The Microbiome-Gut-Brain Axis and Resilience to Developing Anxiety or Depression under Stress
Abstract
1. Introduction
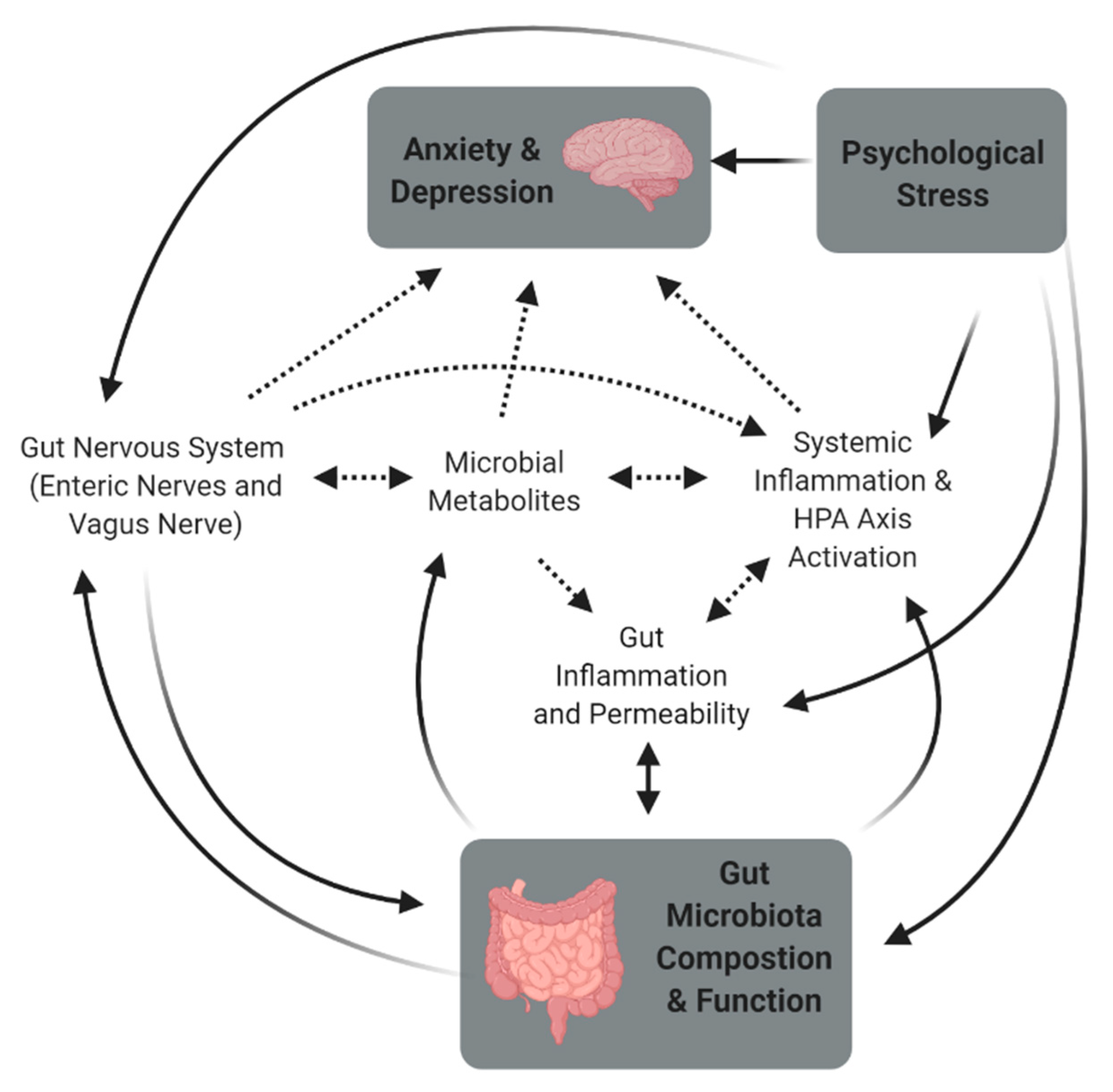
2. Stress and the Microbiome-Gut-Brain Axis
2.1. The Link between the Gut Microbiota and Behavior
2.2. Inconsistencies and Problems with MGBA Research
2.3. Stress, the Gut Microbiota, and Behavior
3. Mechanisms Associated with Stress-Induced Changes in the Gut
3.1. Gut and Systemic Inflammation
3.2. Gut Permeability
3.3. Dysbiosis and Hypothalamic-Pituitary-Adrenal Axis Dysfunction
3.4. Metabolites
3.5. Gut Nervous System—Enteric Nerves and Vagus Nerve
4. Early Life Programming
5. Therefore, Could the Gut Microbiota Be Key in Stress-Resilience?
Considerations for Future Research
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Elwenspoek, M.M.C.; Kuehn, A.; Muller, C.P.; Turner, J.D. The effects of early life adversity on the immune system. Psychoneuroendocrinology 2017, 82, 140–154. [Google Scholar] [CrossRef] [PubMed]
- Kendler, K.S.; Neale, M.C.; Kessler, R.C.; Heath, A.C.; Eaves, L.J. Major depression and generalized anxiety disorder: Same genes, (partly) different environments? Arch. Gen. Psychiatry 1992, 49, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Toomey, R.; Panizzon, M.S.; Kremen, W.S.; Franz, C.E.; Lyons, M.J. A twin-study of genetic contributions to morningness-eveningness and depression. Chronobiol. Int. J. Biol. Med. Rhythm Res. 2015, 32, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.C.; Bland, R.C. Life events and the 1-year prevalence of major depressive episode, generalized anxiety disorder, and panic disorder in a community sample. Comp. Psychiatry 1994, 35, 76–82. [Google Scholar]
- Mazure, C.M. Life stressors as risk factors in depression. Clin. Psychol. Sci. Pract. 1998, 5, 291–313. [Google Scholar] [CrossRef]
- Pfau, M.L.; Russo, S.J. Peripheral and central mechanisms of stress resilience. Neurobiol. Stress 2015, 1, 66–79. [Google Scholar] [CrossRef]
- Franklin, T.B.; Saab, B.J.; Mansuy, I.M. Neural mechanisms of stress resilience and vulnerability. Neuron 2012, 75, 747–761. [Google Scholar] [CrossRef]
- Smith, J.; Prior, M. Temperament and stress resilience in school-age children: A within-families study. J. Am. Acad. Child. Psychiatry 1995, 34, 168–179. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Bailey, M.T.; Dowd, S.E.; Galley, J.D.; Hufnagle, A.R.; Allen, R.G.; Lyte, M. Exposure to a social stressor alters the structure of the intestinal microbiota: Implications for stressor-induced immunomodulation. Brain Behav. Immun. 2011, 25, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Bangsgaard Bendtsen, K.M.; Krych, L.; Sørensen, D.B.; Pang, W.; Nielsen, D.S.; Josefsen, K.; Hansen, L.H.; Sørensen, S.J.; Hansen, A.K. Gut microbiota composition is correlated to grid floor induced stress and behavior in the balb/c mouse. PLoS ONE 2012, 7, e46231. [Google Scholar]
- Galley, J.D.; Yu, Z.; Kumar, P.; Dowd, S.E.; Lyte, M.; Bailey, M.T. The structures of the colonic mucosa-associated and luminal microbial communities are distinct and differentially affected by a prolonged murine stressor. Gut Microbes 2014, 5, 748–760. [Google Scholar] [CrossRef] [PubMed]
- Tannock, G.W.; Savage, D.C. Influences of dietary and environmental stress on microbial populations in the murine gastrointestinal tract. Infect. Immun. 1974, 9, 591–598. [Google Scholar] [CrossRef]
- Marin, I.A.; Goertz, J.E.; Ren, T.; Rich, S.S.; Onengut-Gumuscu, S.; Farber, E.; Wu, M.; Overall, C.C.; Kipnis, J.; Gaultier, A. Microbiota alteration is associated with the development of stress-induced despair behavior. Sci. Rep. 2017, 7, 43859. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, S.M.; Marchesi, J.R.; Scully, P.; Codling, C.; Ceolho, A.M.; Quigley, E.M.; Cryan, J.F.; Dinan, T.G. Early life stress alters behavior, immunity, and microbiota in rats: Implications for irritable bowel syndrome and psychiatric illnesses. Biol. Psychiatry 2009, 65, 263–267. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59. [Google Scholar] [CrossRef]
- Kurina, L.; Goldacre, M.; Yeates, D.; Gill, L. Depression and anxiety in people with inflammatory bowel disease. J. Epidemiol. Community Health 2001, 55, 716–720. [Google Scholar] [CrossRef]
- Addolorato, G.; Capristo, E.; Stefanini, G.F.; Gasbarrini, G. Inflammatory bowel disease: A study of the association between anxiety and depression, physical morbidity, and nutritional status. Scand. J. Gastroenterol. 1997, 32, 1013–1021. [Google Scholar] [CrossRef]
- Lydiard, R.B. Irritable bowel syndrome, anxiety, and depression: What are the links? J. Clin. Psychiatry 2001, 62, 38–47. [Google Scholar]
- Masand, P.S.; Kaplan, D.S.; Gupta, S.; Bhandary, A.N.; Nasra, G.S.; Kline, M.D.; Margo, K.L. Major depression and irritable bowel syndrome: Is there a relationship? J. Clin. Psychiatry 1995, 56, 363–367. [Google Scholar]
- Naseribafrouei, A.; Hestad, K.; Avershina, E.; Sekelja, M.; Linlokken, A.; Wilson, R.; Rudi, K. Correlation between the human fecal microbiota and depression. J. Neurogastroenterol. Motil. 2014, 26, 1155–1162. [Google Scholar] [CrossRef]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-Y.; Zhang, X.; Yu, Z.-H.; Zhang, Z.; Deng, M.; Zhao, J.-H.; Ruan, B. Altered gut microbiota profile in patients with generalized anxiety disorder. J. Psychiatry Res. 2018, 104, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Tillisch, K.; Mayer, E.; Gupta, A.; Gill, Z.; Brazeilles, R.; Le Nevé, B.; van Hylckama Vlieg, J.E.; Guyonnet, D.; Derrien, M.; Labus, J.S. Brain structure and response to emotional stimuli as related to gut microbial profiles in healthy women. Psychosom. Med. 2017, 79, 905. [Google Scholar] [CrossRef] [PubMed]
- Lyte, M.; Li, W.; Opitz, N.; Gaykema, R.P.; Goehler, L.E. Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium. Physiol. Behav. 2006, 89, 350–357. [Google Scholar] [CrossRef]
- Lyte, M.; Varcoe, J.J.; Bailey, M.T. Anxiogenic effect of subclinical bacterial infection in mice in the absence of overt immune activation. Physiol. Behav. 1998, 65, 63–68. [Google Scholar] [CrossRef]
- Bercik, P.; Verdu, E.F.; Foster, J.A.; Macri, J.; Potter, M.; Huang, X.; Malinowski, P.; Jackson, W.; Blennerhassett, P.; Neufeld, K.-A.M.; et al. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology 2010, 139, 2102. [Google Scholar] [CrossRef]
