Multi Locus Sequence Typing and spa Typing of Staphylococcus aureus Isolated from the Milk of Cows with Subclinical Mastitis in Croatia
Abstract
1. Introduction
2. Methods
2.1. DNA Isolation
2.2. Multiplex Polymerase Chain Reaction (PCR)
2.3. Spa Typing
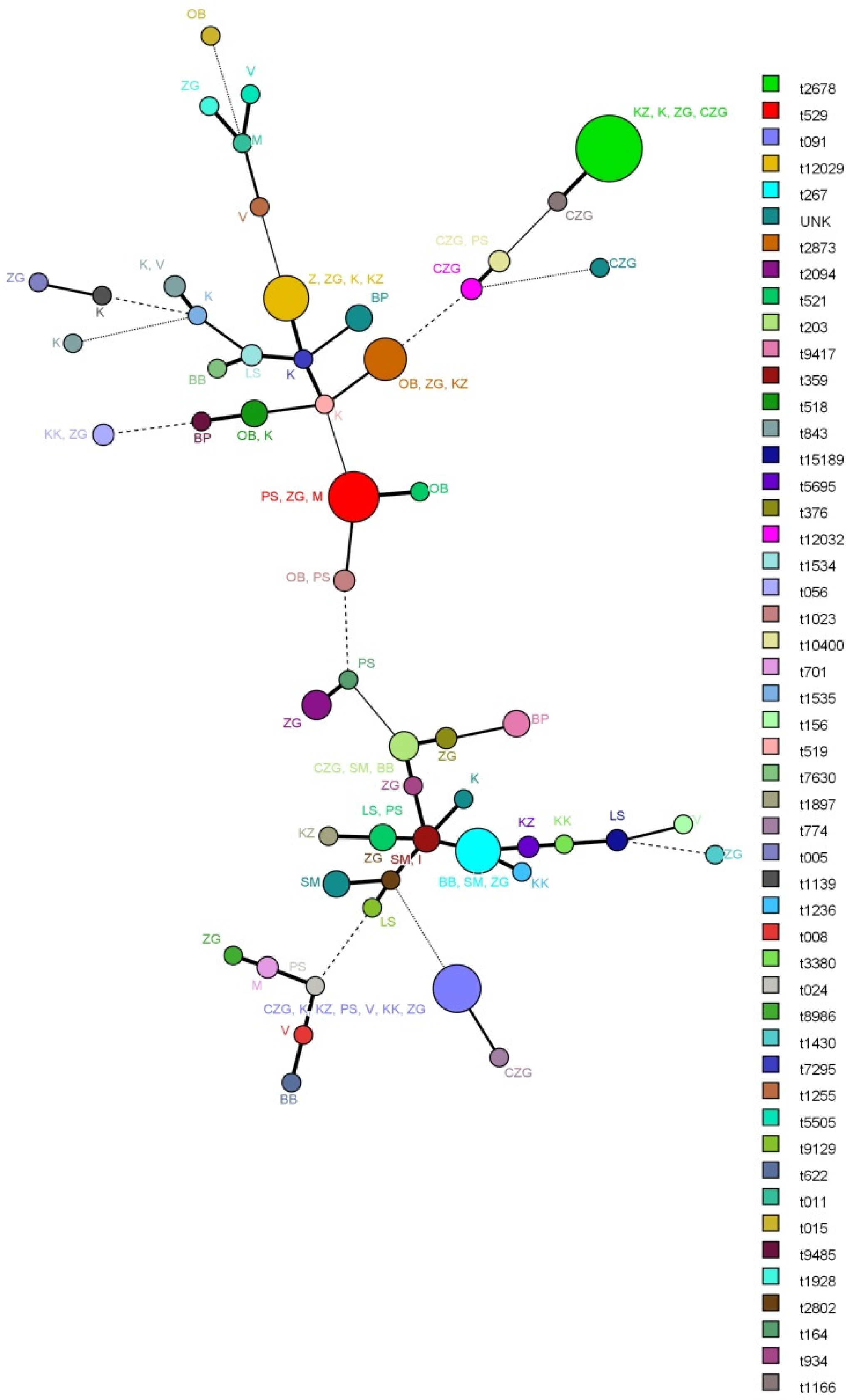
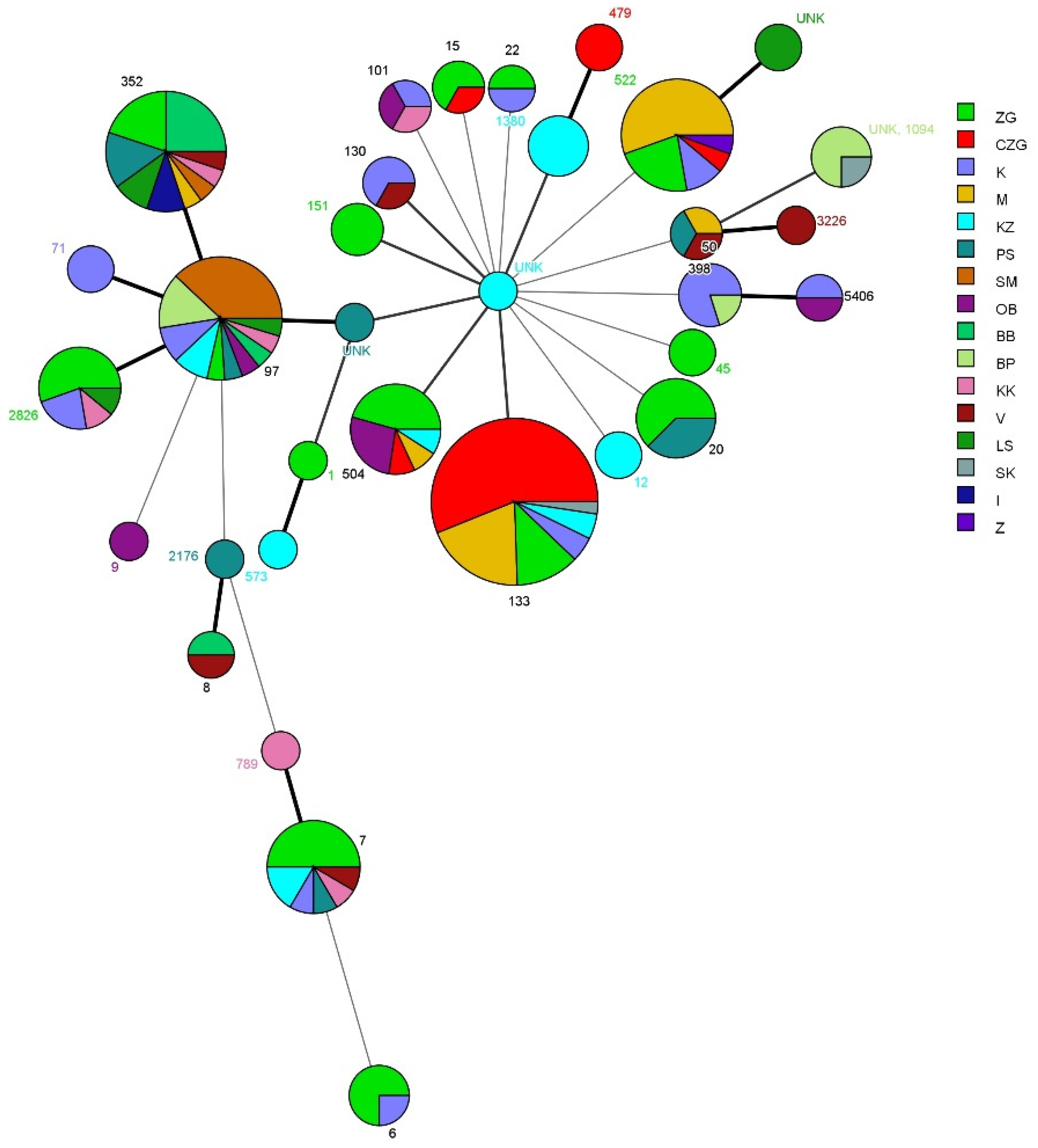
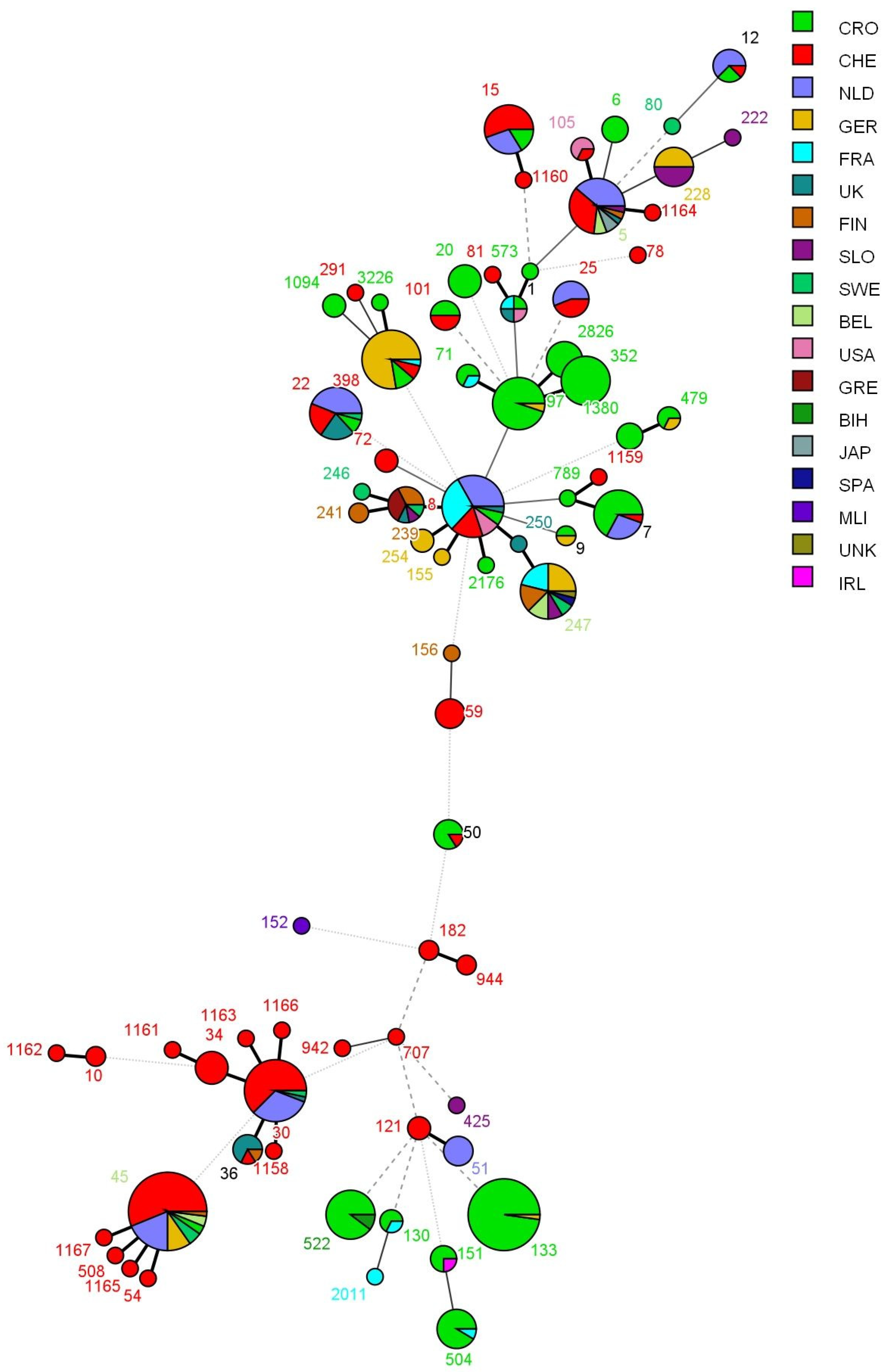
2.4. Multi-Locus Sequence Typing (MLST)
3. Results
3.1. Multiplex PCR
3.2. Results of Multi-Locus Sequence Typing (MLST)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tenhagen, B.A.; Hansen, I.; Reinecke, A.; Heuwieser, W. Prevalence of pathogens in milk samples of dairy cows with clinical mastitis and in heifers at first parturition. J. Dairy Res. 2009, 76, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Petersson-Wolfe, C.S.; Mullarky, I.K.; Jones, G.M. Staphylococcus aureus mastitis: Cause, detection, and control. VCE Publ. 2010, 404, 404-229. [Google Scholar]
- Maćešić, N.; Karadjole, T.; Bačić, G.; Benić, M.; Karadjole, M.; Vince, S.; Lipar, M.; Cergolj, M. Aetiology and prevalence of bovine intramammary infection at drying off. Vet. Arh. 2012, 82, 125–131. [Google Scholar]
- Cvetnić, L.; Samardžija, M.; Habrun, B.; Kompes, G.; Benić, M. Microbiological monitoring of mastitis pathogens in the control of udder health in dairy cows. Slov. Vet. Res. 2016, 53, 131–140. [Google Scholar]
- Devriese, L.A.; Van Damme, L.R.; Fameree, L. Methicillin-(cloxacillin)-resistant Staphylococcus aureus strains isolated from bovine mastitis cases. Zentralbl. Veterinärmed. B 1972, 19, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Juhász-Kaszanyitzky, É.; Jánosi, S.; Somogyi, P.; Dán, Á.; Van der Graaf-Van Bloois, L.; Van Duijkeren, E.; Wagenaar, J.A. MRSA transmission between cows and humans. Emerg. Infect. Dis. 2007, 13, 630–632. [Google Scholar] [CrossRef]
- Monecke, S.; Kuhner, P.; Hotzel, H.; Slickers, P.; Ehricht, R. Microarray based study on virulence-associated genes and resistance determinants of Staphylococcus aureus isolates from cattle. Vet. Microbiol. 2007, 125, 128–140. [Google Scholar] [CrossRef]
- Vanderhaeghen, W.; Cerpetier, T.; Adriaensen, C.; Vicca, J.; Hermans, K.; Butaye, P. Methicillin-resistant Staphylococcus aureus (MRSA) ST398 associated with clinical and subclinical mastitis in Belgian cows. Vet. Microbiol. 2010, 144, 166–171. [Google Scholar] [CrossRef]
- Spoor, L.E.; McAdam, P.R.; Weinert, L.A.; Rambault, A.; Hasman, H.; Aarestrup, F.M.; Kearms, A.M.; Larsen, A.R.; Skov, R.L.; Fitgerald, J.R. Livestock origin for a human pandemic clone of Community-Associated Methicillin-Resistant Staphylococcus aureus. mBio 2013, 4, e00356-13. [Google Scholar] [CrossRef]
- Deurenberg, R.H.; Vink, C.; Kalenić, S.; Friedrich, A.W.; Bruggeman, C.A.; Stobberingh, E.E. The molecular evolution of methicillin-resistant Staphylococcus aureus. Clin. Microbiol. Infect. 2007, 13, 222–235. [Google Scholar] [CrossRef]
