Human Papillomavirus Distribution in Women with Abnormal Pap Smear and/or Cervical Intraepithelial Neoplasia in Vaccination Era. A Single-Center Study in the North Italian Population
Abstract
1. Introduction
2. Materials and Methods
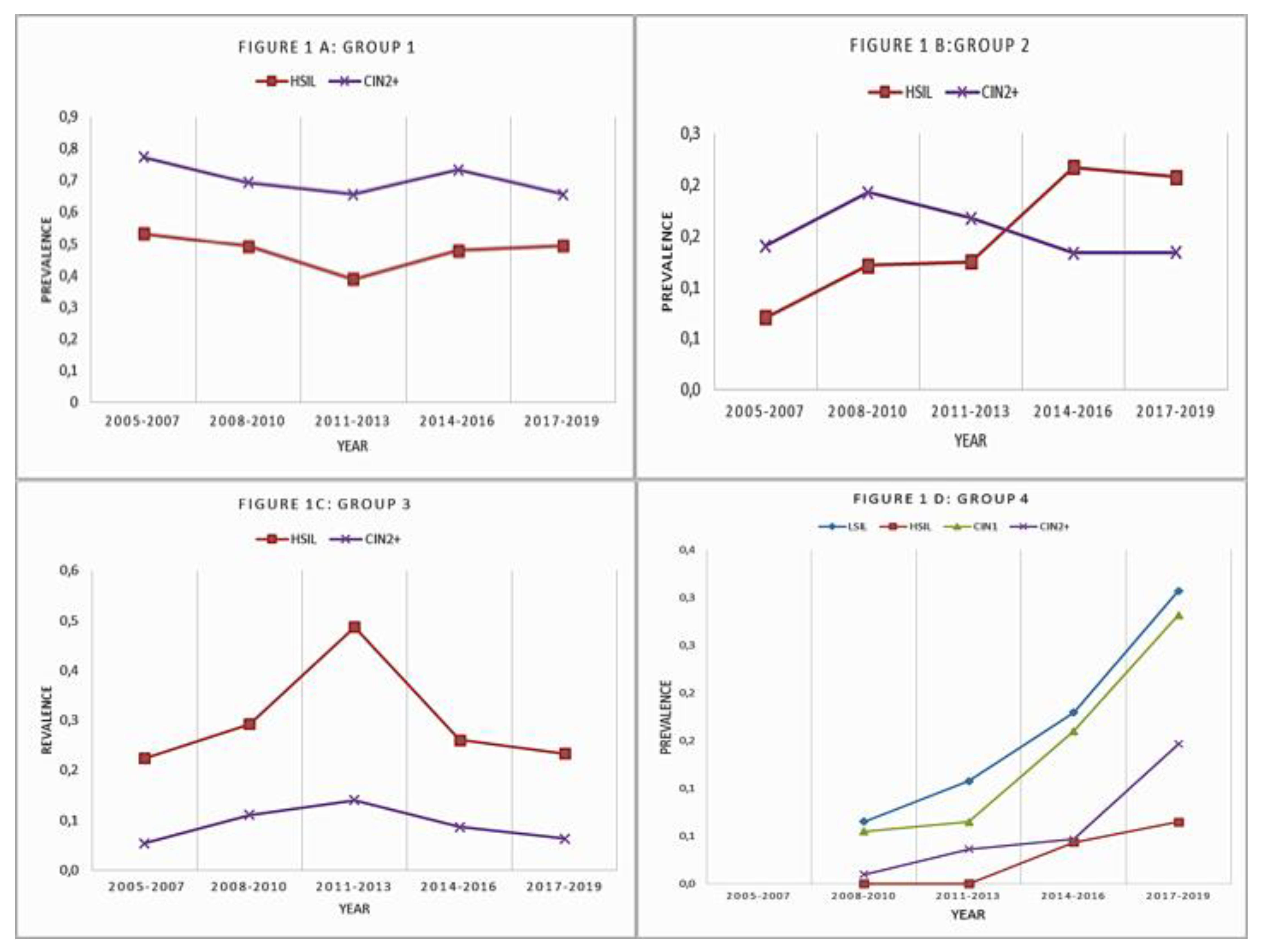
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Future II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N. Engl. J. Med. 2007, 356, 1915–1927. [Google Scholar] [CrossRef]
- Aro, K.; Nieminen, P.; Louvanto, K.; Jakobsson, M.; Virtanen, S.; Lehtinen, M.; Dillner, J.; Kalliala, I. Age-specific HPV type distribution in high-grade cervical disease in screened and unvaccinated women. Gynecol. Oncol. 2019, 154, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Dal Bello, B.; Spinillo, A.; Alberizzi, P.; Cesari, S.; Gardella, B.; Silini, E.M. Time trends of human papillomavirus type distribution in Italian women with cervical intraepithelial neoplasia (CIN). Gynecol. Oncol. 2009, 115, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Dickson, E.L.; Vogel, R.I.; Luo, X.; Downs, L.S. Recent trends in type-specific HPV infection rates in the United States. Epidemiol. Infect. 2014, 143, 1042–1047. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, H.; Clifford, G.; Franceschi, S. HPV infection in Europe. Eur. J. Cancer 2009, 45, 2632–2639. [Google Scholar] [CrossRef]
- Bogani, G.; Serati, M.; Maggiore, U.L.R.; Ditto, A.; Gardella, B.; Ferrero, S.; Spinillo, A.; Ghezzi, F.; Raspagliesi, F. Cervical intraepithelial neoplasia in women who had vaccination against HPV. Int. J. Gynaecol. Obstet. 2019, 147, 233–237, Erratum in 2020, 148, 133. [Google Scholar] [CrossRef]
- Spinillo, A.; Gardella, B.; Roccio, M.; Alberizzi, P.; Silini, E.M.; Bello, B.D. Untypable human papillomavirus infection and risk of cervical intraepithelial neoplasia among women with abnormal cervical cytology. J. Med. Virol. 2014, 86, 1145–1152. [Google Scholar] [CrossRef]
- Molet, L.; Girlich, D.; Bonnin, R.A.; Proust, A.; Bouligand, J.; Bachelerie, F.; Hantz, S.; Deback, C. Identification by high-throughput sequencing of HPV variants and quasispecies that are untypeable by linear reverse blotting assay in cervical specimens. Papillomavirus Res. 2019, 8, 100169. [Google Scholar] [CrossRef]
- Hesselink, A.T.; Van Ham, M.A.P.C.; Heideman, D.A.M.; Groothuismink, Z.M.A.; Rozendaal, L.; Berkhof, J.; van Kemenade, F.J.; Massuger, L.A.F.G.; Melchers, W.J.G.; Meijer, C.J.L.M.; et al. Comparison of GP5+/6+-PCR and SPF10-line blot assays for detection of high-risk human papillomavirus in samples from women with normal cytology results who develop grade 3 cervical intraepithelial neoplasia. Comp. Study J. Clin. Microbiol. 2008, 46, 3215–3221. [Google Scholar] [CrossRef]
