J. 2008, 2, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.J.; Waidner, L.A.; Cottrell, M.T.; Kirchman, D.L. Abundant proteorhodopsin genes in the North Atlantic Ocean. Environ. Microbiol. 2008, 10, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Bohorquez, L.C.; Ruiz-Pérez, C.A.; Zambrano, M.M. Proteorhodopsin-like genes present in thermoacidophilic high-mountain microbial communities. Appl. Environ. Microbiol. 2012, 78, 7813–7817. [Google Scholar] [CrossRef] [PubMed]
- Sabehi, G.; Massana, R.; Bielawski, J.P.; Rosenberg, M.; Delong, E.F.; Béjà, O. Novel proteorhodopsin variants from the Mediterranean and Red Seas. Environ. Microbiol. 2003, 5, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Sabehi, G.; Loy, A.; Jung, K.H.; Partha, R.; Spudich, J.L.; Isaacson, T.; Hirschberg, J.; Wagner, M.; Béjà, O. New insights into metabolic properties of marine bacteria encoding proteorhodopsins. PLoS Biol. 2005, 3. [Google Scholar] [CrossRef]
- Sharma, A.K.; Zhaxybayeva, O.; Papke, R.T.; Doolittle, W.F. Actinorhodopsins: Proteorhodopsin-like gene sequences found predominantly in non-marine environments. Environ. Microbiol. 2008, 10, 1039–1056. [Google Scholar] [CrossRef]
- Sharma, A.K.; Sommerfeld, K.; Bullerjahn, G.S.; Matteson, A.R.; Wilhelm, S.W.; Jezbera, J.; Brandt, U.; Doolittle, W.F.; Hahn, M.W. Actinorhodopsin genes discovered in diverse freshwater habitats and among cultivated freshwater Actinobacteria. ISME J. 2009, 3, 726–737. [Google Scholar] [CrossRef]
- Galazi, G.I. Lake Baikal: Atlas; Roskartografiya: Omsk, Russia, 1993; p. 7.
- Galachyants, A.D.; Bel’kova, N.L.; Sukhanova, E.V.; Blinov, V.V.; Parfenova, V.V. Methods of neuston sampling for the quantitative characteristicof microbial communities of Lake Baikal. Inland Water Biol. 2016, 9, 329–336. [Google Scholar] [CrossRef]
- Maniatis, T.; Fritsch, E.F.; Sambrock, J. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor: New York, NY, USA, 1984. [Google Scholar]
- Wetzel, R.G.; Likens, G.E. Limnological Analyses; Springer: New York, NY, USA, 2000; pp. 85–111. [Google Scholar]
- Bolleter, W.T.; Bushman, C.J.; Tidwell, P.W. Spectrophotometric determination of ammonia as indophenols. Anal. Chem. 1961, 33, 592–594. [Google Scholar] [CrossRef]
- Khodzher, T.V.; Domysheva, V.M.; Sorokovikova, L.M.; Golobokova, L.P. Methods for monitoring the chemical composition of Lake Baikal water. In Novel Methods for Monitoring and Managing Land and Water Resources in Siberia; Mueller, L., Sheudshen, A.K., Eulenstein, F., Eds.; Springer: Cham, Switzerland, 2016; pp. 113–132. [Google Scholar]
- Kahlert, M.; Hasselrot, A.T.; Hillebrand, H.; Pettersson, K. Spatial and temporal variation in the biomass and nutrient status of epilithic algae in Lake Erken, Sweden. Freshw. Biol. 2002, 47, 1191–1215. [Google Scholar] [CrossRef]
- Liess, A.; Drakare, S.; Kahlert, M. Atmospheric nitrogen-deposition may intensify phosphorus limitation of shallow epilithic periphyton in unproductive lakes. Freshw. Biol. 2009, 54, 1759–1773. [Google Scholar] [CrossRef]
- Qin, P.; Mayer, C.M.; Schulz, K.L.; Ji, X.; Ritchie, M.E. Ecological stoichiometry in benthic food webs: Effect of light and nutrients on periphyton food quantity and quality in lakes. Limnol. Oceanogr. 2007, 52, 1728–1734. [Google Scholar] [CrossRef]