- Nishino, R.; Mikami, K.; Takahashi, H.; Tomonaga, S.; Furuse, M.; Hiramoto, T.; Aiba, Y.; Koga, Y.; Sudo, N. Commensal microbiota modulate murine behaviors in a strictly contamination-free environment confirmed by culture-based methods. J. Neurogastroenterol. Motil. 2013, 25, 521. [Google Scholar] [CrossRef] [PubMed]
- Crumeyrolle-Arias, M.; Jaglin, M.; Bruneau, A.; Vancassel, S.; Cardona, A.; Dauge, V.; Naudon, L.; Rabot, S. Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats. Psychoneuroendocrinology 2014, 42, 207–217. [Google Scholar] [CrossRef]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 2013, 18, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, K.M.; Kang, N.; Bienenstock, J.; Foster, J.A. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. J. Neurogastroenterol. Motil. 2011, 23, 255–264.e119. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W.J. Psychobiotics and the manipulation of bacteria–gut–brain signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef] [PubMed]
- Abildgaard, A.; Elfving, B.; Hokland, M.; Wegener, G.; Lund, S. Probiotic treatment reduces depressive-like behaviour in rats independently of diet. Psychoneuroendocrinology 2017, 79, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central gaba receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, C.; Ronaghan, N.; Zaheer, R.; Dicay, M.; Le, T.; MacNaughton, W.K.; Surrette, M.G.; Swain, M.G. Probiotics improve inflammation-associated sickness behavior by altering communication between the peripheral immune system and the brain. J. Neurosci. 2015, 35, 10821–10830. [Google Scholar] [CrossRef]
- Smith, C.J.; Emge, J.R.; Berzins, K.; Lung, L.; Khamishon, R.; Shah, P.; Rodrigues, D.M.; Sousa, A.J.; Reardon, C.; Sherman, P.M. Probiotics normalize the gut-brain-microbiota axis in immunodeficient mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G793–G802. [Google Scholar] [CrossRef]
- Bercik, P.; Park, A.; Sinclair, D.; Khoshdel, A.; Lu, J.; Huang, X.; Deng, Y.; Blennerhassett, P.; Fahnestock, M.; Moine, D. The anxiolytic effect of Bifidobacterium longum ncc3001 involves vagal pathways for gut–brain communication. J. Neurogastroenterol. Motil. 2011, 23, 1132–1139. [Google Scholar] [CrossRef]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Kiely, B.; Cryan, J.F.; Dinan, T.G. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 2010, 170, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Wang, T.; Hu, X.; Luo, J.; Li, W.; Wu, X.; Duan, Y.; Jin, F. Administration of Lactobacillus helveticus ns8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience 2015, 310, 561–577. [Google Scholar] [CrossRef] [PubMed]
- Barrera-Bugueño, C.; Realini, O.; Escobar-Luna, J.; Sotomayor-Zárate, R.; Gotteland, M.; Julio-Pieper, M.; Bravo, J.A. Anxiogenic effects of a Lactobacillus, inulin and the synbiotic on healthy juvenile rats. Neuroscience 2017, 359, 18–29. [Google Scholar] [CrossRef]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Bienenstock, J.; Dinan, T.G. The probiotic bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. J. Psychiatry Res. 2009, 43, 164–174. [Google Scholar] [CrossRef]
- Kelly, J.R.; Allen, A.P.; Temko, A.; Hutch, W.; Kennedy, P.J.; Farid, N.; Murphy, E.; Boylan, G.; Bienenstock, J.; Cryan, J.F.; et al. Lost in translation? The potential psychobiotic Lactobacillus rhamnosus (jb-1) fails to modulate stress or cognitive performance in healthy male subjects. Brain Behav. Immun. 2017, 61, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Simrén, M.; Ohman, L.; Olsson, J.; Svensson, U.; Ohlson, K.; Posserud, I.; Strid, H. Clinical trial: The effects of a fermented milk containing three probiotic bacteria in patients with irritable bowel syndrome—A randomized, double-blind, controlled study. Aliment. Pharmacol. Ther. 2010, 31, 218–227. [Google Scholar] [CrossRef]
- Akkasheh, G.; Kashani-Poor, Z.; Tajabadi-Ebrahimi, M.; Jafari, P.; Akbari, H.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z.; Esmaillzadeh, A. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. Nutrition 2016, 32, 315–320. [Google Scholar] [CrossRef]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.-F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus r0052 and Bifidobacterium longum r0175) in rats and human subjects. Br. J. Nutr. 2011, 105, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, M.; Violle, N.; Bisson, J.-F.; Desor, D.; Javelot, H.; Rougeot, C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus r0052 and Bifidobacterium longum r0175) in healthy human volunteers. Gut Microbes 2011, 2, 256–261. [Google Scholar] [CrossRef]
- Steenbergen, L.; Sellaro, R.; van Hemert, S.; Bosch, J.A.; Colzato, L.S. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav. Immun. 2015, 48, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Bested, A.C.; Beaulne, T.M.; Katzman, M.A.; Iorio, C.; Berardi, J.M.; Logan, A.C. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog. 2009, 1, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Benton, D.; Williams, C.; Brown, A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur. J. Clin. Nutr. 2006, 61, 355. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, S.; Mian, F.M.; Stanisz, A.M.; Bindels, L.B.; Cambier, E.; Ben-Amram, H.; Koren, O.; Forsythe, P.; Bienenstock, J. Low-dose penicillin in early life induces long-term changes in murine gut microbiota, brain cytokines and behavior. Nat. Commun. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; Macri, J.; McCoy, K.D. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 2011, 141, 599–609.e593. [Google Scholar] [CrossRef] [PubMed]
- Desbonnet, L.; Clarke, G.; Traplin, A.; O’Sullivan, O.; Crispie, F.; Moloney, R.D.; Cotter, P.D.; Dinan, T.G.; Cryan, J.F. Gut microbiota depletion from early adolescence in mice: Implications for brain and behaviour. Brain Behav. Immun. 2015, 48, 165–173. [Google Scholar] [CrossRef]
- Pirbaglou, M.; Katz, J.; de Souza, R.J.; Stearns, J.C.; Motamed, M.; Ritvo, P. Probiotic supplementation can positively affect anxiety and depressive symptoms: A systematic review of randomized controlled trials. Nutr. Res. 2016, 36, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Galley, J.D.; Nelson, M.C.; Yu, Z.; Dowd, S.E.; Walter, J.; Kumar, P.S.; Lyte, M.; Bailey, M.T. Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. BMC Microbiol. 2014, 14, 189. [Google Scholar] [CrossRef]
