Seroprevalence and Risk Factors of Brucella Infection in Dairy Animals in Urban and Rural Areas of Bihar and Assam, India
Abstract
1. Introduction
2. Materials and Methods
2.1. Data and Sampling Procedure
2.2. Biological Sample Collection and Laboratory Analysis
2.3. Data Analyses
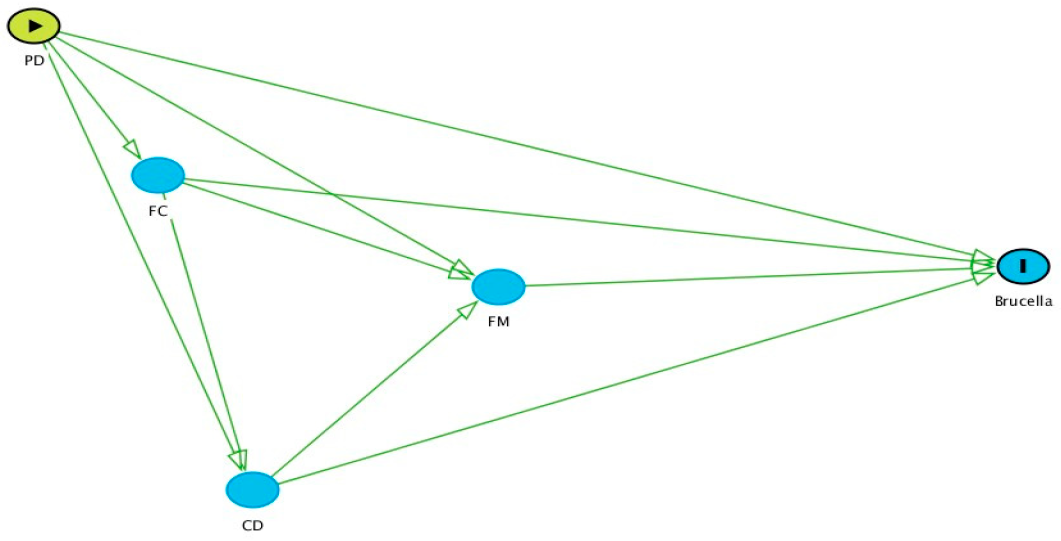
2.4. Criteria Followed for Selection of the Variables for Multivariable Analysis
- For all pairs of variables within a group (e.g., FC, FM, etc.) in which both variables in the pair were either continuous or dichotomous, correlations were computed;
- For all pairs of variables within a group (e.g., FC, FM, etc.) in which one variable was continuous and the other was categorical (the continuous variable was considered as the outcome and the categorical variable was converted to a set of dummy variables), R2 and a p-value were computed using linear regression;
- For all pairs of variables within a group (e.g., FC, FM, etc.) in which one variable was categorical and the other variable was either categorical or dichotomous, a p-value was computed using a Chi-square test.
- A variable that did not have a plausible biological relationship to the outcome variable was excluded;
- A variable that did not have substantial variability (e.g., species of dairy animals) was excluded;
- A variable that had more than 10% missing values was excluded;
- If correlation between two variables was greater than 0.7 or R2 > 0.5 (as computed above), the following selection criteria were used to exclude one variable in favour of the other: (1) the variable that had a weaker association with outcome variable was excluded, (2) the variable that had higher missing value was excluded, (3) the variable for which it was relatively difficult to collect accurate data was excluded;
- A variable that had no significant association with the outcome variable (p > 0.3) was excluded.
2.5. Steps Followed for Model Building for Multivariable Analyses
- Separate models were built for each group of variables (i.e., PD, FC, CD, and FM).
- Following the causal diagram, initially, an analysis was conducted using variables in the PD group. This was followed by the FC, CD, and FM groups in sequence. For each group, significant variables from the antecedent groups (i.e., to the left of the group of interest) were retained to see if there was any confounding effect. No variables from subsequent groups were included, as these would be intervening (intermediate) variables. For example, when analysing variables in the CD group, significant variables from the PD and FC models were retained, but all variables in the FM group were excluded.
- During analyses, variables were manually eliminated one at a time. The variable for elimination was selected based on its p-value, absence of evidence of confounding effects (i.e., changes in the magnitude of other coefficients), and the plausibility of its causal association with Brucella seropositivity.