- Martemyanov, V.V.; Belousova, I.A.; Pavlushin, S.V.; Dubovskiy, I.M.; Ershov, N.I.; Alikina, T.Y.; Kabilov, M.R.; Glupov, V.V. Phenological asynchrony between host plant and gypsy moth reduces insect gut microbiota and susceptibility to Bacillus thuringiensis. Ecol. Evol. 2016, 6, 7298–7310. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and Metagenomic sequencing platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef]
- Allgaier, M.; Uphoff, H.; Felske, A.; Wagner-Döbler, I. Aerobic anoxygenic photosynthesis in Roseobacter clade bacteria from diverse marine habitats. Appl. Environ. Microbiol. 2003, 69, 5051–5059. [Google Scholar] [CrossRef]
- Oz, A.; Sabehi, G.; Koblížek, M.; Massana, R.; Béjà, O. Roseobacter-like bacteria in Red and Mediterranean sea aerobic anoxygenic photosynthetic populations. Appl. Environ. Microbiol. 2005, 71, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Yutin, N.; Suzuki, M.T.; Béjà, O. Novel primers reveal wider diversity among marine aerobic anoxygenic phototrophs. Appl. Environ. Microbiol. 2005, 71, 8958–8962. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high- throughput sequencing reads. EMBnet J. 2011, 17. [Google Scholar] [CrossRef]
- Callahan, B.; McMurdie, P.; Rosen, M.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Posada, D. jModelTest: Phylogenetic model averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef]
- Suchard, M.A.; Lemey, P.; Baele, G.; Ayres, D.L.; Drummond, A.J.; Rambaut, A. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol. 2018, 4. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarization in bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Simpson, G.L.; Solymos, P.R.; Stevens, M.H.H.; Wagner, H. The Vegan Package. Community Ecology Package Version 1.15-1 10.631-637, 2007; License GPL-2. Available online: https://www.um.es/docencia/geobotanica/ficheros/programas/vegan.pdf (accessed on 11 March 2021).
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria 2013. Available online: http://www.R-project.org/ (accessed on 11 March 2021).
- Belykh, O.I.; Tikhonova, I.V.; Krasnopeev, A.Y.; Galachyants, A.D.; Sukhanova, E.V.; Suslova, M.Y.; Gorshkova, A.S.; Gevorgyan, G.; Potapov, S.A. Characterization of photoautotrophic microbial communities in the coastal water of Lake Baikal. Limnol. Freshw. Biol. 2020, 3, 966–968. [Google Scholar] [CrossRef]
- Galach’yants, A.D.; Bel’kova, N.L.; Sukhanova, E.V.; Galach’yants, Y.P.; Morozov, A.A.; Parfenova, V.V. Taxonomic composition of Lake Baikal bacterioneuston communities. Microbiology 2017, 86, 241–249. [Google Scholar] [CrossRef]
- Mikhailov, I.S.; Zakharova, Y.R.; Galachyants, Y.P.; Usoltseva, M.V.; Petrova, D.P.; Sakirko, M.V.; Likhoshway, Y.V.; Grachev, M.A. Similarity of structure of taxonomic bacterial communities in the photic layer of Lake Baikal’s three basins differing in spring phytoplankton composition and abundance. Dokl. Biochem. Biophys. 2015, 465, 413–419. [Google Scholar] [CrossRef]
- Mikhailov, I.S.; Bukin, Y.S.; Zakharova, Y.R.; Usoltseva, M.V.; Galachyants, Y.P.; Sakirko, M.V.; Blinov, V.V.; Likhoshway, Y.V. Co-occurrence patterns between phytoplankton and bacterioplankton across the pelagic zone of Lake Baikal during spring. J. Microbiol. 2019, 57, 252–262. [Google Scholar] [CrossRef]