- Gautam, A.; Kumar, R.; Chakraborty, N.; Muhie, S.; Hoke, A.; Hammamieh, R.; Jett, M. Altered fecal microbiota composition in all male aggressor-exposed rodent model simulating features of post-traumatic stress disorder. J. Neurosci. Res. 2018, 96, 1311–1323. [Google Scholar] [CrossRef]
- Tsilimigras, M.C.B.; Gharaibeh, R.Z.; Sioda, M.; Gray, L.; Fodor, A.A.; Lyte, M. Interactions between stress and sex in microbial responses within the microbiota-gut-brain axis in a mouse model. Psychosom. Med. 2018, 80, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Gareau, M.G.; Jury, J.; MacQueen, G.; Sherman, P.M.; Perdue, M.H. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut 2007, 56, 1522. [Google Scholar] [CrossRef]
- Bailey, M.T.; Dowd, S.E.; Parry, N.M.; Galley, J.D.; Schauer, D.B.; Lyte, M. Stressor exposure disrupts commensal microbial populations in the intestines and leads to increased colonization by Citrobacter rodentium. Infect. Immun. 2010, 78, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Burokas, A.; Arboleya, S.; Moloney, R.D.; Peterson, V.L.; Murphy, K.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Targeting the microbiota-gut-brain axis: Prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol. Psychiatry 2017, 82, 472–487. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Fujita, Y.; Ren, Q.; Ma, M.; Dong, C.; Hashimoto, K. Bifidobacterium in the gut microbiota confer resilience to chronic social defeat stress in mice. Sci. Rep. 2017, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Galley, J.D.; Parry, N.M.; Ahmer, B.M.M.; Fox, J.G.; Bailey, M.T. The commensal microbiota exacerbate infectious colitis in stressor-exposed mice. Brain Behav. Immun. 2017, 60, 44–50. [Google Scholar] [CrossRef] [PubMed]
- McVey Neufeld, K.-A.; O’Mahony, S.M.; Hoban, A.E.; Waworuntu, R.V.; Berg, B.M.; Dinan, T.G.; Cryan, J.F. Neurobehavioural effects of Lactobacillus rhamnosus gg alone and in combination with prebiotics polydextrose and galactooligosaccharide in male rats exposed to early-life stress. Nutr. Neurosci. 2019, 22, 425–434. [Google Scholar] [CrossRef]
- Gur, T.L.; Shay, L.; Palkar, A.V.; Fisher, S.; Varaljay, V.A.; Dowd, S.; Bailey, M.T. Prenatal stress affects placental cytokines and neurotrophins, commensal microbes, and anxiety-like behavior in adult female offspring. Brain Behav. Immun. 2017, 64, 50–58. [Google Scholar] [CrossRef]
- Jašarević, E.; Howard, C.D.; Misic, A.M.; Beiting, D.P.; Bale, T.L. Stress during pregnancy alters temporal and spatial dynamics of the maternal and offspring microbiome in a sex-specific manner. Sci. Rep. 2017, 7, 44182. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.T.; Coe, C.L. Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Dev. Psychobiol. 1999, 35, 146–155. [Google Scholar] [CrossRef]
- Holdeman, L.V.; Good, I.J.; Moore, W.E. Human fecal flora: Variation in bacterial composition within individuals and a possible effect of emotional stress. App. Environ. Microbiol. 1976, 31, 359. [Google Scholar] [CrossRef]
- Zijlmans, M.A.C.; Korpela, K.; Riksen-Walraven, J.M.; de Vos, W.M.; de Weerth, C. Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology 2015, 53, 233–245. [Google Scholar] [CrossRef]
- Mayer, E.A. The neurobiology of stress and gastrointestinal disease. Gut 2000, 47, 861. [Google Scholar] [CrossRef]
- Castagliuolo, I.; Lamont, J.T.; Qiu, B.; Fleming, S.M.; Bhaskar, K.R.; Nikulasson, S.T.; Kornetsky, C.; Pothoulakis, C. Acute stress causes mucin release from rat colon: Role of corticotropin releasing factor and mast cells. Am. J. Physiol. Gastrointest. Liver Physiol. 1996, 271, G884–G892. [Google Scholar] [CrossRef]
- Santos, J.; Yang, P.; Soderholm, J.; Benjamin, M.; Perdue, M. Role of mast cells in chronic stress induced colonic epithelial barrier dysfunction in the rat. Gut 2001, 48, 630–636. [Google Scholar] [CrossRef]
- Lyte, M.; Vulchanova, L.; Brown, D.R. Stress at the intestinal surface: Catecholamines and mucosa-bacteria interactions. Cell Tissue Res. 2011, 343, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Spitz, J.; Hecht, G.; Taveras, M.; Aoys, E.; Alverdy, J. The effect of dexamethasone administration on rat intestinal permeability: The role of bacterial adherence. Gastroenterology 1994, 106, 35–41. [Google Scholar] [CrossRef]
- Vogelzangs, N.; Beekman, A.T.F.; de Jonge, P.; Penninx, B.W.J.H. Anxiety disorders and inflammation in a large adult cohort. Transl. Psychiatry 2013, 3, e249. [Google Scholar] [CrossRef] [PubMed]
- Kohler, C.A.; Freitas, T.H.; Maes, M.; de Andrade, N.Q.; Liu, C.S.; Fernandes, B.S.; Stubbs, B.; Solmi, M.; Veronese, N.; Herrmann, N.; et al. Peripheral cytokine and chemokine alterations in depression: A meta-analysis of 82 studies. Acta Psychiatry Scand. 2017, 135, 373–387. [Google Scholar] [CrossRef]
- O’Brien, S.M.; Scully, P.; Fitzgerald, P.; Scott, L.V.; Dinan, T.G. Plasma cytokine profiles in depressed patients who fail to respond to selective serotonin reuptake inhibitor therapy. J. Psychiatry Res. 2007, 41, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Mikocka-Walus, A.; Knowles, S.R.; Keefer, L.; Graff, L. Controversies revisited: A systematic review of the comorbidity of depression and anxiety with inflammatory bowel diseases. Inflamm. Bowel Dis. 2016, 22, 752–762. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Hylemon, P.B.; Ridlon, J.M.; Heuman, D.M.; Daita, K.; White, M.B.; Monteith, P.; Noble, N.A.; Sikaroodi, M.; Gillevet, P.M. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G675–G685. [Google Scholar] [CrossRef]
- De Angelis, M.; Francavilla, R.; Piccolo, M.; De Giacomo, A.; Gobbetti, M. Autism spectrum disorders and intestinal microbiota. Gut Microbes 2015, 6, 207–213. [Google Scholar] [CrossRef]
- Shen, L.; Liu, L.; Ji, H.-F. Alzheimer’s disease histological and behavioral manifestations in transgenic mice correlate with specific gut microbiome state. J. Alzheimer’s Dis. 2017, 56, 385–390. [Google Scholar] [CrossRef]
- Goehler, L.E.; Gaykema, R.P.; Opitz, N.; Reddaway, R.; Badr, N.; Lyte, M. Activation in vagal afferents and central autonomic pathways: Early responses to intestinal infection with campylobacter jejuni. Brain Behav. Immun. 2005, 19, 334–344. [Google Scholar] [CrossRef]