- Enright, M.C.; Day, N.P.; Davies, C.E.; Peacock, J.; Spratt, B.G. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 2000, 38, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Koreen, L.; Ramaswamy, S.V.; Gravis, E.A.; Naidich, S.; Musser, J.M.; Kreiswirth, N.N. Spa typing method for discriminating among Staphylococcus aureus isolates: Implications for use of a single marker to detect genetic micro and macrovariation. J. Clin. Microbiol. 2004, 42, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Rabello, R.F.; Moreira, B.M.; Lopes, R.M.; Teixeira, L.M.; Riley, L.W.; Castro, A.C. Multilocus sequence typing of Staphylococcus aureus isolates recovered from cows with mastitis in Brazilian dairy herds. J. Med. Microbiol. 2007, 56, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Modley, A.; Espinosa-Gongora, C.; Nielsen, S.S.; McCarthy, A.J.; Lindsay, J.A.; Guardabassi, L. Comparative host specificity of human and pig associated Staphylococcus aureus clonal lineages. PLoS ONE 2012, 7, e49344. [Google Scholar]
- Lowder, B.V.; Guinane, C.M.; Ben Zakour, N.L.; Weinert, L.A.; Conway-Morris, A.; Cartwright, R.A.; Simpson, A.J.; Rambaut, A.; Nübel, U.; Fitzgerald, J.R. Recent human-to-poultry host jump, adaptation, and pandemic spread of Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 2009, 106, 19545–19550. [Google Scholar] [CrossRef]
- Armand-Lefevre, L.; Ruimy, R.; Andremont, A. Clonal comparison of Staphylococcus aureus isolates from healthy pig farmers, human controls, and pigs. Emerg. Infect. Dis. 2005, 11, 711–714. [Google Scholar] [CrossRef]
- Deurenberg, R.H.; Stobberingh, E.E. The evolution of Staphylococcus aureus. Infect. Genet. Evol. 2008, 8, 747–763. [Google Scholar] [CrossRef] [PubMed]
- Walther, B.; Monecke, S.; Ruscher, C.; Friedrich, A.W.; Ehricht, R.; Slickers, P.; Soba, A.; Wleklinski, C.G.; Wieler, L.H.; Lübke-Becker, A. Comparative molecular analysis substantiates zoonotic potential of equine methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 2009, 47, 704–710. [Google Scholar] [CrossRef]
- McCarthy, A.J.; Lindsday, J.A.; Loeffler, A. Are all meticillin-resistant Staphylococcus aureus (MRSA) equal in all hosts? Epidemiological and genetic comparison between animal and human MRSA. Vet. Dermatol. 2012, 23, 267–275. [Google Scholar] [CrossRef]
- Sheet, O.H.; Grabowski, N.T.; Klein, G.; Reich, F.; Abdulmawjood, A. Characterisation of mecA gene negative Staphylococcus aureus isolated from bovine mastitis milk from Northern Germany. Folia Microbiol. 2019, 64, 845–855. [Google Scholar] [CrossRef]
- Ronco, T.; Stegger, M.; Pedersen, K. Draft genome sequence of a sequence type 398 methicillin-resistant Staphylococcus aureus isolate from a Danish dairy cow with mastitis. Genome Announc. 2017, 5, e00492-17. [Google Scholar] [CrossRef] [PubMed]
- Akkou, M.; Bouchiat, C.; Antri, K.; Bes, M.; Tristan, A.; Dauwalder, O.; Martins-Simoes, P.; Rasigade, J.P.; Etiennne, J.; Vandenesch, F.; et al. New host shift from human cows within Staphylococcus aureus involved in mastitis and nasal carriage of animal’s caretakers. Vet. Microbiol. 2018, 223, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.A.; Zadoks, R.N. Methicillin resistant S. aureus in human and bovine mastitis. J. Mammary Gland Biol. Neoplasia 2011, 16, 373–382. [Google Scholar] [CrossRef]