- Muñoz, N.; Castellsagué, X.; de González, A.B.; Gissmann, L. Chapter 1: HPV in the etiology of human cancer. Vaccine 2006, 24, S1–S10. [Google Scholar] [CrossRef]
- StataCorp, L.P. Stata Statistical Software: Release 13; StataCorp LP: College Station, TX, USA, 2013. [Google Scholar]
- Pimenoff, V.N.; Tous, S.; Benavente, Y.; Alemany, L.; Quint, W.; Bosch, F.X.; Bravo, I.G.; De Sanjosé, S. Distinct geographic clustering of oncogenic human papillomaviruses multiple infections in cervical cancers: Results from a worldwide cross-sectional study. Int. J. Cancer 2018, 144, 2478–2488. [Google Scholar] [CrossRef]
- Alemany, L.; De Sanjosé, S.; Tous, S.; Quint, W.; Vallejos, C.; Shin, H.R.; Bravo, L.E.; Alonso, P.; Lima, M.A.; Guimerà, N.; et al. Time trends of human papillomavirus types in invasive cervical cancer, from 1940 to 2007. Int. J. Cancer 2014, 135, 88–95. [Google Scholar] [CrossRef] [PubMed]
- McClung, N.M.; Gargano, J.W.; Park, I.U.; Whitney, E.; Abdullah, N.; Ehlers, S.; Bennett, N.M.; Scahill, M.; Niccolai, L.M.; Brackney, M.; et al. Estimated Number of Cases of High-Grade Cervical Lesions Diagnosed Among Women—United States, 2008 and 2016. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Saslow, D.; Solomon, D.; Lawson, H.W.; Killackey, M.; Kulasingam, S.L.; Cain, J.; Garcia, F.A.; Moriarty, A.T.; Waxman, A.G.; Wilbur, D.C.; et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Pract. Guidel. Am. J. Clin. Pathol. 2012, 137, 516–542. [Google Scholar] [CrossRef]
- Tornesello, M.L.; Cassese, R.; De Rosa, N.; Buonaguro, L.; Masucci, A.; Vallefuoco, G.; Palmieri, S.; Schiavone, V.; Piccoli, R.; Buonaguro, F.M. High prevalence of human papillomavirus infection in Eastern European and West African women immigrants in South Italy. APMIS 2011, 119, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Spinillo, A.; Gardella, B.; Roccio, M.; Alberizzi, P.; Cesari, S.; Patrizia, M.; Silini, E. Multiple human papillomavirus infection with or without type 16 and risk of cervical intraepithelial neoplasia among women with cervical cytological abnormalities. Cancer Causes Control. 2014, 25, 1669–1676. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Myers, L.; Hammons, A.F.; Clark, R.A.; Dunlap, K.; Kissinger, P.J.; Hagensee, M.E. Prevalence and clustering patterns of human papillomavirus genotypes in multiple infections. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2439–2445. [Google Scholar] [CrossRef]