- Kurilkina, M.I.; Zakharova, Y.R.; Galachyants, Y.P.; Petrova, D.P.; Bukin, Y.S.; Domysheva, V.M.; Blinov, V.V.; Likhoshway, Y.V. Bacterial community composition in the water column of the deepest freshwater Lake Baikal as determined by next-generation sequencing. FEMS Microbiol. Ecol. 2016, 92, fiw094. [Google Scholar] [CrossRef]
- Newton, R.J.; Jones, S.E.; Eiler, A.; McMahon, K.D.; Bertilsson, S. A guide to the natural history of freshwater lake bacteria. Microbiol. Mol. Biol. Rev. 2011, 75, 14–49. [Google Scholar] [CrossRef]
- Zwart, G.; Crump, B.C.; Kamst-van Agterveld, M.P.; Hagen, F.; Han, S.-K. Typical freshwater bacteria: An analysis of available 16S rRNA gene sequences from plankton of lakes and rivers. Aquat. Microb. Ecol. 2002, 28, 141–155. [Google Scholar] [CrossRef]
- Hecky, R.E.; Hesslein, R.H. Contributions of benthic algae to lake food webs as revealed by stable isotope analysis. J. N. Am. Bentholl. Soc. 1995, 14, 631–653. [Google Scholar] [CrossRef]
- Romaní, A.M.; Guasch, H.; Muñoz, I.; Ruana, J.; Vilalta, E.; Schwartz, T.; Emtiazi, F.; Sabater, S. Biofilm structure and function and possible implications for riverine DOC dynamics. Microb. Ecol. 2004, 47, 316–328. [Google Scholar] [CrossRef]
- Parfenova, V.V.; Gladkikh, A.S.; Belykh, O.I. Comparative analysis of biodiversity in the planktonic and biofilm bacterial communities in Lake Baikal. Microbiology 2013, 82, 91–101. [Google Scholar] [CrossRef]
- Sorokovikova, E.G.; Belykh, O.I.; Gladkikh, A.S.; Kotsar, O.V.; Tikhonova, I.V.; Timoshkin, O.A.; Parfenova, V.V. Diversity of cyanobacterial species and phylotypes in biofilms from the littoral zone of Lake Baikal. J. Microbiol. 2013, 51, 757–765. [Google Scholar] [CrossRef]
- Bartrons, M.; Catalan, J.; Casamayor, E.O. High bacterial diversity in epilithic biofilms of oligotrophic mountain lakes. Microb. Ecol. 2012, 64, 860–869. [Google Scholar] [CrossRef]
- Romero, F.; Acuña, V.; Sabater, S. Multiple stressors determine community structure and estimated function of river biofilm bacteria. Appl. Environ. Microbiol. 2020, 86. [Google Scholar] [CrossRef]
- Nagata, T.; Takai, K.; Kawanobe, K.; Kim, D.-S.; Nakazato, R.; Guselnikova, N.; Bondarenko, N.; Mologawaya, O.; Kostrnova, T.; Drucker, V.; et al. Autotrophic picoplankton in southern Lake Baikal: Abundance, growth and grazing mortality during summer. J. Plankton Res. 1994, 16, 945–959. [Google Scholar] [CrossRef]
- Belykh, O.I.; Sorokovikova, E.G. Autotrophic picoplankton in Lake Baikal: Abundance, dynamics, and distribution. Aquat. Ecosyst. Health Manag. 2003, 6, 251–261. [Google Scholar] [CrossRef]
- Belykh, O.I.; Gladkikh, A.S.; Sorokovikova, E.G.; Tikhonova, I.V.; Potapov, S.A.; Butina, T.V. Saxitoxin-producing cyanobacteria in Lake Baikal. Contemp. Probl. Ecol. 2015, 8, 186–192. [Google Scholar] [CrossRef]
- Horňák, K.; Kasalický, V.; Šimek, K.; Grossart, H.P. Strain-specific consumption and transformation of alga-derived dissolved organic matter by members of the Limnohabitans-C and Polynucleobacter-B clusters of Betaproteobacteria. Environ. Microbiol. 2017, 19, 4519–4535. [Google Scholar] [CrossRef] [PubMed]
- Madigan, M.T.; Jung, D.O. An Overview of purple bacteria: Systematics, physiology, and habitats. In The Purple Phototrophic Bacteria. Advances in Photosynthesis and Respiration; Hunter, C.N., Daldal, F., Thurnauer, M.C., Beatty, J.T., Eds.; Springer: Dordrecht, The Netherlands, 2009; Volume 28, pp. 1–15. [Google Scholar] [CrossRef]