- Nemeghaire, S.; Argudin, M.A.; Haesebrouck, F.; Butaye, P. Epidemiology and molecular characterization of methicillin-resistant Staphylococcus aureus nasal carriage isolates from bovines. BMC Vet. Res. 2014, 10, 153. [Google Scholar] [CrossRef]
- Schmidt, T.; Koch, M.M.; Ehlers, M.M. Molecular characterisation of Staphylococcus aureus isolated from bovine mastitis and close human contact in South African dairy herds: Genetic diversity and inter-species host transmission. Front. Microbiol. 2017, 8, 511. [Google Scholar] [CrossRef]
- National Mastitis Council. Staphylococci. In Laboratory Handbook on Bovine Mastitis, 3rd ed.; National Mastitis Council: Verona, WI, USA, 2017. [Google Scholar]
- Stegger, M.; Anderson, P.S.; Kearns, A.; Pichon, B.; Holmes, M.A.; Edwards, G.; Laurent, F.; Teale, C.; Skov, R.; Larsen, A.R. Rapid detection, differentiation and typing of methicillin-resistant Staphylococcus aureus harbouring either mecA or the new mecA homologue mecA (LGA251). Clin. Microbiol. Infect. 2012, 18, 395–400. [Google Scholar] [CrossRef]
- Hasman, H.; Moodley, A.; Guardabassi, L.; Stegger, M.; Skov, R.L.; Aerestrup, F.M. Spa type distribution in Staphylococcus aureus originating from pigs, cattle and poultry. Vet. Microbiol. 2010, 141, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Tianming, L.; Lu, H.; Wang, X.; Gao, Q.; Dai, Y.; Shang, J.; Li, M. Molecular characteristics of Staphylococcus aureus causing bovine mastitis between 2014 and 2015. Front. Cell. Infect. Microbiol. 2017, 7, 127. [Google Scholar]
- Luini, M.; Cremonesi, P.; Magro, G.; Bianchini, V.; Minozzi, G.; Castiglioni, B.; Piccinini, R. Methicilin-resistant Staphylococcus aureus (MRSA) is associated with low within-herd prevalence of intra-mammaryinfections in dairy cows: Genotypping isolates. Vet. Microbiol. 2015, 178, 270–274. [Google Scholar] [CrossRef]
- Hamid, S.; Bhat, M.A.; Mir, I.A.; Taku, A.; Badroo, G.A.; Nazki, S.; Malik, A. Phenotypic and genotypic characterization of methicilin-resistant Staphylococcus aureus from bovine mastitis. Vet. World 2017, 10, 363–367. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Z.; Yan, Z.; Wu, J.; Ali, T.; Li, J.; Lu, Y.; Han, B. Bovine mastitis Staphylococcus aureus: Antibiotic susceptibility profile, resistance genes and molecular typing of methicillin-resistant and methicillin-sensitive strains in China. Infect. Genet. Evol. 2015, 31, 9–16. [Google Scholar] [CrossRef]
- Haran, K.P.; Godden, S.M.; Boxrud, D.; Jawahir, S.; Sreevatsan, S. Prevalence and Characterization of Staphylococcus aureus, Including Methicillin-Resistant Staphylococcus aureus, Isolated from Bulk Tank Milk from Minnesota Dairy Farms. J. Clin. Microbiol. 2012, 50, 688–695. [Google Scholar] [CrossRef]
- Tenhagen, B.A.; Alt, K.; Pfefferkorn, B.; Wiehle, B.; Käsbohrer, A.; Fetsch, A. Short communication: Methicilin-resistant Staphylococcus aureus in conventional and organic dairy herds in Germany. J. Dairy Sci. 2018, 101, 3380–3386. [Google Scholar] [CrossRef] [PubMed]