- Gaykema, R.P.; Goehler, L.E.; Tilders, F.J.; Bol, J.G.; McGorry, M.; Fleshner, M.; Maier, S.F.; Watkins, L.R. Bacterial endotoxin induces fos immunoreactivity in primary afferent neurons of the vagus nerve. Neuroimmunomodulation 1998, 5, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Erny, D.; Hrabe de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the cns. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Gaykema, R.P.; Goehler, L.E.; Lyte, M. Brain response to cecal infection with campylobacter jejuni: Analysis with fos immunohistochemistry. Brain Behav. Immun. 2004, 18, 238–245. [Google Scholar] [CrossRef]
- Rajkumar, H.; Kumar, M.; Das, N.; Kumar, S.N.; Challa, H.R.; Nagpal, R. Effect of probiotic Lactobacillus salivarius ubl s22 and prebiotic fructo-oligosaccharide on serum lipids, inflammatory markers, insulin sensitivity, and gut bacteria in healthy young volunteers: A randomized controlled single-blind pilot study. J. Cardiovasc. Pharmacol. Ther. 2015, 20, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Ait-Belgnaoui, A.; Durand, H.; Cartier, C.; Chaumaz, G.; Eutamene, H.; Ferrier, L.; Houdeau, E.; Fioramonti, J.; Bueno, L.; Theodorou, V. Prevention of gut leakiness by a probiotic treatment leads to attenuated hpa response to an acute psychological stress in rats. Psychoneuroendocrinology 2012, 37, 1885–1895. [Google Scholar] [CrossRef] [PubMed]
- Kittana, H.; Gomes-Neto, J.C.; Heck, K.; Geis, A.L.; Segura Muñoz, R.R.; Cody, L.A.; Schmaltz, R.J.; Bindels, L.B.; Sinha, R.; Hostetter, J.M.; et al. Commensal Escherichia coli strains can promote intestinal inflammation via differential interleukin-6 production. Front. Immunol. 2018, 9, 2318. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Kubera, M.; Leunis, J.-C. The gut-brain barrier in major depression: Intestinal mucosal dysfunction with an increased translocation of lps from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol. Lett. 2008, 29, 117–124. [Google Scholar]
- Bharwani, A.; Mian, M.F.; Surette, M.G.; Bienenstock, J.; Forsythe, P. Oral treatment with Lactobacillus rhamnosus attenuates behavioural deficits and immune changes in chronic social stress. BMC Med. 2017, 15, 7. [Google Scholar] [CrossRef]
- Smith, P.; Garrett, W. The gut microbiota and mucosal t cells. Front. Microbiol. 2011, 2, 111. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.S.; Vallance, B.A.; Blennerhassett, P.A.; Collins, S.M. The role of cd4+ lymphocytes in the susceptibility of mice to stress-induced reactivation of experimental colitis. Nat. Med. 1999, 5, 1178–1182. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Kubera, M.; Leunis, J.C.; Berk, M.; Geffard, M.; Bosmans, E. In depression, bacterial translocation may drive inflammatory responses, oxidative and nitrosative stress (o&ns), and autoimmune responses directed against o&ns-damaged neoepitopes. Acta Psychiatry Scand. 2012, 127, 344–354. [Google Scholar]
- Demaude, J.; Salvador-Cartier, C.; Fioramonti, J.; Ferrier, L.; Bueno, L. Phenotypic changes in colonocytes following acute stress or activation of mast cells in mice: Implications for delayed epithelial barrier dysfunction. Gut 2006, 55, 655. [Google Scholar] [CrossRef]
- Saunders, P.R.; Kosecka, U.; McKay, D.M.; Perdue, M.H. Acute stressors stimulate ion secretion and increase epithelial permeability in rat intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 1994, 267, G794–G799. [Google Scholar] [CrossRef] [PubMed]
- Lasselin, J.; Elsenbruch, S.; Lekander, M.; Axelsson, J.; Karshikoff, B.; Grigoleit, J.-S.; Engler, H.; Schedlowski, M.; Benson, S. Mood disturbance during experimental endotoxemia: Predictors of state anxiety as a psychological component of sickness behavior. Brain Behav. Immun. 2016, 57, 30–37. [Google Scholar] [CrossRef]
- Zhao, Q.Y.; Wu, X.H.; Yan, S.; Xie, X.F.; Fan, Y.H.; Zhang, J.Q.; Peng, C.; You, Z.L. The antidepressant-like effects of pioglitazone in a chronic mild stress mouse model are associated with ppar gamma-mediated alteration of microglial activation phenotypes. J. Neuroinflamm. 2016, 13, 259. [Google Scholar] [CrossRef]
- Qin, L.; Wu, X.; Block, M.L.; Liu, Y.; Breese, G.R.; Hong, J.-S.; Knapp, D.J.; Crews, F.T. Systemic lps causes chronic neuroinflammation and progressive neurodegeneration. Glia 2007, 55, 453–462. [Google Scholar] [CrossRef]
- Maes, M.; Kubera, M.; Leunis, J.C.; Berk, M. Increased iga and igm responses against gut commensals in chronic depression: Further evidence for increased bacterial translocation or leaky gut. J. Affect. Disord. 2012, 141, 55–62. [Google Scholar] [CrossRef]
- Kiliaan, A.J.; Saunders, P.R.; Bijlsma, P.B.; Berin, M.C.; Taminiau, J.A.; Groot, J.A.; Perdue, M.H. Stress stimulates transepithelial macromolecular uptake in rat jejunum. Am. J. Physiol. Gastrointest. Liver Physiol. 1998, 275, G1037. [Google Scholar] [CrossRef] [PubMed]
- Meddings, J.B.; Swain, M.G. Environmental stress–induced gastrointestinal permeability is mediated by endogenous glucocorticoids in the rat. Gastroenterology 2000, 119, 1019–1028. [Google Scholar] [CrossRef]
- Soderholm, J.D.; Yang, P.C.; Ceponis, P.; Vohra, A.; Riddell, R.; Sherman, P.M.; Perdue, M.H. Chronic stress induces mast cell-dependent bacterial adherence and initiates mucosal inflammation in rat intestine. Gastroenterology 2002, 123, 1099–1108. [Google Scholar] [CrossRef]
- Bhatia, V.; Tandon, R.K. Stress and the gastrointestinal tract. J. Gastroenterol. Hepatol. 2005, 20, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, S.; Matamoros, S.; Cani, P.D.; Neyrinck, A.M.; Jamar, F.; Starkel, P.; Windey, K.; Tremaroli, V.; Backhed, F.; Verbeke, K.; et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc. Natl. Acad. Sci. USA 2014, 111, E4485–E4493. [Google Scholar] [CrossRef] [PubMed]
- Eutamene, H.; Lamine, F.; Chabo, C.; Theodorou, V.; Rochat, F.; Bergonzelli, G.E.; Corthésy-Theulaz, I.; Fioramonti, J.; Bueno, L. Synergy between Lactobacillus paracasei and its bacterial products to counteract stress-induced gut permeability and sensitivity increase in rats. J. Nutr. 2007, 137, 1901–1907. [Google Scholar] [CrossRef]
- Arborelius, L.; Owens, M.; Plotsky, P.; Nemeroff, C. The role of corticotropin-releasing factor in depression and anxiety disorders. J. Endocrin. 1999, 160, 1–12. [Google Scholar] [CrossRef]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.-N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Pusceddu, M.M.; El Aidy, S.; Crispie, F.; O’Sullivan, O.; Cotter, P.; Stanton, C.; Kelly, P.; Cryan, J.F.; Dinan, T.G. N-3 polyunsaturated fatty acids (pufas) reverse the impact of early-life stress on the gut microbiota. PLoS ONE 2015, 10, e0139721. [Google Scholar] [CrossRef]