- A variable was dropped from the analysis if it had strong collinearity with another variable that had more plausible association with Brucella seropositivity.
- In the final model, only significant variables (p < 0.1) identified through multivariable regression analysis were kept, along with the pre-selected confounders, if any. A cut point of p < 0.1 was chosen in light of the relatively small sample size and low prevalence of Brucella seropositivity.
3. Results
3.1. Brief Profile of Farming System and Dairy Animals
3.2. Laboratory Results of Serum Samples
3.3. Univariable Analysis
3.4. Multivariable Analysis of Risk Factors
4. Discussion
4.1. Sero-Prevalence
4.2. Risk Factors
4.3. Comments on Study Design
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization; Food and Agricultural Organization; World Organization for Animal Health. Brucellosis in Humans and Animals; World Health Organization Press: Geneva, Switzerland, 2006. [Google Scholar]
- McDermott, J.; Grace, D.; Zinsstag, J. Economics of brucellosis impact and control in low-income countries. Rev. Sci. Tech. l’OIE 2013, 32, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Ul-Islam, M.R.; Gupta, M.P.; Filia, G.; Sidhu, P.K.; Shafi, T.A.; Bhat, S.A.; Hussain, S.A.; Mustafa, R.; Verma, A.K.; Sinha, D.K. Seroepidemiology of brucellosis in organized cattle and buffaloes in Punjab, India. Adv. Anim. Vet. Sci. 2013, 1, 5–8. [Google Scholar]
- World Organization for Animal Health. OIE Bovine Brucellosis: OIE Terrestrial Manual; World Organization for Animal Health Press: Rome, Italy, 2009. [Google Scholar]
- Rajala, E.L. Brucella in Tajikistan: Zoonotic Risks of Urbanized Livestock in a Low-Income Country. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2016. [Google Scholar]
- Godfroid, J.; Scholz, H.; Barbier, T.; Nicolas, C.; Wattiau, P.; Fretin, D.; Whatmore, A.; Cloeckaert, A.; Blasco, J.; Moriyon, I.; et al. Brucellosis at the animal/ecosystem/human interface at the beginning of the 21st century. Prev. Veter. Med. 2011, 102, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Londhe, S.P.; Bannalikar, A.S.; Dighe, V.D. Serodetection of bovine brucellosis by RBPT and AB-ELISA. Anim. Sci. Rep. 2011, 5, 69–73. [Google Scholar]
- Nielsen, K.; Yu, W.L. Serological diagnosis of brucellosis. Prilozi 2010, 31, 65–89. [Google Scholar]
- Deka, R.P.; Magnusson, U.; Grace, D.; Lindahl, J. Bovine brucellosis: Prevalence, risk factors, economic cost and control options with particular reference to India: A review. Infect. Ecol. Epidemiol. 2018, 8, 1556548. [Google Scholar] [CrossRef]
- Deka, R.P.; Magnusson, U.; Grace, D.; Shome, R.; Lindahl, J.F. Knowledge and practices of dairy farmers relating to brucellosis in urban, periurban and rural areas of Assam and Bihar, India. Infect. Ecol. Epidemiol. 2020, 10, 1769531. [Google Scholar] [CrossRef]
- Naing, L.; Winn, T.; Rusli, B. Practical Issues in Calculationg the Sample Size Calculator for Prevalence Studies. Archv. Orofac. Sci. 2006, 1, 9–14. [Google Scholar]
- Sharma, V.P.; Delgado, C.L.; Stall, S.; Singh, R.V. Annex III: Livestock Industrialization Project: Phase II—Policy, Technical, and Environmental Determinants and Implications of the Scaling-Up of Milk Production in India; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003. [Google Scholar]
- International Livestock Research Institute. Comprehensive Study of the Assam Dairy Sector: Action Plan for Pro-Poor Dairy Development; International Livestock Research Institute: Nairobi, Kenya, 2007. [Google Scholar]
- Department of Home Affairs. Census of India. In Handbook of Dist. Census, Kamrup Metropolitan, Assam; Series-12; Government of India: New Delhi, India, 2011. [Google Scholar]
- Department of Animal Husbandry and Dairying. National Action Plan for Dairy Development; Vision-2022; Press Information Bureau Government of India: New Delhi, India, 2018.