- Spohr, M.; Rau, J.; Friedrich, A.; Kitich, G.; Fetsch, A.; Guerra, B.; Hammerl, J.A.; Tenhagen, B.A. Methicillin-resistent Staphylococcus aureus (MRSA) in three dairy herds in sothwest Germany. Zoonoses Public Health 2011, 58, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, C.; Cremonesi, P.; Caprioli, A.; Carfora, V.; Lanzano, A.; Barbeiro, A.; Morandi, S.; Casula, A.; Castiglioni, B.; Bronzo, V.; et al. Occurence of methicilin-resistant Staphylococcus aureus in dairy cattle herds, related swine farms, and humans in contact with herds. J. Dairy Sci. 2017, 100, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Basanisi, M.G.; La Bella, G.; Nobili, G.; Franconieri, L.; La Salandra, G. Genotyping of methicillin-resistant Staphylococcus aureus (MRSA) isolated from milk and dairy products in South Italy. Food Microbiol. 2017, 62, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Pajić, M.J.; Rašić, Z.B.; Velebit, B.M.; Bološ, S.P.; Mihajlović-Ukropina, M.M.; Radinović, M.Ž.; Galfi, A.L.; Petković, J.M.; Trojačanec, S.I. The prevalence of mecA, mecC and PVL genes in Staphylococcus aureus of bovine and human origin. Vet. Arh. 2014, 84, 205–214. [Google Scholar]
- Garcia-Alvarez, L.; Holden, M.T.; Lindsay, H.; Webb, C.R.; Brown, D.F.; Curran, M.D.; Walpole, E.; Brooks, K.; Pickard, D.J.; Teale, C.; et al. Meticillin-resistant Staphylococcus aureus with a novel mecA homologue in human and bovine populations in the UK and Denmark: A descriptive study. Lancet Infect. Dis. 2011, 11, 595–603. [Google Scholar] [CrossRef]
- Bietrix, J.; Kolenda, C.; Sapin, A.; Madec, J.Y.; Bes, M.; Dupieux, C.; Tasse, J.; Laurent, F. Persistence and diffusion of mecC-positive CC130 MRSA Isolates in dairy farms in Meurthe-et-Moselle County (France). Front. Microbiol. 2019, 10, 47. [Google Scholar] [CrossRef]
- Becker, K.; Ballhausen, B.; Köch, R.; Kriegeskorte, A. Methicillin resistance in Staphylococcus isolates: The “mec alphabet” with specific consideration of mecC, a mec homolog associated with zoonotic lineages. Int. J. Med. Microbiol. 2014, 304, 794–804. [Google Scholar] [CrossRef]
- Aires de Sousa, M. Methicillin-resistant Staphylococcus aureus among animals: Current overview. Clin. Microb. Infect. 2017, 23, 373–380. [Google Scholar] [CrossRef]
- Ikawaty, R.; Brouwer, E.C.; Jansen, M.D.; Van Duijkeren, E.; Mevius, D.; Verhoef, J.; Flui, A.C. Characterization of Dutch Staphylococcus aureus from bovine mastitis using a Multiple Locus Variable Number Tandem Repeat Analysis. Vet. Microbiol. 2009, 136, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Van Duijkeren, E.; Hengeveld, P.D.; Albers, M.; Pluister, G.; Jacobs, P.; Heres, L.; Van der Giessen, A.W. Prevalence of methicillin-resistant Staphylococcus aureus carrying mecA or mecC in dairy cattle. Vet. Microbiol. 2014, 171, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Jaki Tkalec, V.; Majnarić, D.; Jurmanović, J.; Habrun, B.; Cvetnić, Ž.; Zadravec, M.; Šeol Martinec, B. Methicillin-resistant Staphylococcus aureus in cows with mastitis, the presence of the mecA gene and the gene for virulence. Mljekarstvo 2015, 65, 259–268. (In Croatian) [Google Scholar]
- Strommenger, B.; Kettlitz, C.; Weniger, T.; Harmsen, D.; Friedrich, A.W.; Witte, W. Assignment of staphylococcus isolates to groups by spa typing, smaI macrorestriction analysis, and multilocus sequence typing. J. Clin. Microbiol. 2006, 44, 2533–2540. [Google Scholar] [CrossRef] [PubMed]