- Kalyuzhnaya, M.G.; Bowerman, S.; Lara, J.C.; Lidstrom, M.E.; Chistoserdova, L. Methylotenera mobilis gen. nov., sp. nov., an obligately methylamine-utilizing bacterium within the family Methylophilaceae. Int. J. Syst. Evol. Microbiol. 2006, 56, 2819–2823. [Google Scholar] [CrossRef]
- Morris, R.M.; Rappe, M.S.; Connon, S.A.; Vergin, K.L.; Siebold, W.A.; Carlson, C.A.; Giovannoni, S.J. SAR11 clade dominates ocean surface bacterioplankton communities. Nature 2002, 420, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Tahon, G.; Willems, A. Isolation and characterization of aerobic anoxygenic phototrophs from exposed soils from the Sør Rondane Mountains, East Antarctica. Syst. Appl. Microbiol. 2017, 40, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Bowman, J.P.; Nichols, C.M.; Gibson, J.A.E. Algoriphagus ratkowskyi gen. nov., sp. nov., Brumimicrobium glaciale gen. nov., sp. nov., Cryomorpha ignava gen. nov., sp. nov. and Crocinitomix catalasitica gen. nov., sp. nov., novel flavobacteria isolated from various polar habitats. Int. J. Syst. Evol. Microbiol. 2003, 53, 1343–1355. [Google Scholar] [CrossRef]
- De Wever, A.; Van der Gucht, K.; Muylaert, K.; Cousin, S.; Vyverman, W. Clone library analysis reveals an unusual composition and strong habitat partitioning of pelagic bacterial communities in Lake Tanganyika. Aquat. Microb. Ecol. 2008, 50, 113–122. [Google Scholar] [CrossRef][Green Version]
- Lindström, E.S.; Vrede, K.; Leskinen, E. Response of a member of the Verrucomicrobia, among the dominating bacteria in a hypolimnion, to increased phosphorus availability. J. Plankton Res. 2004, 26, 241–246. [Google Scholar] [CrossRef]
- Pascual, J.; García-López, M.; González, I.; Genilloud, O. Luteolibacter gellanilyticus sp. nov., a gellan-gum-degrading bacterium of the phylum Verrucomicrobia isolated from miniaturized diffusion chambers. Int. J. Syst. Evol. Microbiol. 2017, 67, 3951–3959. [Google Scholar] [CrossRef] [PubMed]
- Jovel, J.; Patterson, J.; Wang, W.; Hotte, N.; O’Keefe, S.; Mitchel, T.; Perry, T.; Kao, D.; Mason, A.L.; Madsen, K.L.; et al. Characterization of the gut microbiome using 16S or shotgun metagenomics. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Suslova, M.Y.; Pestunova, O.S.; Parfenova, V.V. Water quality assessment in the Selenga river and its delta in terms of sanitary and microbiological indices. Hydrobiol. J. 2017, 53, 70–81. [Google Scholar] [CrossRef]
- Shi, L.; Cai, Y.; Chen, Z.; Zhou, Y.; Li, P.; Kong, F. Diversity and abundance of aerobic anoxygenic phototrophic bacteria in two cyanobacterial bloom-forming lakes in China. Ann. Limnol. Int. J. Lim. 2010, 46, 233–239. [Google Scholar] [CrossRef]
- Piwosz, K.; Vrdoljak, A.; Frenken, T.; González-Olalla, J.M.; Šantić, D.; McKay, R.M.; Spilling, K.; Guttman, L.; Znachor, P.; Mujakić, I.; et al. Light and primary production shape bacterial activity and community composition of aerobic anoxygenic phototrophic bacteria in a microcosm experiment. mSphere 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, S.; Jogler, M.; Jogler, C. On the maverick Planctomycetes. FEMS Microbiol. Rev. 2018, 42, 739–760. [Google Scholar] [CrossRef]