- Renukaradhya, G.; Isloor, S.; Rajasekhar, M. Epidemiology, zoonotic aspects, vaccination and control/eradication of brucellosis in India. Veter. Microbiol. 2002, 90, 183–195. [Google Scholar] [CrossRef]
- Pandian, S.J.; Ray, P.K.; Chandran, P.C.; Kumar, M. Seroprevalence of Brucella abortus and Leptospira hardjo in cattle. Veter. World 2015, 8, 217–220. [Google Scholar] [CrossRef]
- Kaushik, P.; Singh, D.K.; Tiwari, A.K. Comparison of PCR with conventional techniques for the diagnosis of brucellosis in cattle. Indian J. Anim. Sci. 2010, 80, 326–328. [Google Scholar]
- Bhattacharya, H.; Ahmed, D.K.; Rahman, H. Studies on sero prevalence of bovine brucellosis by different tests. J. Vet. Public Health 2005, 3, 131–133. [Google Scholar]
- Gogoi, S.B.; Hussain, P.; Sarma, P.C.; Barua, A.G.; Mahato, G.; Bora, D.P.; Konch, P.; Gogoi, P. Prevalence of bovine brucellosis in Assam, India. J. Entomol. Zool. Stud. 2017, 5, 179–185. [Google Scholar]
- Aulakh, H.K.; Patil, P.K.; Sharma, S.; Kumar, H.; Mahajan, V.; Sandhu, K.S. A Study on the Epidemiology of Bovine Brucellosis in Punjab (India) Using Milk-ELISA. Acta Veter. Brno 2008, 77, 393–399. [Google Scholar] [CrossRef]
- Muma, J.; Samui, K.; Oloya, J.; Munyeme, M.; Skjerve, E. Risk factors for brucellosis in indigenous cattle reared in livestock–wildlife interface areas of Zambia. Prev. Veter. Med. 2007, 80, 306–317. [Google Scholar] [CrossRef]
- Mugizi, D.R.; Boqvist, S.; Nasinyama, G.W.; Waiswa, C.; Ikwap, K.; Rock, K.; Lindahl, E.; Magnusson, U.; Erume, J. Prevalence of and factors associated with Brucella sero-positivity in cattle in urban and peri-urban Gulu and Soroti towns of Uganda. J. Veter. Med Sci. 2015, 77, 557–564. [Google Scholar] [CrossRef]
- Lindahl, J.F.; Gill, J.P.S.; Hazarika, R.A.; Fairoze, N.M.; Bedi, J.S.; Dohoo, I.; Chauhan, A.S.; Grace, D.; Kakkar, M. Risk Factors for Brucella Seroprevalence in Peri-Urban Dairy Farms in Five Indian Cities. Trop. Med. Infect. Dis. 2019, 4, 70. [Google Scholar] [CrossRef]
- Makita, K.; Fèvre, E.M.; Waiswa, C.; Eisler, M.C.; Thrusfield, M.; Welburn, S.C. Herd prevalence of bovine brucellosis and analysis of risk factors in cattle in urban and peri-urban areas of the Kampala economic zone, Uganda. BMC Veter. Res. 2011, 7, 60. [Google Scholar] [CrossRef]
- Lindahl, E.; Sattorov, N.; Boqvist, S.; Sattori, I.; Magnusson, U. Seropositivity and risk factors for Brucella in dairy cows in urban and pe-ri-urban small-scale farming in Tajikistan. Trop. Anim. Health Prod. 2014, 46, 563–569. [Google Scholar] [CrossRef][Green Version]