- Mollers, M.; Vriend, H.J.; van der Sande, M.A.; van Bergen, J.E.; King, A.J.; Lenselink, C.H.; Bekkers, R.L.; Meijer, C.J.; de Melker, H.E.; Bogaards, J.A. Population- and type-specific clustering of multiple HPV types across diverse risk populations in the Netherlands. Am. J. Epidemiol. 2014, 179, 1236–1246. [Google Scholar] [CrossRef][Green Version]
- Hariri, S.; Bennett, N.M.; Niccolai, L.M.; Schafer, S.; Park, I.U.; Bloch, K.C.; Unger, E.R.; Whitney, E.; Julian, P.; Scahill, M.W.; et al. Reduction in HPV 16/18-associated high grade cervical lesions following HPV vaccine introduction in the United States–2008–2012. Vaccine 2015, 33, 1608–16613. [Google Scholar] [CrossRef]
- Cameron, R.L.; Kavanagh, K.; Pan, J.; Love, J.; Cuschieri, K.; Robertson, C.; Ahmed, S.; Palmer, T.; Pollock, K.G. Human Papillomavirus Prevalence and Herd Immunity after Introduction of Vaccination Program, Scotland, 2009–2013. Emerg. Infect. Dis. 2016, 22, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.E.; Unger, E.R.; Lewis, R.; McDaniel, D.; Gargano, J.W.; Steinau, M.; Markowitz, L.E. Prevalence of Human Papillomavirus Among Females After Vaccine Introduction—National Health and Nutrition Examination Survey, United States, 2003–2014. J. Infect. Dis. 2017, 216, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Ciavattini, A.; Giannella, L.; De Vincenzo, R.; Di Giuseppe, J.; Papiccio, M.; Lukic, A.; Carpini, G.D.; Perino, A.; Frega, A.; Sopracordevole, F.; et al. HPV Vaccination: The Position Paper of the Italian Society of Colposcopy and Cervico-Vaginal Pathology (SICPCV). Vaccines 2020, 8, 354. [Google Scholar] [CrossRef] [PubMed]
- Eltoum, I.A.; Chhieng, D.C.; Crowe, D.R.; Roberson, J.; Jin, G.; Broker, T.R. Significance and possible causes of false-negative results of reflex human Papillomavirus infection testing. Cancer 2007, 111, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Kaliff, M.; Karlsson, M.G.; Sorbe, B.; Mordhorst, L.B.; Helenius, G.; Lillsunde-Larsson, G. HPV-negative Tumors in a Swedish Cohort of Cervical Cancer. Int. J. Gynecol. Pathol. 2020, 39, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Petry, K.U.; Liebrich, C.; Luyten, A.; Zander, M.; Iftner, T. Surgical staging identified false HPV-negative cases in a large series of invasive cervical cancers. Papillomavirus Res. 2017, 4, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Barzon, L.; Militello, V.; Pagni, S.; Palù, G. Comparison of INNO-LiPA genotyping extra and hybrid capture 2 assays for detection of carcinogenic human papillomavirus genotypes. J. Clin. Virol. 2012, 55, 256–261. [Google Scholar] [CrossRef]