- Pusceddu, M.M.; Kelly, P.; Ariffin, N.; Cryan, J.F.; Clarke, G.; Dinan, T.G. N-3 pufas have beneficial effects on anxiety and cognition in female rats: Effects of early life stress. Psychoneuroendocrinology 2015, 58, 79–90. [Google Scholar] [CrossRef]
- Kelly, J.R.; Borre, Y.; O’Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatry Res. 2016, 82, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Silverman, M.N.; Pearce, B.D.; Biron, C.A.; Miller, A.H. Immune modulation of the hypothalamic-pituitary-adrenal (hpa) axis during viral infection. Viral Immunol. 2005, 18, 41–78. [Google Scholar] [CrossRef]
- Turnbull, A.V.; Rivier, C.L. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: Actions and mechanisms of action. Physiol. Rev. 1999, 79, 1–71. [Google Scholar] [CrossRef] [PubMed]
- Silverman, M.N.; Sternberg, E.M. Glucocorticoid regulation of inflammation and its functional correlates: From hpa axis to glucocorticoid receptor dysfunction. Ann. N. Y. Acad. Sci. 2012, 1261, 55–63. [Google Scholar] [CrossRef]
- Asano, Y.; Hiramoto, T.; Nishino, R.; Aiba, Y.; Kimura, T.; Yoshihara, K.; Koga, Y.; Sudo, N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G1288–G1295. [Google Scholar] [CrossRef] [PubMed]
- Tsavkelova, E.; Klimova, S.Y.; Cherdyntseva, T.; Netrusov, A. Hormones and hormone-like substances of microorganisms: A review. Appl. Biochem. Microbiol. 2006, 42, 229–235. [Google Scholar] [CrossRef]
- Roshchina, V.V. Evolutionary considerations of neurotransmitters in microbial, plant, and animal cells. In Microbial Endocrinology; Springer: New York, NY, USA, 2010; pp. 17–52. [Google Scholar]
- Ross, R.P.; Mills, S.; Hill, C.; Fitzgerald, G.F.; Stanton, C. Specific metabolite production by gut microbiota as a basis for probiotic function. Int. Dairy J. 2010, 20, 269. [Google Scholar] [CrossRef]
- Lyte, M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: Microbial endocrinology in the design and use of probiotics. Bioessays 2011, 33, 574–581. [Google Scholar] [CrossRef]
- Holzer, P.; Farzi, A. Neuropeptides and the microbiota-gut-brain axis. In Microbial Endocrinology: The microbiota-Gut-Brain Axis in Health and Disease; Lyte, M., Cryan, J.F., Eds.; Springer: New York, NY, USA, 2014; Volume 817, pp. 195–219. [Google Scholar]
- Matsumoto, M.; Kibe, R.; Ooga, T.; Aiba, Y.; Kurihara, S.; Sawaki, E.; Koga, Y.; Benno, Y. Impact of intestinal microbiota on intestinal luminal metabolome. Sci. Rep. 2012, 2, 233. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; David, H.M.; et al. Activation of gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor gpr43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef]
- Stilling, R.M.; van de Wouw, M.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem. Int. 2016, 99, 110–132. [Google Scholar] [CrossRef] [PubMed]
- Skonieczna-Zydecka, K.; Grochans, E.; Maciejewska, D.; Szkup, M.; Schneider-Matyka, D.; Jurczak, A.; Loniewski, I.; Kaczmarczyk, M.; Marlicz, W.; Czerwinska-Rogowska, M.; et al. Faecal short chain fatty acids profile is changed in polish depressive women. Nutrients 2018, 10, 1939. [Google Scholar] [CrossRef] [PubMed]
- Maltz, R.M.; Keirsey, J.; Kim, S.C.; Mackos, A.R.; Gharaibeh, R.Z.; Moore, C.C.; Xu, J.; Somogyi, A.; Bailey, M.T. Social stress affects colonic inflammation, the gut microbiome, and short-chain fatty acid levels and receptors. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 533–540. [Google Scholar] [CrossRef] [PubMed]
- van de Wouw, M.; Boehme, M.; Lyte, J.M.; Wiley, N.; Strain, C.; O’Sullivan, O.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Short-chain fatty acids: Microbial metabolites that alleviate stress-induced brain–gut axis alterations. J. Physiol. 2018, 596, 4923–4944. [Google Scholar] [CrossRef] [PubMed]
- Bharwani, A.; Mian, M.F.; Foster, J.A.; Surette, M.G.; Bienenstock, J.; Forsythe, P. Structural & functional consequences of chronic psychosocial stress on the microbiome & host. Psychoneuroendocrinology 2016, 63, 217–227. [Google Scholar] [PubMed]
- Michels, N.; Van de Wiele, T.; De Henauw, S. Chronic psychosocial stress and gut health in children: Associations with calprotectin and fecal short-chain fatty acids. Psychosom. Med. 2017, 79, 927–935. [Google Scholar] [CrossRef]
- Marques, A.H.; Silverman, M.N.; Sternberg, E.M. Evaluation of stress systems by applying noninvasive methodologies: Measurements of neuroimmune biomarkers in the sweat, heart rate variability and salivary cortisol. Neuroimmunomodulation 2010, 17, 205–208. [Google Scholar] [CrossRef]
- West, C.; Wu, R.Y.; Wong, A.; Stanisz, A.M.; Yan, R.; Min, K.K.; Pasyk, M.; McVey Neufeld, K.A.; Karamat, M.I.; Foster, J.A.; et al. Lactobacillus rhamnosus strain jb-1 reverses restraint stress-induced gut dysmotility. Neurogastroenterol. Motil 2017, 29, e12903. [Google Scholar] [CrossRef]
- Dalile, B.; Vervliet, B.; Bergonzelli, G.; Verbeke, K.; Van Oudenhove, L. Colon-delivered short-chain fatty acids attenuate the cortisol response to psychosocial stress in healthy men: A randomized, placebo-controlled trial. Neuropsychopharmacology 2020, 45, 2257–2266. [Google Scholar] [CrossRef]
- Bassett, S.A.; Young, W.; Fraser, K.; Dalziel, J.E.; Webster, J.; Ryan, L.; Fitzgerald, P.; Stanton, C.; Dinan, T.G.; Cryan, J.F.; et al. Metabolome and microbiome profiling of a stress-sensitive rat model of gut-brain axis dysfunction. Sci. Rep. 2019, 9, 14026. [Google Scholar] [CrossRef] [PubMed]
- Hill, M. Intestinal flora and endogenous vitamin synthesis. Eur. J. Cancer Prev. 1997, 6, S43–S45. [Google Scholar] [CrossRef]
- Burgess, C.M.; Smid, E.J.; Van Sinderen, D. Bacterial vitamin b2, b11 and b12 overproduction: An overview. Int. J. Food. Microbiol. 2009, 133, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sumi, Y.; Miyakawa, M.; Kanzaki, M.; Kotake, Y. Vitamin b-6 deficiency in germfree rats. J. Nutr. 1977, 107, 1707–1714. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Milani, C.; de Giori, G.S.; Sesma, F.; van Sinderen, D.; Ventura, M. Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Curr. Opin. Biotech. 2013, 24, 160–168. [Google Scholar] [CrossRef]