- Kumar, N.; Pal, B.C.; Yadav, S.K.; Verma, A.K.; Jain, U.; Yadav, G. Prevalence of bovine brucellosis in Uttar Pradesh, India. J. Vet. Public Health 2009, 7, 129–131. [Google Scholar]
- Jagapur, R.; Rathore, R.; Karthik, K.; Somavanshi, R. Seroprevalence studies of bovine brucellosis using indirect-enzyme-linked immuno-sorbent assay (i-ELISA) at organized and unorganized farms in three. Vet. World 2013, 6, 550–553. [Google Scholar] [CrossRef]
- Anka, M.S.; Hassan, L.; Khairani-Bejo, S.; Zainal, M.A. A Case-Control Study of Risk Factors for Bovine Brucellosis Seropositivity in Peninsular Malaysia. PLoS ONE 2014, 9, e108673. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Gupta, V.K.; Verma, A.K.; Sahzad; Kumar, V.; Singh, A.; Reddy, N.C.P. Seroprevalence and risk factors associated with bovine brucellosis Uttar Pradesh, India. Indian J. Anim. Sci. 2016, 86, 131–135. [Google Scholar]
- Coelho, A.; Coelho, A.; Roboredo, M.; Rodrigues, J. A case–control study of risk factors for brucellosis seropositivity in Portuguese small ruminants herds. Prev. Veter. Med. 2007, 82, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Wolff, C.; Boqvist, S.; Ståhl, K.; Masembe, C.; Sternberg-Lewerin, S. Biosecurity aspects of cattle production in Western Uganda, and associations with seroprevalence of brucellosis, salmonellosis and bovine viral diarrhoea. BMC Veter. Res. 2017, 13, 382. [Google Scholar] [CrossRef] [PubMed]
- Sagamiko, F.; Muma, J.; Karimuribo, E.; Mwanza, A.; Sindato, C.; Hang’Ombe, B. Sero-prevalence of Bovine Brucellosis and associated risk factors in mbeya region, Southern highlands of Tanzania. Acta Trop. 2018, 178, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Shome, R.; Shankaranarayan, B.; Krithiga, N.; Triveni, K.M.; Sahays, S.; Shome, B.R.; Singh, P.; Rahman, H. Bovine brucellosis in organised farms of Indian assessment of diagnostic assays and risk factors. Adv. Anim. Vet. Sci. 2014, 2, 557–564. [Google Scholar] [CrossRef]
- Boukary, A.R.; Saegerman, C.; Abatih, E.; Fretin, D.; Alambédji Bada, R.; De Deken, R.; Harouna, H.A.; Yenikoye, A.; Thys, E. Seroprevalence and Potential Risk Factors for Brucella Spp. Infection in Traditional Cattle, Sheep and Goats Reared in Urban, Periurban and Rural Areas of Niger. PLoS ONE 2013, 8, e83175. [Google Scholar] [CrossRef]