- Zeng, Y.; Chen, X.; Madsen, A.M.; Zervas, A.; Nielsen, T.K.; Andrei, A.-S.; Lund-Hansen, L.C.; Liu, Y.; Hansen, L.H. Potential rhodopsin- and bacteriochlorophyll-based dual phototrophy in a high Arctic glacier. mBio 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.-X.; He, Y.-B.; Wang, M.-H.; Zhang, S.-F.; Kong, L.-F.; Lin, L.; Liu, S.-Q.; Wang, D.-Z. Dissecting microbial community structure and metabolic activities at an oceanic deep chlorophyll maximum layer by size-fractionated metaproteomics. Prog. Oceanogr. 2020, 188. [Google Scholar] [CrossRef]
- Shiba, T.; Simidu, U. Erythrobacter longus gen. nov., sp. nov., an aerobic bacterium which contains bacteriochlorophyll a. Int. J. Syst. Bacteriol. 1982, 32, 211–217. [Google Scholar] [CrossRef]
- Yabuuchi, E.; Kosako, Y.; Fujiwara, N.; Naka, T.; Matsunaga, I.; Ogura, H.; Kobayashi, K. Emendation of the genus Sphingomonas Yabuuchi et al. 1990 and junior objective synonymy of the species of three genera, Sphingobium, Novosphingobium and Sphingopyxis, in conjunction with Blastomonas ursincola. Int. J. Syst. Evol. Microbiol. 2002, 52, 1485–1496. [Google Scholar] [CrossRef]
- Xiao, N.; Liu, Y.; Liu, X.; Gu, Z.; Jiao, N.; Liu, H.; Zhou, Y.; Shen, L. Blastomonas aquatica sp. nov., a bacteriochlorophyll-containing bacterium isolated from lake water. Int. J. Syst. Evol. Microbiol. 2015, 65, 1653–1658. [Google Scholar] [CrossRef] [PubMed]
- Tarhriz, V.; Thiel, V.; Nematzadeh, G.; Hejazi, M.A.; Imhoff, J.F.; Hejazi, M.S. Tabrizicola aquatica gen. nov. sp. nov., a novel alphaproteobacterium isolated from Qurugol Lake nearby Tabriz city, Iran. Antonie Van Leeuwenhoek 2013, 104, 1205–1215. [Google Scholar] [CrossRef]
- Phurbu, D.; Wang, H.; Tang, Q.; Lu, H.; Zhu, H.; Jiang, S.; Xing, P.; Wu, Q.L. Tabrizicola alkalilacus sp. nov., isolated from alkaline Lake Dajiaco on the Tibetan Plateau. Int. J. Syst. Evol. Microbiol. 2019, 69, 3420–3425. [Google Scholar] [CrossRef] [PubMed]
- Sheu, C.; Li, Z.H.; Sheu, S.Y.; Yang, C.C.; Chen, W.M. Tabrizicola oligotrophica sp. nov. and Rhodobacter tardus sp. nov., two new species of bacteria belonging to the family Rhodobacteraceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 6266–6283. [Google Scholar] [CrossRef] [PubMed]
- Haro-Moreno, J.M.; Rodriguez-Valera, F.; López-Pérez, M. Prokaryotic population dynamics and viral predation in a marine succession experiment using metagenomics. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Vigneron, A.; Cruaud, P.; Mohit, V.; Martineau, M.-J.; Culley, A.I.; Lovejoy, C.; Vincent, W.F. Multiple strategies for light-harvesting, photoprotection, and carbon flow in high latitude microbial mats. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef]
- Rieck, A.; Herlemann, D.P.R.; Jürgens, K.; Grossart, H.-P. Particle-associated differ from free-living bacteria in surface waters of the Baltic Sea. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef]




| Sample | PO43−, mg/L | NH4+, mg/L | NO2−, mg/L | NO3−, mg/L | N Total, mg/L | TOC, mg C/L | P Total, mg/L | T, °C |
|---|---|---|---|---|---|---|---|---|
| BK1 | 0.023 | 0.0125 | 0.0025 | 0.075 | 0.031 | 35.3 | 0.0225 | 13.7 |
| BK2 | 0.008 | 0.002 | 0.001 | 0.08 | 0.022 | 5.8 | 0.007 | 10.0 |
| BK4 | 0.016 | 0.004 | 0.001 | 0.29 | 0.073 | 5.4 | 0.008 | 6.0 |
| G1 | 0.018 | 0.0085 | 0.0005 | 0.115 | 0.0315 | 3.1 | 0.015 | 15.4 |
| G2 | 0.007 | 0.004 | 0.002 | 0.12 | 0.034 | 3.8 | 0.007 | 14.0 |
| G4 | 0.009 | 0.007 | 0.002 | 0.17 | 0.046 | 3.7 | 0.007 | 12.0 |