- Rosenberg, J.; Ischebeck, T.; Commichau, F.M. Vitamin b6 metabolism in microbes and approaches for fermentative production. Biotechnol. Adv. 2017, 35, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Gougeon, L.; Payette, H.; Morais, J.A.; Gaudreau, P.; Shatenstein, B.; Gray-Donald, K. Intakes of folate, vitamin b6 and b12 and risk of depression in community-dwelling older adults: The quebec longitudinal study on nutrition and aging. Eur. J. Clin. Nutr. 2016, 70, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Jacka, F.N.; Maes, M.; Pasco, J.A.; Williams, L.J.; Berk, M. Nutrient intakes and the common mental disorders in women. J. Affect. Disord. 2012, 141, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Vulser, H.; Wiernik, E.; Hoertel, N.; Thomas, F.; Pannier, B.; Czernichow, S.; Hanon, O.; Simon, T.; Simon, J.M.; Danchin, N.; et al. Association between depression and anemia in otherwise healthy adults. Acta Psychiatry Scand. 2016, 134, 150–160. [Google Scholar] [CrossRef]
- Alpert, J.E.; Fava, M. Nutrition and depression: The role of folate. Nutr. Rev. 1997, 55, 145–149. [Google Scholar] [CrossRef]
- Coppen, A.; Bolander-Gouaille, C. Treatment of depression: Time to consider folic acid and vitamin b12. J. Psychopharm. 2005, 19, 59–65. [Google Scholar] [CrossRef]
- Paul, R.T.P.; McDonnell, A.P.; Kelly, C.B. Folic acid: Neurochemistry, metabolism and relationship to depression. Hum. Psychopharm. Clin. 2004, 19, 477–488. [Google Scholar] [CrossRef]
- Spedding, S. Vitamin d and depression: A systematic review and meta-analysis comparing studies with and without biological flaws. Nutrients 2014, 6, 1501–1518. [Google Scholar] [CrossRef] [PubMed]
- Partonen, T. Vitamin d and serotonin in winter. Med. Hypotheses 1998, 51, 267–268. [Google Scholar] [CrossRef]
- Hartvig, P.; Lindner, K.J.; Bjurling, P.; Långström, B.; Tedroff, J. Pyridoxine effect on synthesis rate of serotonin in the monkey brain measured with positron emission tomography. J. Neural Transm. 1995, 102, 91–97. [Google Scholar] [CrossRef]
- Dakshinamurti, K.; Sharma, S.K.; Bonke, D. Influence of b vitamins on binding properties of serotonin receptors in the cns of rats. Klin. Wochenschr. 1990, 68, 142–145. [Google Scholar] [CrossRef]
- Wang, J.; Um, P.; Dickerman, B.A.; Liu, J. Zinc, magnesium, selenium and depression: A review of the evidence, potential mechanisms and implications. Nutrients 2018, 10, 584. [Google Scholar] [CrossRef]
- Rybka, J.; Kedziora-Kornatowska, K.; Banas-Lezanska, P.; Majsterek, I.; Carvalho, L.A.; Cattaneo, A.; Anacker, C.; Kedziora, J. Interplay between the pro-oxidant and antioxidant systems and proinflammatory cytokine levels, in relation to iron metabolism and the erythron in depression. Free Radic. Biol. Med. 2013, 63, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Agrawal, A.; Said, H.M. Biotin deficiency enhances the inflammatory response of human dendritic cells. Am. J. Physiol. Cell Physiol. 2016, 311, C386–C391. [Google Scholar] [CrossRef] [PubMed]
- Pfalzer, A.C.; Choi, S.-W.; Tammen, S.A.; Park, L.K.; Bottiglieri, T.; Parnell, L.D.; Lamon-Fava, S. S-adenosylmethionine mediates inhibition of inflammatory response and changes in DNA methylation in human macrophages. Physiol. Genom. 2014, 46, 617–623. [Google Scholar] [CrossRef]
- Myint, A.-M.; Kim, Y.K.; Verkerk, R.; Scharpé, S.; Steinbusch, H.; Leonard, B. Kynurenine pathway in major depression: Evidence of impaired neuroprotection. J. Affect. Disord. 2007, 98, 143–151. [Google Scholar] [CrossRef]
- Takeuchi, F.; Shibata, Y. Kynurenine metabolism in vitamin-b-6-deficient rat liver after tryptophan injection. Biochem. J. 1984, 220, 693–699. [Google Scholar] [CrossRef]
- Willner, P.; Scheel-Kruger, J.; Belzung, C. The neurobiology of depression and antidepressant action. Neurosci. Biobehav. Rev. 2013, 37, 2331–2371. [Google Scholar] [CrossRef] [PubMed]
- Gershon, M.D. 5-hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, K.; Engdahl, C.; Henning, P.; Lerner, U.H.; Tremaroli, V.; Lagerquist, M.K.; Bäckhed, F.; Ohlsson, C. The gut microbiota regulates bone mass in mice. J. Bone Miner. Res. 2012, 27, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Mazmanian, S.K.; Hsiao, E.Y.; Ma, L.; Ismagilov, R.F.; Nagler, C.R. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.F.; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015, 29, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, S.; Tatewaki, M.; Yamada, T.; Fujimiya, M.; Mantyh, C.; Voss, M.; Eubanks, S.; Harris, M.; Pappas, T.N.; Takahashi, T. Short-chain fatty acids stimulate colonic transit via intraluminal 5-ht release in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R1269. [Google Scholar] [CrossRef]
- Krantis, A. Gaba in the mammalian enteric nervous system. Physiology 2000, 15, 284–290. [Google Scholar] [CrossRef]
- Matsumoto, M.; Kibe, R.; Ooga, T.; Aiba, Y.; Sawaki, E.; Koga, Y.; Benno, Y. Cerebral low-molecular metabolites influenced by intestinal microbiota: A pilot study. Front. Syst. Neurosci. 2013, 7, 9. [Google Scholar] [CrossRef]
- Wikoff, W.R.; Anfora, A.T.; Liu, J.; Schultz, P.G.; Lesley, S.A.; Peters, E.C.; Siuzdak, G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA 2009, 106, 3698. [Google Scholar] [CrossRef]
- Young, L.W.; Darios, E.S.; Watts, S.W. An immunohistochemical analysis of sert in the blood–brain barrier of the male rat brain. Histochem. Cell Biol. 2015, 144, 321–329. [Google Scholar] [CrossRef]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef] [PubMed]
- de Jonge, W.J. The gut’s little brain in control of intestinal immunity. ISRN Gastroenterol. 2013, 2013, 630159. [Google Scholar] [CrossRef] [PubMed]
- Bjurstöm, H.; Wang, J.; Ericsson, I.; Bengtsson, M.; Liu, Y.; Kumar-Mendu, S.; Issazadeh-Navikas, S.; Birnir, B. Gaba, a natural immunomodulator of t lymphocytes. J. Neuroimmunol. 2008, 205, 44–50. [Google Scholar] [CrossRef]
- O’Connor, J.C.; Lawson, M.A.; Andre, C.; Moreau, M.; Lestage, J.; Castanon, N.; Kelley, K.W.; Dantzer, R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry 2008, 14, 511–522. [Google Scholar] [CrossRef]
- Ondicova, K.; Pecenak, J.; Mravec, B. The role of the vagus nerve in depression. Neuro Endocrinol. Lett. 2010, 31, 602–608. [Google Scholar]