- Ali, S.; Akhter, S.; Neubauer, H.; Melzer, F.; Khan, I.; Abatih, E.N.; El-Adawy, H.; Irfan, M.; Muhammad, A.; Akbar, M.W.; et al. Seroprevalence and risk factors associated with bovine brucellosis in the Potohar Plateau, Pakistan. BMC Res. Notes 2017, 10, 1–11. [Google Scholar] [CrossRef]
- Patel, M.D.; Patel, P.R.; Prajapati, M.G.; Kanani, A.N.; Tyagi, K.K.; Fulsoundar, A.B. Prevalence and risk factor’s analysis of bovine brucellosis in peri-urban areas under intensive system of production in Gujarat, India. Veter. World 2014, 7, 509–516. [Google Scholar] [CrossRef]

| Variables | Particulars | Bihar | Assam | Total | p-Value |
|---|---|---|---|---|---|
| Mean Herd Size per Farm (± standard error (SE)) | 2.8 ± 0.2 | 4.1 ± 0.4 | 3.4 ± 0.2 | 0.009 | |
| Breed of the animals surveyed | High-producing (exotic or crossbred) | 345/376 (91.8) * | 186/364 (51.1) | 531/740 (71.8) | <0.001 |
| Non-descript indigenous | 31/376 (8.2) | 178/364 (48.9) | 209/740 (28.2) | ||
| Species of the animals | Cattle | 354/376 (94.1) | 364/364 (100.0) | 718/740 (97.0) | <0.001 |
| Buffalo | 22/376 (5.9) | 0/364 | 22/740 (2.9) | ||
| Mean age of animals surveyed (± SE) | 4.7 ± 0.1 | 6.2 ± 0.1 | 5.4 ± 0.1 | <0.001 | |
| Breeding method followed by households | Followed artificial insemination (AI) | 266/292 (91.1) | 128/242 (52.9) | 394/534 (73.8) | <0.001 |
| Variables | Particulars | Kamrup (Metropolitan) | Golaghat | Baska | p-Value |
|---|---|---|---|---|---|
| Mean herd size of dairy animals | Per farm | 8.8 ± 1.1 | 1.7 ± 0.1 | 1.6 ± 0.1 | 0.01 |
| Rearing system | Fully stall-fed | 141/178 (79.2) * | 9/105 (8.6) | 6/94 (6.4) | <0.001 |
| Partly stall-fed | 37/178 (20.8) | 96/105 (91.4) | 88/94 (93.6) | ||
| Breed | Improved | 148/176 (84.1) | 18/96 (18.7) | 20/92 (21.7) | <0.001 |
| Non-descript | 28/176 (15.9) | 78/96 (81.2) | 72/92 (78.3) | ||
| Adoption of AI | Yes | 152/178 (85.4) | 45/105 (42.8) | 34/94 (36.2) | <0.001 |
| Animal movement | Yes | 39/178 (21.9) | 96/105 (91.4) | 90/94 (95.7) | <0.001 |
| New animal introduced | Yes | 71/178 (39.9) | 6/105 (5.7) | 4/94 (4.2) | <0.001 |
| Animals belonging to trained farmers | Yes | 41/178 (23.0) | 5/105 (4.8) | 5/94 (5.3) | <0.001 |
| Animals belonging to farmers who had consultation with veterinarian | Yes | 158/178 (88.8) | 77/105 (73.3) | 70/94 (74.50) | 0.001 |
| Use of disinfectants in cleaning the farms | Used | 52/176 (29.5) | 2/96 (2.1) | 4/92 (4.3) | <0.001 |
| Variables | Description of the Variables | Sero-Positive/Total (%) | Coefficient of Unconditional Association with Brucella Sero-Positivity | p-Value of Unconditional Association with Brucella Sero-Positivity | Missing Value | Kept for Multivariable Model |