| Sample | N Total, mg/cm2 | TOC, mg C/cm2 | P Total, mg/cm2 |
|---|---|---|---|
| BK51 + BK53 | 0.6 | 4.2 | 0.053 |
| G51 + G52 | 0.4 | 2.7 | 0.024 |
| ESV | Genus | Number of Seqs in Water Microbiomes | Number of Seqs in Epilithon | Closest Homologue Accession Number |
|---|---|---|---|---|
| 278, 860, 1839, 2009 | Tabrizicola | 0 | 19507 | QBQ34549 |
| 150, 2012, 2249 | Erythrobacter | 0 | 4033 | AGK27892 |
| 2758 | Blastomonas | 4 | 1572 | AGK27877 |
| 1430 | Blastomonas | 0 | 411 | AGK27877 |
| 3609 | Sphingomonas | 0 | 855 | AGK27878 |
| Microbial Community | S.chao1 | Shannon | Simpson |
|---|---|---|---|
| BK1 | 49 | 3.2 | 0.9 |
| BK2 | 40 | 2.9 | 0.9 |
| BK4 | 46 | 3.2 | 0.9 |
| BK51 | 43 | 3.2 | 0.9 |
| BK53 | 35 | 2.8 | 0.9 |
| G1 | 47 | 3.1 | 0.9 |
| G2 | 50 | 3.0 | 0.9 |
| G4 | 34 | 2.7 | 0.9 |
| G51 | 65 | 3.5 | 1.0 |
| G52 | 52 | 3.0 | 0.9 |
| ESV | Genus | Number of Seqs in Water Microbiomes | Number of Seqs in Epilithon | Closest Homologue Accession Number |
|---|---|---|---|---|
| 1901, 2299 | Aquirufa | 821 | 0 | WP_130895144 |
| 678 | Runella | 0 | 1330 | TAG64876 |
| 1360 | Runella | 703 | 0 | TAG64876 |
| Microbial Community | S.chao1 | Shannon | Simpson |
|---|---|---|---|
| BK1 | 29 | 3.1 | 0.9 |
| BK2 | 10 | 2.1 | 0.8 |
| BK4 | 70 | 4.0 | 1.0 |
| BK51 | 61 | 3.8 | 1.0 |
| BK53 | 70 | 4.1 | 1.0 |
| G1 | 133 | 4.6 | 1.0 |
| G2 | 26 | 3.0 | 0.9 |
| G4 | 26 | 2.9 | 0.9 |
| G51 | 20 | 2.7 | 0.9 |
| G52 | 46 | 3.5 | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galachyants, A.D.; Krasnopeev, A.Y.; Podlesnaya, G.V.; Potapov, S.A.; Sukhanova, E.V.; Tikhonova, I.V.; Zimens, E.A.; Kabilov, M.R.; Zhuchenko, N.A.; Gorshkova, A.S.; et al. Diversity of Aerobic Anoxygenic Phototrophs and Rhodopsin-Containing Bacteria in the Surface Microlayer, Water Column and Epilithic Biofilms of Lake Baikal. Microorganisms 2021, 9, 842. https://doi.org/10.3390/microorganisms9040842
Galachyants AD, Krasnopeev AY, Podlesnaya GV, Potapov SA, Sukhanova EV, Tikhonova IV, Zimens EA, Kabilov MR, Zhuchenko NA, Gorshkova AS, et al. Diversity of Aerobic Anoxygenic Phototrophs and Rhodopsin-Containing Bacteria in the Surface Microlayer, Water Column and Epilithic Biofilms of Lake Baikal. Microorganisms. 2021; 9(4):842. https://doi.org/10.3390/microorganisms9040842
Chicago/Turabian StyleGalachyants, Agnia Dmitrievna, Andrey Yurjevich Krasnopeev, Galina Vladimirovna Podlesnaya, Sergey Anatoljevich Potapov, Elena Viktorovna Sukhanova, Irina Vasiljevna Tikhonova, Ekaterina Andreevna Zimens, Marsel Rasimovich Kabilov, Natalia Albertovna Zhuchenko, Anna Sergeevna Gorshkova, and et al. 2021. "Diversity of Aerobic Anoxygenic Phototrophs and Rhodopsin-Containing Bacteria in the Surface Microlayer, Water Column and Epilithic Biofilms of Lake Baikal" Microorganisms 9, no. 4: 842. https://doi.org/10.3390/microorganisms9040842
APA StyleGalachyants, A. D., Krasnopeev, A. Y., Podlesnaya, G. V., Potapov, S. A., Sukhanova, E. V., Tikhonova, I. V., Zimens, E. A., Kabilov, M. R., Zhuchenko, N. A., Gorshkova, A. S., Suslova, M. Y., & Belykh, O. I. (2021). Diversity of Aerobic Anoxygenic Phototrophs and Rhodopsin-Containing Bacteria in the Surface Microlayer, Water Column and Epilithic Biofilms of Lake Baikal. Microorganisms, 9(4), 842. https://doi.org/10.3390/microorganisms9040842