- Boss, R.; Cosandey, A.; Luini, M.; Arturson, K.; Bardiau, M.; Breitenwieser, F.; Hehenberger, E.; Lam, T.; Mansfeld, M.; Michel, A.; et al. Bovine Staphylococcus aureus: Subtyping, evolution, and zoonotic transfer. J. Dairy Sci. 2016, 99, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.M.; Green, L.E.; Medley, G.F.; Bird, H.E.; Fox, L.K.; Schukken, Y.H.; Kruze, J.V.; Bradley, A.J.; Zadoks, R.N.; Dowson, C.G. Sequence typing of intercontinental bovine Staphylococcus aureus isolates. J. Clin. Microbiol. 2005, 43, 4737–4743. [Google Scholar] [CrossRef] [PubMed]
- Smyth, D.S.; Feil, E.J.; Meaney, W.J.; Hartigan, P.J.; Tollersrud, T.; Fitzgerald, J.R.; Enright, M.C.; Smyth, C.J. Molecular genetic typing reveals further insights into the diversity of animal-associated Staphylococcus aureus. J. Med. Microbiol. 2009, 58, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, H.J.; Mork, T.; Caugant, D.A.; Kearns, A.; Rorvik, L.M. Genetic Variation among Staphylococcus Aureus Strains from Norwegian Bulk Milk. Appl. Environ. Microbiol. 2005, 71, 8352–8361. [Google Scholar] [CrossRef]
- Bergonier, D.; Sobral, D.; Feßler, A.T.; Jacquet, E.; Gibert, F.B.; Schwartz, S.; Treilles, M.; Bouloc, P.; Pourcel, C.; Vergnaud, G. Staphylococcus aureus from 152 cases of bovine, ovine and caprine mastitis investigated by Multiple-locus variable number of tandem repeat analysis (MLVA). Vet. Res. 2014, 45, 97. [Google Scholar] [CrossRef]
- Voss, A.; Loeffen, F.; Bakker, J.; Klaassen, C.; Wulf, M. Methicillin resistant Staphylococcus aureus in pig farming. Emerg. Infect. Dis. 2005, 11, 1965–1966. [Google Scholar] [CrossRef]
- Van Cleef, B.A.G.; Monnet, D.L.; Voss, A.; Krzivanek, K.; Allerberger, F.; Struelens, M.; Zemlickova, H.; Skov, R.L.; Vuopio-Varkila, J.; Cuny, C.; et al. Livestock associated methicillin-resistant Staphylococcus aureus in humans, Europe. Emer. Infect. Dis. 2011, 17, 502–505. [Google Scholar] [CrossRef]
- Bal, A.M.; Coombs, G.W.; Holden, M.T.G.; Lindsay, J.A.; Nimmo, G.R.; Tattevin, P.; Skov, R.L. Genomic insights into the emergence and spread of international clones of healthcare-, community- and livestock-associated meticillin-resistant Staphylococcus aureus: Blurring of the traditional definitions. J. Glob. Antim. Resist. 2016, 6, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Ben Zakour, N.L.; Guinane, C.M.; Fitzgerald, J.R. Pathogenomics of the staphylococci: Insights into niche adaptation and the emergence of new virulent strains. FEMS Microbiol. Lett. 2008, 289, 1–12. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cremonesi, P.; Pozzi, F.; Raschetti, M.; Bignoli, G.; Capra, E.; Graber, H.U.; Vezzoli, F.; Piccinin, R.; Bertasi, B.; Biffani, S.; et al. Genomic characteristics of Staphylococcus aureus strains associated with high within-herd prevalence of intramammary infections in dairy cows. J. Dairy Sci. 2015, 98, 6828–6838. [Google Scholar] [CrossRef] [PubMed]
- Dastmalchi, S.H.; Panahi, M. Genotyping and antimicrobial resistance of Staphylococcus aureus isolates from dairy ruminants: Differences in the distribution of clonal types between cattle and small ruminants. Arch. Microbiol. 2020, 202, 115–125. [Google Scholar] [CrossRef]