|---|---|---|---|---|---|---|
| Outcome | ||||||
| ELISA results of Brucella infection | Positive | 58 | 13 | |||
| Negative | 306 | |||||
| Identifiers | ||||||
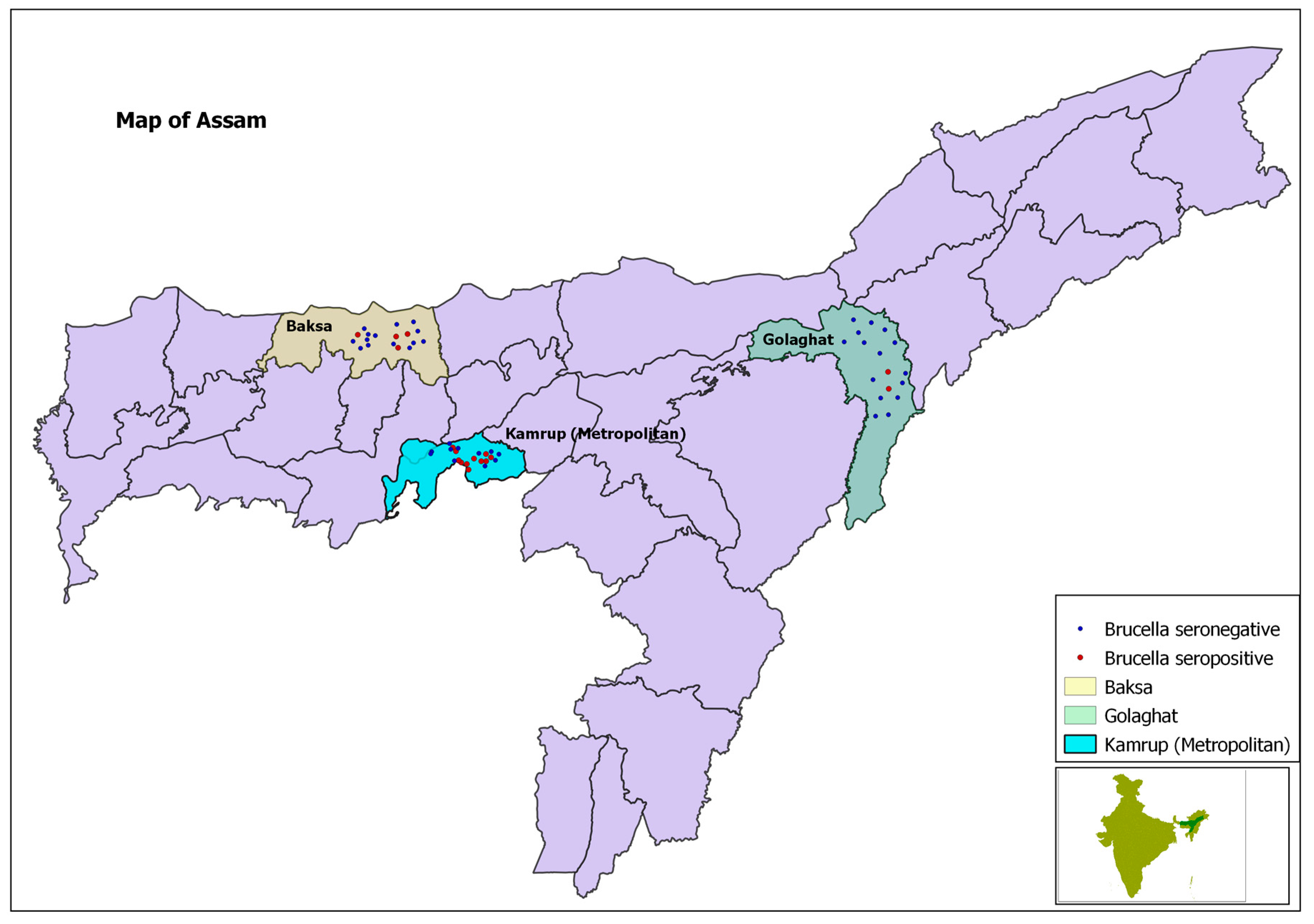
| Districts | Kamrup (large farms) | 49/135 (18.9) | Ref. * | <0.001 | 0 | Yes |
| Kamrup (small farms) | 3/41 (7.3) | −2.19 | ||||
| Golaghat | 2/96 (2.1) | −2.77 | ||||
| Baska | 4/92 (4.3) | −3.55 | ||||
| Farm Characteristics (FC) | ||||||
| Location of the farm in rural or urban areas | Rural CDB | 20/161 (12.4) | Ref. | 0.160 | 0 | Yes |
| Urban CDB | 38/203 (18.7) | 0.43 | ||||
| Category of farms | Small (1–3 dairy animals), | 8/223 (3.6) | Ref. | <0.001 | 0 | Yes |
| Medium (4–10 dairy animals) | 19/81 (23.4) | 2.19 | ||||
| Large (>10 dairy animals) | 31/60 (51.7) | 3.58 | ||||
| Dairy animals in contact with goats | Yes | 9/106 (8.5) | −1.19 | 0.030 | 0 | Yes |
| No | 49/258 (19.0) | Ref. | ||||
| Type of floor | Concrete | 20/67 (29.8) | Ref. | <0.001 | 0 | Yes |
| Earthen | 9/202 (4.4) | −2.42 | ||||
| Others | 29/95 (30.5) | 0.10 | ||||
| Farm Management (FM) | ||||||
| Adoption of AI | Yes | 46/225 (20.4) | 1.16 | 0.020 | 0 | Yes |
| No | 12/139 (8.6) | Ref. | ||||
| Introduction of new animals | Introduced | 22/79 (27.8) | 1.54 | 0.006 | 0 | Yes |
| Not introduced | 36/285 (12.6) | Ref. | ||||
| Animal movement | Animal moved | 9/213 (4.2) | −2.67 | <0.001 | 0 | Yes |
| Not moved | 49/151 (32.4) | Ref. | ||||
| Use of disinfectant in cleaning farms | Used disinfectant | 35/160 (21.9) | 1.06 | 0.020 | 0 | Yes |
| Not used disinfectant | 23/204 (11.3) | Ref. | ||||
| Producer Demographics (PD) | ||||||
| Education of farmers | No education | 17/66 (25.7) | Ref. | 0.14 | 0 | Yes |
| Class I–V | 11/49 (22.4) | −0.15 | ||||
| Class VI–X | 18/149 (12.1) | −1.16 | ||||
| Class XI and above | 12/100 (12.0) | −1.13 | ||||
| Age of farmers | 20–40 years | 21/89 (23.6) | Ref. | 0.10 | 0 | Yes |
| 41–60 years | 26/191 (13.6) | −1.15 | ||||
| 60 years and above | 11/84 (13.1) | −1.15 | ||||
| Training completed by farmers | Completed | 15/50 (30.0) | 1.52 | 0.02 | 0 | Yes |
| Not completed | 43/314 (13.7) | Ref. | ||||
| Interaction had with the veterinarians | Had interaction | 55/297 (18.5) | 1.85 | 0.005 | 0 | Yes |
| No interaction | 3/67 (4.5) | Ref. | ||||
| Cow Demographics (CD) | ||||||
| Breed of animal | Non-descript indigenous | 7/178 (3.9) | Ref. | <0.001 | 13 | Yes |