- Geraets, D.T.; Struijk, L.; Kleter, B.; Molijn, A.; Van Doorn, L.-J.; Quint, W.G.; Colau, B. The original SPF10 LiPA25 algorithm is more sensitive and suitable for epidemiologic HPV research than the SPF10 INNO-LiPA Extra. J. Virol. Methods 2015, 215–216, 22–29. [Google Scholar] [CrossRef]
- Meijer, C.J.; Berkhof, J.; Castle, P.E.; Hesselink, A.T.; Franco, E.L.; Ronco, G.; Arbyn, M.; Bosch, F.X.; Cuzick, J.; Dillner, J.; et al. Guidelines for human papillomavirus DNA test requirements for primary cervical cancer screening in women 30 years and older. Int. J. Cancer 2008, 124, 516–520. [Google Scholar] [CrossRef]
- Coquillard, G.; Palao, B.; Patterson, B.K. Quantification of intracellular HPV E6/E7 mRNA expression increases the specificity and positive predictive value of cervical cancer screening compared to HPV DNA. Gynecol. Oncol. 2011, 120, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Varnai, A.D.; Bollmann, M.; Bankfalvi, A.; Speich, N.; Schmitt, C.; Griefingholt, H.; Kovács, K.; Klozoris, C.; Bollmann, R. Predictive testing of early cervical pre-cancer by detecting human papillomavirus E6/E7 mRNA in cervical cytologies up to high-grade squamous intraepithelial lesions: Diagnostic and prognostic implications. Oncol. Rep. 2008, 19, 457–465. [Google Scholar] [CrossRef][Green Version]
| 2005–2007 N = 1163 (%) | 2008–2010 N = 1592 (%) | 2011–2013 N = 1349 (%) | 2014–2016 N = 913 (%) | 2017–2019 N = 790 (%) | |
|---|---|---|---|---|---|
| Age (years) | |||||
| 21–29 | 333 (28.6) | 374 (23.5) | 341 (25.3) | 256 (28) | 206 (26.1) |
| 30–45 | 590 (50.7) | 848 (53.3) | 680 (50.4) | 402 (44) | 341 (43.2) |
| >45 | 240 (20.6) | 370 (23.2) | 328 (24.3) | 355 (27.9) | 243 (30.8) |
| HIV positive | 76 (6.5) | 127 (8) | 78 (5.8) | 42 (4.6) | 22 (2.8) * |
| Parous | 707 (60.8) | 864 (54.3) | 520 (38.5) | 433 (47.4) | 296 (37.5) * |
| Nationality | |||||
| Italy | 1062 (91.3) | 1419 (89.1) | 1156 (85.7) | 774 (84.8) | 690 (87.3) |
| Europe | 51 (4.4) | 80 (5) | 104 (7.7) | 78(8.5) | 52 (6.6) |
| Extra-europe | 50 (4.3) | 93 (5.8) | 89(6.6) | 61(6.7) | 48 (6.1) * |
| Non-smokers | 860 (73.9) | 1220 (76.6) | 977 (72.4) | 692 (75.8) | 608 (77) |
| <10 cig/day | 157 (13.5) | 185 (11.6) | 209 (15.5) | 100 (11) | 107 (13.5) |
| ≥10 cig/day | 146 (12.6) | 187 (11.7) | 163 (12.1) | 121 (13.3) | 75 (9.5) |
| Contraceptive use | |||||
| No | 544 (46.8) | 848 (53.3) | 709 (52.6) | 526 (57.6) | 421 (53.3) |
| Barrier | 112 (9.6) | 127 (8) | 139 (10.3) | 121 (13.3) # | 67 (8.5) |
| Hormonal | 482 (41.4) | 601 (37.8) | 491 (36.4) | 257 (28.1) # | 300 (38) |
| IUD | 25 (2.1) | 16 (1) | 10 (0.7) | 9 (1) | 2 (0.3) * |
| 2005–2007 N = 1163 (%) | 2008–2010 N = 1592 (%) | 2011–2013 N = 1349 (%) | 2014–2016 N = 913 (%) | 2017–2019 N = 790 (%) | |