- Grundmann, H.; Schouls, L.M.; Aanensen, D.M.; Pluister, G.N.; Tami, A.; Chlebowicz, M.; Glasner, C.; Sabat, A.J.; Weist, K.; Heuer, O.; et al. The dynamic changes of dominant clones of Staphylococcus aureus causing bloodstream infections in the European region: Results of a second structured survey. EuroSurveillance 2014, 19, 20987. [Google Scholar] [CrossRef]
- Sakwinska, O.; Giddey, M.; Morreillon, M.; Morisset, D.; Waldvogel, A.; Moreillon, P. Staphylococcus aureus host range and human-bovine host shift. Appl. Environ. Microbiol. 2011, 77, 5908–5915. [Google Scholar] [CrossRef]
- Magro, G.; Rebolini, M.; Beretta, D.; Picinini, R. Methicillin-resistant Staphylococcus aureus CC-MRSA-IV as an agent of dairy cow intramammary infections. Vet. Microbiol. 2018, 227, 29–33. [Google Scholar] [CrossRef]
| County of | Year | Total | ||||
|---|---|---|---|---|---|---|
| 2014 | 2015 | 2016 | 2017 | 2018 | ||
| Osijek-Baranja | 4 | 3 | 2 | 4 | 2 | 15 |
| Zagreb | 3 | 37 | 18 | 1 | - | 59 |
| Karlovac | 2 | 1 | 20 | - | 1 | 24 |
| Varaždin | 1 | - | 5 | - | 1 | 7 |
| Požega-Slavonija | 4 | - | 9 | - | - | 13 |
| Međimurje | 1 | 20 | 2 | - | - | 23 |
| City of Zagreb | 4 | 3 | 8 | 19 | - | 34 |
| Bjelovar-Bilogora | 1 | 1 | 5 | - | 2 | 9 |
| Koprivnica-Križevci | 1 | 1 | 7 | - | - | 9 |
| Krapina-Zagorje | - | 2 | 6 | 4 | 4 | 16 |
| Brod-Posavina | - | - | 8 | - | - | 8 |
| Sisak-Moslavina | - | - | 5 | 4 | - | 9 |
| Lika-Senj | - | - | 3 | 3 | - | 6 |
| Šibenik-Knin | - | - | 2 | - | - | 2 |
| Zadar | - | - | - | 1 | - | 1 |
| Istra | - | - | - | - | 2 | 2 |
| Total | 21 | 68 | 100 | 36 | 12 | 237 |
| Method | Primer | Sequence |
|---|---|---|
| Multiplex PCR | spa-1113F | 5′-TAAAGACGATCCTTCGGTGAGC-3′ |
| spa-1514R | 5′-CAGCAGTAGTGCCGTTTGCTT-3′ | |
| mecA P4 | 5′-TCCAGATTACAACTTCACCAGG-3′ | |
| mecA P7 | 5′-CCACTTCATATCTTGTAACG-3′ | |
| pvl-F | 5′-GCTGGACAAAACTTCTTGGAATAT-3′ | |
| pvl-R | 5′-GATAGGACACCAATAAATTCTGGATTG-3′ | |
| mecALGA251 MultiFP | 5′-GAAAAAAAGGCTTAGAACGCCTC-3′ | |
| mecALGA251 MultiRP | 5′-GAAGATCTTTTCCGTTTTCAGC-3′ | |
| Spa typing | spaAF1 | 5′-GACGATCCTTCGGTGAGC-3′ |
| spaAR1 | 5′-CAGCAGTAGTGCCGTTTGC-3′ | |
| MLST | arc up | 5′-TTGATTCACCAGCGCGTATTGTC-3′ |
| arc dn | 5′-AGGTATCTGCTTCAATCAGCG-3′ | |
| aro up | 5′-ATCGGAAATCCTATTTCACATTC-3′ | |
| aro dn | 5′-GGTGTTGTATTAATAACGATATC-3′ | |
| glp up | 5′-CTAGGAACTGCAATCTTAATCC-3′ | |
| glp dn | 5′-TGGTAAAATCGCATGTCCAATTC-3′ | |
| gmk up | 5′-ATCGTTTTATCGGGACCATC-3′ | |
| gmk dn | 5′-TCATTAACTACAACGTAATCGTA-3′ | |
| pta up | 5′-GTTAAAATCGTATTACCTGAAGG-3′ | |
| pta dn | 5′-GACCCTTTTGTTGAAAAGCTTAA-3′ | |
| tpi up | 5′-TCGTTCATTCTGAACGTCGTGAA-3′ | |
| tpi dn | 5′-TTTGCACCTTCTAACAATTGTAC-3′ | |
| yqi up | 5′-CAGCATACAGGACACCTATTGGC-3′ | |
| yqi dn | 5′-CGTTGAGGAATCGATACTGGAAC-3′ |
| spa-Type | N Strains | % | N Counties | MRSA | MSSA | spa-Type Repeats |
|---|---|---|---|---|---|---|
| NEW 1 | 1 | 0.67 | 1 | - | 1 | 07-23-12-21-22-17-34-34-34-34-33-34 |
| NEW 2 | 1 | 0.67 | 1 | - | 1 | 08-25-51-68-02-24-02-24 |
| NEW 3 | 3 | 2.00 | 1 | - | 3 | 08-16-16-17 |
| NEW 4 | 3 | 2.00 | 1 | - | 3 | 07-23-21-17-13-34-33-34 |