- Agelink, M.W.; Boz, C.; Ullrich, H.; Andrich, J. Relationship between major depression and heart rate variability.: Clinical consequences and implications for antidepressive treatment. Psychiatry Res. 2002, 113, 139–149. [Google Scholar] [CrossRef]
- Schlaepfer, T.E.; Frick, C.; Zobel, A.; Maier, W.; Heuser, I.; Bajbouj, M.; O’Keane, V.; Corcoran, C.; Adolfsson, R.; Trimble, M.; et al. Vagus nerve stimulation for depression: Efficacy and safety in a european study. Psychol. Med. 2008, 38, 651–661. [Google Scholar] [CrossRef]
- Lv, H.; Zhao, Y.-H.; Chen, J.-G.; Wang, D.-Y.; Chen, H. Vagus nerve stimulation for depression: A systematic review. Front. Psychol. 2019, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Goehler, L.E.; Gaykema, R.P.; Hansen, M.K.; Anderson, K.; Maier, S.F.; Watkins, L.R. Vagal immune-to-brain communication: A visceral chemosensory pathway. Autonom. Neurosci. 2000, 85, 49–59. [Google Scholar] [CrossRef]
- Tracey, K.J. The inflammatory reflex. Nature 2002, 420, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, V.A.; Tracey, K.J. The vagus nerve and the inflammatory reflex--linking immunity and metabolism. Nat. Rev. Endocrinol. 2012, 8, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Daskalakis, N.P.; Bagot, R.C.; Parker, K.J.; Vinkers, C.H.; de Kloet, E.R. The three-hit concept of vulnerability and resilience: Toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology 2013, 38, 1858–1873. [Google Scholar] [CrossRef]
- Tanida, M.; Yamano, T.; Maeda, K.; Okumura, N.; Fukushima, Y.; Nagai, K. Effects of intraduodenal injection of Lactobacillus johnsonii la1 on renal sympathetic nerve activity and blood pressure in urethane-anesthetized rats. Neurosci. Lett. 2005, 389, 109–114. [Google Scholar] [CrossRef]
- Sominsky, L.; Fuller, E.A.; Bondarenko, E.; Ong, L.K.; Averell, L.; Nalivaiko, E.; Dunkley, P.R.; Dickson, P.W.; Hodgson, D.M. Functional programming of the autonomic nervous system by early life immune exposure: Implications for anxiety. PLoS ONE 2013, 8, e57700. [Google Scholar] [CrossRef] [PubMed]
- Réus, G.Z.; Stringari, R.B.; Ribeiro, K.F.; Cipriano, A.L.; Panizzutti, B.S.; Stertz, L.; Lersch, C.; Kapczinski, F.; Quevedo, J. Maternal deprivation induces depressive-like behaviour and alters neurotrophin levels in the rat brain. Neurochem. Res. 2011, 36, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.; Robbins, T.W. Early experience as a determinant of adult behavioural responses to reward: The effects of repeated maternal separation in the rat. Neurosci. Biobehav. Rev. 2003, 27, 45–55. [Google Scholar] [CrossRef]
- Montalvo-Ortiz, J.L.; Bordner, K.A.; Carlyle, B.C.; Gelernter, J.; Simen, A.A.; Kaufman, J. The role of genes involved in stress, neural plasticity, and brain circuitry in depressive phenotypes: Convergent findings in a mouse model of neglect. Behav. Brain Res. 2016, 315, 71–74. [Google Scholar] [CrossRef]
- Yohn, N.L.; Blendy, J.A. Adolescent chronic stress and adult anxiety adolescent chronic unpredictable stress exposure is a sensitive window for long-term changes in adult behavior in mice. Neuropsychopharmacology 2017, 42, 1670–1678. [Google Scholar] [CrossRef]
- Jaffee, S.R. Sensitive, stimulating caregiving predicts cognitive and behavioral resilience in neurodevelopmentally at-risk infants. Dev. Psychopathol. 2007, 19, 631–647. [Google Scholar] [CrossRef]
- Del Giudice, M.; Ellis, B.J.; Shirtcliff, E.A. The adaptive calibration model of stress responsivity. Neurosci. Biobehav. Rev. 2011, 35, 1562–1592. [Google Scholar] [CrossRef]
- Nesse, R.M.; Bhatnagar, S.; Ellis, B. Chapter 11—Evolutionary origins and functions of the stress response system. In Stress: Concepts, Cognition, Emotion, and Behavior; Fink, G., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 95–101. [Google Scholar]
- Heim, C.; Binder, E.B. Current research trends in early life stress and depression: Review of human studies on sensitive periods, gene–environment interactions, and epigenetics. Exp. Neurol. 2012, 233, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Schapiro, S. Some physiological, biochemical, and behavioral consequences of neonatal hormone administration: Cortisol and thyroxine. Gen. Comp. Endocrinol. 1968, 10, 214–228. [Google Scholar] [CrossRef]
- Tambs, K.; Czajkowsky, N.; Røysamb, E.; Neale, M.C.; Reichborn-Kjennerud, T.; Aggen, S.H.; Harris, J.R.; Ørstavik, R.E.; Kendler, K.S. Structure of genetic and environmental risk factors for dimensional representations of dsm–iv anxiety disorders. Br. J. Psychiatry 2009, 195, 301–307. [Google Scholar] [CrossRef] [PubMed]
- On Wah, D.T.; Kavaliers, M.; Bishnoi, I.R.; Ossenkopp, K.-P. Lipopolysaccharide (lps) induced sickness in early adolescence alters the behavioral effects of the short-chain fatty acid, propionic acid, in late adolescence and adulthood: Examining anxiety and startle reactivity. Behav. Brain Res. 2019, 360, 312–322. [Google Scholar] [CrossRef]
- Aguilera, M.; Vergara, P.; Martínez, V. Stress and antibiotics alter luminal and wall-adhered microbiota and enhance the local expression of visceral sensory-related systems in mice. J. Neurogastroenterol. Motil. 2013, 25, e515–e529. [Google Scholar] [CrossRef]
- Brenes, J.C.; Fornaguera, J. Effects of environmental enrichment and social isolation on sucrose consumption and preference: Associations with depressive-like behavior and ventral striatum dopamine. Neurosci. Lett. 2008, 436, 278–282. [Google Scholar] [CrossRef]
- Bear, T.L.K.; Dalziel, J.E.; Coad, J.; Roy, N.C.; Butts, C.A.; Gopal, P.K. The role of the gut microbiota in dietary interventions for depression and anxiety. Adv. Nutr. 2020, 11, 890–907. [Google Scholar] [CrossRef]
- Taylor, A.M.; Thompson, S.V.; Edwards, C.G.; Musaad, S.M.A.; Khan, N.A.; Holscher, H.D. Associations among diet, the gastrointestinal microbiota, and negative emotional states in adults. Nutr. Neurosci. 2019, 23, 983–992. [Google Scholar] [CrossRef]
| Subject, Study Design and Model | Probiotic | Dose and Administration | Treatment Duration | Effect on Mood | Reference | |
|---|---|---|---|---|---|---|
| Animal Studies | ||||||
| Male AKR mice with parasite-induced (Trichuris muris) chronic gastrointestinal inflammation | Bifidobacterium longum NCC3001 and Lactobacillus rhamnosus NCC4007 | Gavaged daily, dose not specified | 10 days | Reduction in anxiety-like behaviors in the LDB | + | [29] |