|---|---|---|---|---|---|
| Pap-smear | |||||
| ASCUS/LSIL | 1007 (86.6) | 1452 (91.2) | 1197 (88.7) | 890 (97.5) # | 713 (90.3) |
| HSIL | 156 (13.4) # | 140 (8.8) | 152 (11.3) | 23 (2.5) # | 77 (9.7) |
| Type of virus | |||||
| LR-HPV | 366 (31.5) | 653 (41) | 536 (39.7) | 167 (18.3) # | 124 (15.7) # |
| HR-HPV vaccine | 542 (46.6) | 631 (39.6) | 488 (36.2) # | 440 (48.2) # | 301 (38.1) |
| HR-HPV others | 93 (8) # | 216 (13.6) | 196 (14.5) | 145 (15.9) | 141 (17.8) # |
| Negative | 105 (9) | 83 (5.2) # | 81 (6) # | 96 (10.5) | 108 (13.7) # |
| Untypable | 57 (4.9) | 9 (0.6) # | 48 (3.6) | 65 (7.1) | 116 (14.7) #* |
| Multiple HPV infection: | 471 (40.5) | 813 (51.1) | 663 (49.1) | 156 (17.1) | 133 (16.8) * |
| Colposcopic lesion >50% | 193 (16.59) | 236 (14.9) | 309 (22.9) | 101 (11.1) | 199 (21.8) * |
| Excisional cervical treatment | 268 (23) | 357 (22.4) | 244 (18.1) | 157 (17.2) | 138 (17.5) * |
| Histology | 2005–2007 N = 693 (%) | 2008–2010 N = 934 (%) | 2011–2013 N = 739 (%) | 2014–2016 N = 525 (%) | 2017–2019 N = 513 (%) |
| Negative | 240 (34.6) # | 274 (29.3) | 179 (24.2) | 96 (18.3) # | 104 (20.3) # |
| CIN1 | 269 (38.8) # | 411 (44) | 346 (46.8) | 282 (53.7) | 258 (50.3) |
| CIN2 | 47 (6.8) # | 85 (9.1) | 70 (9.5) | 70 (13.3) | 73 (14.2) |
| CIN3 | 124 (17.9) | 149 (16) | 128 (17.3) | 70 (13.3) | 75 (14.6) |
| Squamous Invasive Cancer | 13 (1.9) | 15 (1.6) | 16 (2.2) | 7 (1.3) | 3 (0.6) * |
| Multiple HPV infection: | 471 (40.5) | 813 (51.1) | 663 (49.1) | 156 (17.1) | 133 (16.8) * |
| 2005–2007 N = 693 (%) | 2008–2010 N = 934 (%) | 2011–2013 N = 739 (%) | 2014–2016 N = 525 (%) | 2017–2019 N = 513 (%) | ||
|---|---|---|---|---|---|---|
| Negative/CIN1 | HPV16 | 125 (18) | 135 (14.5) | 114 (15.4) | 78 (14.9) | 39 (7.6) * |
| HPV18 | 37 (5.3) | 27 (2.9) | 21 (2.8) | 22 (4.2) | 16 (3.1) | |
| HPV31 | 89 (12.8) | 48 (5.1) | 25 (3.4) | 48 (9.1) | 25 (4.9) * | |
| HPV33 | 13 (1.9) | 18 (1.9) | 13 (1.8) | 18 (3.4) | 4 (0.8) | |
| HPV45 | 10 (1.4) | 11 (1.2) | 6 (0.8) | 10 (1.9) | 5 (1) | |
| HPV52 | 53 (7.6) | 145 (15.5) | 96 (13) | 64 (12.2) | 29 (5.7) | |
| HPV 58 | 9 (1.3) | 8 (0.9) | 9 (1.2) | 20 (3.8) | 24 (4.7) | |
| CIN2+ | HPV16 | 80 (11.5) | 85 (9.1) | 77 (10.4) | 65 (12.4) | 56 (10.9) |
| HPV18 | 42 (6.1) | 27 (2.9) | 6 (0.8) | 11 (2.1) | 9 (1.8) * | |
| HPV31 | 57 (8.2) | 39 (4.2) | 33 (4.5) | 22 (4.2) | 20 (3.9) * | |
| HPV33 | 7 (1) | 23 (2.5) | 10 (1.4) | 11 (2.1) | 11 (2.1) | |
| HPV45 | 15 (2.2) | 7 (0.7) | 2 (0.3) | 7 (1.3) | 7 (1.4) | |
| HPV52 | 47 (6.8) | 79 (8.5) | 67 (9.1) | 14 (2.7) | 15 (2.9) * | |
| HPV 58 | 6 (0.9) | 7 (0.7) | 3 (0.4) | 10 (1.9) | 6 (1.2) |