| t005 | 1 | 0.67 | 1 | - | 1 | 26-23-13-23-31-05-17-25-17-25-16-28 |
| t008 | 1 | 0.67 | 1 | - | 1 | 11-19-12-21-17-34-24-34-22-25 |
| t011 | 1 | 0.67 | 1 | 1 | - | 08-16-02-25-34-24-25 |
| t015 | 1 | 0.67 | 1 | - | 1 | 08-16-02-16-34-13-17-34-16-34 |
| t024 | 1 | 0.67 | 1 | - | 1 | 11-12-21-17-34-24-34-22-25 |
| t056 | 2 | 1.33 | 2 | - | 2 | 04-20-12-17-20-17-12-17-17 |
| t091 | 11 | 7.33 | 7 | - | 11 | 07-23-21-17-34-12-23-02-12-23 |
| t1023 | 2 | 1.33 | 2 | - | 1 | 07-06-34 * |
| t10400 | 2 | 1.33 | 2 | - | 2 | 03-16-16-17-17-17-17-23-24 |
| t1139 | 1 | 0.67 | 1 | - | 1 | 26-23-13-17-25-17-25-16-28 |
| t1166 | 1 | 0.67 | 1 | - | 1 | 03-16-21-17-23-13-17-17-17-23-24 |
| t12029 | 10 | 6.67 | 4 | - | 10 | 04-31-17-24-17 |
| t12032 | 2 | 1.33 | 1 | - | 2 | 03-16-16-17-17-17-23-24 |
| t1236 | 1 | 0.67 | 1 | - | 1 | 26-23-12-21-17-34-34-34-33-34 |
| t1255 | 1 | 0.67 | 1 | - | 1 | 08-16-34-24-25 |
| t1430 | 1 | 0.67 | 1 | - | 1 | 07-16-23-02-12-23-02-34 |
| t15189 | 2 | 1.33 | 1 | - | 2 | 07-23-12-21-33-34 |
| t1534 | 2 | 1.33 | 1 | - | 2 | 04-31-17-25-17-17 |
| t1535 | 1 | 0.67 | 1 | - | 1 | 04-82-17-25-17-25-16-17 |
| t156 | 1 | 0.67 | 1 | - | 1 | 07-23-12-33-22-17 |
| t164 | 1 | 0.67 | 1 | - | 1 | 07-06-17-21-34-34-22-34 |
| t1897 | 1 | 0.67 | 1 | - | 1 | 26-23-34-21-17-34-34-34-34-33-34 |
| t1928 | 1 | 0.67 | 1 | - | 1 | 08-02-25-02-25-34-24-25 |
| t203 | 4 | 2,67 | 3 | - | 4 | 07-23-12-12-34-34-33-34 |
| t2094 | 4 | 2.67 | 1 | - | 4 | 26-06-17-21-34-34-22-34 |
| t267 | 11 | 7.33 | 3 | - | 11 | 07-23-12-21-17-34-34-34-33-34 |
| t2678 | 21 | 14.00 | 4 | - | 21 | 03-16-12-21-17-23-13-17-17-17-23-24 |
| t2802 | 1 | 0.67 | 1 | 1 | 07-23-21-17-34-34-34-33-34 | |
| t2873 | 8 | 5.33 | 3 | - | 8 | 04-20-17-31-24 |
| t3380 | 1 | 0.67 | 1 | - | 1 | 07-23-12-21-17-34-34 |
| t359 | 3 | 2.00 | 2 | - | 3 | 07-23-12-21-17-34-34-33-34 |
| t376 | 2 | 1.33 | 1 | - | 2 | 07-23-12-34-34-34-33-34 |
| t518 | 3 | 2.00 | 2 | - | 3 | 04-20-17-23-24-20-17-25 |
| t519 | 1 | 0.67 | 1 | - | 1 | 04-20-17-25 |
| t521 | 4 | 2.67 | 3 | - | 4 | 07-23-12-21-17-34-34-34-34-33-34 |
| t529 | 12 | 8.00 | 3 | 1 | 11 | 04-34 |
| t5505 | 1 | 0.67 | 1 | 1 | 08-16-315-25-02-25-34-24-25 | |
| t5695 | 2 | 1.33 | 1 | - | 2 | 07-23-12-21-17-34-34-34-34-34 |
| t622 | 1 | 0.67 | 1 | - | 1 | 11-19-12-21-17-34-22-25 |
| t701 | 2 | 1.33 | 1 | - | 2 | 11-10-21-17-34-24-34-22-25-25 |
| t7295 | 1 | 0.67 | 1 | - | 1 | 04-31-17-17 |
| t7630 | 1 | 0.67 | 1 | - | 1 | 04-31-17-25-17-17-17-17 |
| t774 | 1 | 0.67 | 1 | - | 1 | 07-23-12-34-34-12-12-12-23-02-12-23 |
| t843 | 3 | 2.00 | 2 | 1 | 2 | 04-82-17-25-17-25-25-16-17 |
| t8986 | 1 | 0.67 | 1 | - | 1 | 11-10-21-17-34-24-34-17-25-25 |
| t9129 | 1 | 0.67 | 1 | - | 1 | 07-16-21-17-34-34-34-33-34 |
| t934 | 1 | 0.67 | 1 | - | 1 | 07-23-12-34-34-34-34-33-34 |
| t9417 | 3 | 2.00 | 1 | - | 3 | 15-17-34-34-34-34-33-34 |
| t9485 | 1 | 0.67 | 1 | - | 1 | 04-20-23-24-20-17-25 |
| ST | N Isolates * | ST (%) | Alellic Profile | eBURST | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| arcC | aroE | glpF | gmk | pta | tpi | yqiL | ||||
| 1 | 1 | 0.51 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | G6 |