| Immunodeficient (B and T cell-deficient) male and female Rag 1−/− mice | L. rhamnosus R0011 and Lactobacillus helveticus R0052 | 109 CFU/mL in drinking water daily | 4 weeks | Probiotic supplement normalized deficits in anxiety in LDB tests | + | [38] |
| Male C57BL/6 mice with liver inflammation-induced sickness behavior and brain inflammation | Commercial mixture VSL#3: L. casei, L. plantarum, L. acidophilus and L. delbrueckii subsp. Bulgaricus, B. longum, B. breve and Bifidobacterium infantis, Streptococcus salivarius subsp. Thermophiles. Strains unspecified | 1.7 billion bacteria/day, gavaged daily | 10 days | Prevention of a decrease in social interaction | + | [37] |
| Male AKR mice with chemically induced colitis | B. longum NCC3001 - | 100 µL of 1 × 1010 CFU | 7 days | A probiotic supplementation reduced anxiety-like behavior in SDT, but only when the vagus nerve was intact | +/ | [39] |
| Male Sprague Dawley Rats | B. bifidum W23, B. lactis W52, L. acidophilus W37, L. brevis W63, L. casei W56, L. salivarius W24, L. lactis W19, L. lactis W58 | 4.5 g (2.5 × 109 CFU/g) of freeze-dried powder in 30 mL of tap water per cage (2 rats) daily | 10 weeks | A probiotic mix decreased depressive-like behavior in FST | + | [35] |
| Male Sprague Dawley rats following maternal separation stress | B. infantis 35624 | 1 × 1010 live bacterial cells/100 mL drinking water | 55 days | A probiotic supplement ameriorated MSS induced depressive-like behavior in FST | + | [40] |
| A probiotic given alongside 3 weeks of restraint stress in male Sprague Dawley rats | L. helveticus ns8 | 109 CFU/mL live bacteria in drinking water | 3 weeks | Probiotic ameliorated stress-induced depressive-like behavior in SPT, and anxiety like behavior in EPM | + | [41] |
| Male BALB/c mice | L. rhamnosus JB-1 | 109 CFU, gavaged daily | 28 days | Decreased anxiety-like behaviors in the EPM | + | [36] |
| Male Sprague Dawley rats | L. casei 54-2-33 | 104 CFU/mL in drinking water | 14 days | Increase in anxiety-like behavior in the OFT and no difference in anxiety-like behavior in the EPM | − | [42] |
| Male Sprague Dawley rats | B. infantis 35624 | 1 × 1010 live bacterial cells/100 mL drinking water | 14 days | No decrease in depressive-like behaviors in FST | / | [43] |
| Human Studies | ||||||
| Healthy adult men | L. rhamnosus JB-1 | 109 CFU, probiotic capsule, daily | 8 weeks | No reduction in subjective stress measure, depression or anxiety scores on the PSS, BAI or BDI scales or improve cognitive measures | / | [44] |
| Healthy men and women | L. helveticus R0052 and B. longum R0175 | 3 × 109 CFU probiotic capsule daily | 30 days | Reduction in depression and anxiety scores (HADS). In a subset of people with low baseline urinary cortisol, the perceived stress scores were also reduced by the probiotic | + | [47,48] |
| Healthy men and women | B. bifidum W23, B. lactis W52, L. acidophilus W37, L. brevis W63, L. casei W56, L. salivarius W24, and Lactococcus lactis (W19 and W58) | 2.5 × 109 CFU probiotic capsule daily | 4 weeks | Reduction in participant’s cognitive reactivity to sad mood | + | [49] |
| Men and women with chronic fatigue syndrome | L. casei Shirota | 8 × 109 CFU probiotic capsule daily | 2 months | Improved anxiety (BAI) but not depressive (BDI) symptoms | +/ | [50] |
| Healthy men and women | Milk drink containing probiotic L. casei Shirota | 6.5 × 109 CFU in a milk drink | Improvement in mood in POMS only in those who already had low mood | +/ | [51] | |
| Men and women with irritable bowel syndrome | Yohgurt containing Lactobacillus paracasei, ssp. paracasei F19, L. acidophilus La5 and B. lactis Bb12 (Cultura; active) | 5 × 107 cfu/mL × 200 mL milk drink, daily | 8 weeks | The probiotic yoghurt drink did not improve mood scores in HADS | / | [45] |
| Men and women with diagnosed depression | B. bifidum, L. acidophilus, and L. casei (strains not specified) | L. acidophilus (2 × 109 CFU/g), L.casei (2 × 109 CFU/g), B. bifidum (2 × 109 CFU/g), amount not specified | 8 weeks | Reduction in symptoms of depression I BDI, along with fasting plasma insulin, glutathione, and C-reactive protein | + | [46] |
| Study Design (Stress, Subjects, Intervention) | Results | SR | SS | Reference |
|---|---|---|---|---|
|
| Probiotic: L. rhamnosus JB-1 | Not applicable | [36] |
|
| Probiotic: L. reuteri ATCC 23272 | Decrease in (fecal) Lactobacillus | [15] |
|
| Not applicable | Bacterioides; Ruminococccaceae | [12] |
|
| Probiotic: L. rhamnosus strain R0011 (95%) and Lactobacillus helveticus strain R0052 (5%) | Not applicable | [59] |
|
| Probiotic: B. infantis 35624 | Not applicable | [40]. |
|
| Not applicable | Not applicable | [16] |
|
| Not applicable | Not applicable | [67] |
|
| Not applicable | Not applicable | [69] |
|
| Not applicable | Not applicable | [66] |
|
| Not applicable | Not applicable | [65] |
|
| Not applicable | Not applicable | [63] |
|
| Probiotic: L. helveticus ns8 | Not applicable | [41] |
|
| Not applicable | Not applicable | [60] |
|
| Not applicable | Not applicable | [13] |
|
| Not applicable | Not applicable | [58] |
|
| Not applicable | Not applicable | [56] |
|
| Bifidobacterium | Not applicable | [62] |
|
| Not applicable | Not applicable | [57] |
|
| Not applicable | Not applicable | [11] |
|
| Not applicable | Not applicable | [190] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bear, T.; Dalziel, J.; Coad, J.; Roy, N.; Butts, C.; Gopal, P. The Microbiome-Gut-Brain Axis and Resilience to Developing Anxiety or Depression under Stress. Microorganisms 2021, 9, 723. https://doi.org/10.3390/microorganisms9040723
Bear T, Dalziel J, Coad J, Roy N, Butts C, Gopal P. The Microbiome-Gut-Brain Axis and Resilience to Developing Anxiety or Depression under Stress. Microorganisms. 2021; 9(4):723. https://doi.org/10.3390/microorganisms9040723
Chicago/Turabian StyleBear, Tracey, Julie Dalziel, Jane Coad, Nicole Roy, Christine Butts, and Pramod Gopal. 2021. "The Microbiome-Gut-Brain Axis and Resilience to Developing Anxiety or Depression under Stress" Microorganisms 9, no. 4: 723. https://doi.org/10.3390/microorganisms9040723
APA StyleBear, T., Dalziel, J., Coad, J., Roy, N., Butts, C., & Gopal, P. (2021). The Microbiome-Gut-Brain Axis and Resilience to Developing Anxiety or Depression under Stress. Microorganisms, 9(4), 723. https://doi.org/10.3390/microorganisms9040723