| 2005–2007 N = 693 (%) | 2008–2010 N = 934 (%) | 2011–2013 N = 739 (%) | 2014–2016 N = 525 (%) | 2017–2019 N = 513 (%) | ||
|---|---|---|---|---|---|---|
| Negative/CIN1 | HPV16 | 125 (18) | 135 (14.5) | 114 (15.4) | 78 (14.9) | 39 (7.6) * |
| HPV18 | 37 (5.3) | 27 (2.9) | 21 (2.8) | 22 (4.2) | 16 (3.1) | |
| HPV31 | 89 (12.8) | 48 (5.1) | 25 (3.4) | 48 (9.1) | 25 (4.9) * | |
| HPV33 | 13 (1.9) | 18 (1.9) | 13 (1.8) | 18 (3.4) | 4 (0.8) | |
| HPV45 | 10 (1.4) | 11 (1.2) | 6 (0.8) | 10 (1.9) | 5 (1) | |
| HPV52 | 53 (7.6) | 145 (15.5) | 96 (13) | 64 (12.2) | 29 (5.7) | |
| HPV58 | 9 (1.3) | 8 (0.9) | 9 (1.2) | 20 (3.8) | 24 (4.7) | |
| CIN2+ | HPV16 | 80 (11.5) | 85 (9.1) | 77 (10.4) | 65 (12.4) | 56 (10.9) |
| HPV18 | 42 (6.1) | 27 (2.9) | 6 (0.8) | 11 (2.1) | 9 (1.8) * | |
| HPV31 | 57 (8.2) | 39 (4.2) | 33 (4.5) | 22 (4.2) | 20 (3.9) * | |
| HPV33 | 7 (1) | 23 (2.5) | 10 (1.4) | 11 (2.1) | 11 (2.1) | |
| HPV45 | 15 (2.2) | 7 (0.7) | 2 (0.3) | 7 (1.3) | 7 (1.4) | |
| HPV52 | 47 (6.8) | 79 (8.5) | 67 (9.1) | 14 (2.7) | 15 (2.9) * | |
| HPV58 | 6 (0.9) | 7 (0.7) | 3 (0.4) | 10 (1.9) | 6 (1.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gardella, B.; Dominoni, M.; Sosso, C.; Arrigo, A.; Gritti, A.; Cesari, S.; Fiandrino, G.; Spinillo, A. Human Papillomavirus Distribution in Women with Abnormal Pap Smear and/or Cervical Intraepithelial Neoplasia in Vaccination Era. A Single-Center Study in the North Italian Population. Microorganisms 2021, 9, 729. https://doi.org/10.3390/microorganisms9040729
Gardella B, Dominoni M, Sosso C, Arrigo A, Gritti A, Cesari S, Fiandrino G, Spinillo A. Human Papillomavirus Distribution in Women with Abnormal Pap Smear and/or Cervical Intraepithelial Neoplasia in Vaccination Era. A Single-Center Study in the North Italian Population. Microorganisms. 2021; 9(4):729. https://doi.org/10.3390/microorganisms9040729
Chicago/Turabian StyleGardella, Barbara, Mattia Dominoni, Cecilia Sosso, Anna Arrigo, Andrea Gritti, Stefania Cesari, Giacomo Fiandrino, and Arsenio Spinillo. 2021. "Human Papillomavirus Distribution in Women with Abnormal Pap Smear and/or Cervical Intraepithelial Neoplasia in Vaccination Era. A Single-Center Study in the North Italian Population" Microorganisms 9, no. 4: 729. https://doi.org/10.3390/microorganisms9040729
APA StyleGardella, B., Dominoni, M., Sosso, C., Arrigo, A., Gritti, A., Cesari, S., Fiandrino, G., & Spinillo, A. (2021). Human Papillomavirus Distribution in Women with Abnormal Pap Smear and/or Cervical Intraepithelial Neoplasia in Vaccination Era. A Single-Center Study in the North Italian Population. Microorganisms, 9(4), 729. https://doi.org/10.3390/microorganisms9040729