| 6 | 4 | 2.02 | 12 | 4 | 1 | 4 | 12 | 1 | 3 | single |
| 7 | 12 | 6.06 | 5 | 4 | 1 | 4 | 4 | 6 | 3 | G4 |
| 8 | 2 | 1.01 | 3 | 3 | 1 | 1 | 4 | 4 | 3 | G3 |
| 9 | 1 | 0.51 | 3 | 3 | 1 | 1 | 1 | 1 | 10 | single |
| 12 | 2 | 1.01 | 1 | 3 | 1 | 8 | 11 | 5 | 11 | single |
| 15 | 3 | 1.52 | 13 | 13 | 1 | 1 | 12 | 11 | 13 | single |
| 20 | 8 | 4.04 | 4 | 9 | 1 | 8 | 1 | 10 | 8 | single |
| 22 | 2 | 1.01 | 7 | 6 | 1 | 5 | 8 | 8 | 6 | single |
| 45 | 2 | 1.01 | 10 | 14 | 8 | 6 | 10 | 3 | 2 | single |
| 50 | 5 | 2.53 | 16 | 16 | 12 | 2 | 13 | 13 | 15 | G7 |
| 71 | 2 | 1.01 | 18 | 1 | 1 | 1 | 1 | 5 | 3 | G1 |
| 97 | 21 (4) | 10.61 | 3 | 1 | 1 | 1 | 1 | 5 | 3 | G1 |
| 101 | 3 | 1.52 | 3 | 1 | 14 | 15 | 11 | 19 | 3 | single |
| 130 | 3 (3) | 1.52 | 6 | 57 | 45 | 2 | 7 | 58 | 52 | single |
| 133 | 41 | 20.71 | 6 | 66 | 46 | 2 | 7 | 50 | 18 | single |
| 151 | 3 | 1.52 | 6 | 72 | 12 | 43 | 49 | 67 | 59 | single |
| 352 | 20 | 10.10 | 3 | 78 | 1 | 1 | 1 | 5 | 3 | G1 |
| 398 | 3 (3) | 1.52 | 3 | 35 | 19 | 2 | 20 | 26 | 39 | G2 |
| 479 | 2 | 1.01 | 52 | 79 | 54 | 18 | 56 | 32 | 65 | G5 |
| 504 | 11 | 5.56 | 6 | 72 | 72 | 43 | 52 | 67 | 59 | single |
| 522 | 18 | 9.09 | 18 | 95 | 45 | 2 | 7 | 15 | 5 | single |
| 573 | 1 | 0.51 | 1 | 1 | 1 | 1 | 12 | 1 | 1 | G6 |
| 789 | 1 | 0.51 | 3 | 4 | 1 | 4 | 4 | 6 | 3 | G4 |
| 1094 | 3 | 1.52 | 10 | 35 | 19 | 2 | 49 | 26 | 39 | single |
| 1380 | 4 | 2.02 | 169 | 79 | 54 | 18 | 56 | 32 | 65 | G5 |
| 2176 | 1 | 0.51 | 3 | 3 | 1 | 1 | 4 | 243 | 3 | G3 |
| 2826 | 10 | 4.55 | 3 | 1 | 1 | 1 | 309 | 5 | 3 | G1 |
| 3226 | 1 | 0.51 | 3 | 35 | 19 | 2 | 20 | 352 | 39 | G2 |
| 5858 | 1 | 0.51 | 52 | 79 | 54 | 18 | 56 | 32 | 18 | G5 |
| 5859 | 1 | 0.51 | 3 | 78 | 1 | 1 | 1 | 45 | 3 | G1 |
| 5406 | 2 | 1.01 | 3 | 16 | 12 | 2 | 13 | 13 | 15 | G7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cvetnić, L.; Samardžija, M.; Duvnjak, S.; Habrun, B.; Cvetnić, M.; Jaki Tkalec, V.; Đuričić, D.; Benić, M. Multi Locus Sequence Typing and spa Typing of Staphylococcus aureus Isolated from the Milk of Cows with Subclinical Mastitis in Croatia. Microorganisms 2021, 9, 725. https://doi.org/10.3390/microorganisms9040725
Cvetnić L, Samardžija M, Duvnjak S, Habrun B, Cvetnić M, Jaki Tkalec V, Đuričić D, Benić M. Multi Locus Sequence Typing and spa Typing of Staphylococcus aureus Isolated from the Milk of Cows with Subclinical Mastitis in Croatia. Microorganisms. 2021; 9(4):725. https://doi.org/10.3390/microorganisms9040725
Chicago/Turabian StyleCvetnić, Luka, Marko Samardžija, Sanja Duvnjak, Boris Habrun, Marija Cvetnić, Vesna Jaki Tkalec, Dražen Đuričić, and Miroslav Benić. 2021. "Multi Locus Sequence Typing and spa Typing of Staphylococcus aureus Isolated from the Milk of Cows with Subclinical Mastitis in Croatia" Microorganisms 9, no. 4: 725. https://doi.org/10.3390/microorganisms9040725
APA StyleCvetnić, L., Samardžija, M., Duvnjak, S., Habrun, B., Cvetnić, M., Jaki Tkalec, V., Đuričić, D., & Benić, M. (2021). Multi Locus Sequence Typing and spa Typing of Staphylococcus aureus Isolated from the Milk of Cows with Subclinical Mastitis in Croatia. Microorganisms, 9(4), 725. https://doi.org/10.3390/microorganisms9040725