| Improved/CB/pure | 51/186 (27.4) | 2.66 | ||||
| Age of animals | With Brucella seropositive | 6.83 ± 0.33 | 0.03 | 13 | Yes | |
| With Brucella sero-negative | 6.09 ± 0.13 | |||||
| Odds Ratio | Standard Error | p-Value | 95% Confidence Interval | |
|---|---|---|---|---|
| Producer Demographics (PD) | ||||
| District | ||||
| Kamrup (large farms) | Ref. * | |||
| Kamrup (small farms) | 0.14 | 0.10 | 0.007 | 0.030–0.590 |
| Baska | 0.07 | 0.04 | <0.001 | 0.020–0.250 |
| Golaghat | 0.03 | 0.03 | <0.001 | 0.006–0.160 |
| Cow Demographics (CD) | ||||
| Age of the dairy animals, in years | 1.23 | 0.10 | 0.008 | 1.050–1.440 |
| Farm Management (FM) | ||||
| Artificial insemination adopted | 0.33 | 0.20 | 0.070 | 0.100–1.100 |
| Variables | Odds Ratio | Standard Error | p-Value | 95% Confidence Interval |
|---|---|---|---|---|
| District | ||||
| Kamrup (large farms) | Ref. * | |||
| Kamrup (small farms) | 0.11 | 0.09 | 0.007 | 0.020–0.540 |
| Baska | 0.03 | 0.02 | <0.001 | 0.005–0.150 |
| Golaghat | 0.01 | 0.01 | <0.001 | 0.002–0.100 |
| Age of dairy animals, in years | 1.24 | 0.10 | 0.007 | 1.060–1.440 |
| Artificial insemination adopted | 0.33 | 0.20 | 0.072 | 0.100–1.100 |
| Random effect of village | 0.40 | 0.49 | ||
| Random effect of household | 0.51 | 0.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deka, R.P.; Shome, R.; Dohoo, I.; Magnusson, U.; Randolph, D.G.; Lindahl, J.F. Seroprevalence and Risk Factors of Brucella Infection in Dairy Animals in Urban and Rural Areas of Bihar and Assam, India. Microorganisms 2021, 9, 783. https://doi.org/10.3390/microorganisms9040783
Deka RP, Shome R, Dohoo I, Magnusson U, Randolph DG, Lindahl JF. Seroprevalence and Risk Factors of Brucella Infection in Dairy Animals in Urban and Rural Areas of Bihar and Assam, India. Microorganisms. 2021; 9(4):783. https://doi.org/10.3390/microorganisms9040783
Chicago/Turabian StyleDeka, Ram Pratim, Rajeswari Shome, Ian Dohoo, Ulf Magnusson, Delia Grace Randolph, and Johanna F. Lindahl. 2021. "Seroprevalence and Risk Factors of Brucella Infection in Dairy Animals in Urban and Rural Areas of Bihar and Assam, India" Microorganisms 9, no. 4: 783. https://doi.org/10.3390/microorganisms9040783
APA StyleDeka, R. P., Shome, R., Dohoo, I., Magnusson, U., Randolph, D. G., & Lindahl, J. F. (2021). Seroprevalence and Risk Factors of Brucella Infection in Dairy Animals in Urban and Rural Areas of Bihar and Assam, India. Microorganisms, 9(4), 783. https://doi.org/10.3390/microorganisms9040783







