Synergistic Effects of Nisin, Lysozyme, Lactic Acid, and CitricidalTM for Enhancing Pressure-Based Inactivation of Bacillus amyloliquefaciens, Geobacillus stearothermophilus, and Bacillus atrophaeus Endospores
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Propagation, and Endospore Suspension Preparation
2.2. High-Pressure Processing and Bacteriocin/Bactericidal Compounds
2.3. Microbiological Enumeration, Neutralization, and pH Analysis
2.4. Design of Trials and Descriptive and Inferential Analytical Methods
3. Results and Discussion
3.1. Pressure-Based Reduction of Three Bacterial Endospores in the Presence of Nisin and Lysozyme
3.2. Pressure-Based Reduction of Three Bacterial Endospores in Presence of Lactic Acid and Citricidal
3.3. Linear and Non-Linear Inactivation Indices for the Reduction of Bacterial Endospores
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United States Centers for Disease Control and Prevention (CDC). Ten great public health achievements- United States, 1900–1999. MMWR Morb. Mortal. Wkly. Rep. 1999, 48, 241–243.
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—major pathogens. Emerg Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015. 2015. Available online: https://apps.who.int/iris/handle/10665/199350 (accessed on 28 February 2021).
- Fouladkhah, A.C.; Thompson, B.; Camp, J.S. Safety of food and water supplies in the landscape of changing climate. Microorganisms 2019, 7, 469. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture. Food Waste FAQ. Available online: https://www.usda.gov/foodwaste/faqs (accessed on 28 February 2021).
- Food and Agriculture Organization of the United States. Food Loss and Food waste. Available online: http://www.fao.org/food-loss-and-food-waste/flw-data (accessed on 28 February 2021).
- Tirado, M.C.; Clarke, R.; Jaykus, L.A.; McQuatters-Gollop, A.; Frank, J.M. Climate change and food safety: A review. Food Res. Int. 2019, 43, 1745–1765. [Google Scholar] [CrossRef]
- Miraglia, M.; Marvin, H.J.P.; Kleter, G.A.; Battilani, P.; Brera, C.; Coni, E.; Cubadda, F.; Croci, L.; De Santis, B.; Dekkers, S.; et al. Climate change and food safety: An emerging issue with special focus on Europe. Food Chem. Toxicol. 2009, 47, 1009–1021. [Google Scholar] [CrossRef]
- Jessberger, N.; Dietrich, R.; Granum, P.E.; Märtlbauer, E. The Bacillus cereus food infection as multifactorial process. Toxins 2020, 12, 701. [Google Scholar] [CrossRef]
- Rana, N.; Panda, A.K.; Pathak, N.; Gupta, T.; Thakur, S.D. Bacillus cereus: Public health burden associated with ready-to-eat foods in Himachal Pradesh, India. J. Food Sci. Technol. 2020, 1, 1–10. [Google Scholar] [CrossRef]
- Logan, N.A. Bacillus and relatives in foodborne illness. J. Appl. Microbiol. 2012, 112, 417–429. [Google Scholar] [CrossRef]
- Sood, B.; Sahota, P.P.; Hunjan, M. Multidrug resistant Bacillus cereus in fresh vegetables: A serious burden to public health. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 649–661. [Google Scholar]
- Sofos, J.N. Challenges to meat safety in the 21st century. Meat Sci. 2008, 78, 3–13. [Google Scholar] [CrossRef]
- Galanakis, C.M. Food waste recovery: Prospects and opportunities. In Sustainable Food Systems from Agriculture to Industry, 1st ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 401–419. [Google Scholar]
- Govaris, A.; Pexara, A. Inactivation of foodborne viruses by high-pressure processing (HPP). Foods 2021, 10, 215. [Google Scholar] [CrossRef] [PubMed]
- Fouladkhah, A. Frontiers in pressure-based pasteurization: Cost optimization by synergism with natural bactericidal and bacteriocin compounds. In International Association for Food Protection 2020 Annual Virtual Meeting; International Association for Food Protection: Des Moines, IA, USA, 2020; Abstract Number T12-04. [Google Scholar]
- Allison, A.; Fouladkhah, A.C. Sensitivity of wild-type and rifampicin-resistant O157 and non-O157 Shiga toxin-producing Escherichia coli to elevated hydrostatic pressure and lactic acid in ground meat and meat homogenate. PLoS ONE 2021, 16, e0246735. [Google Scholar] [CrossRef] [PubMed]
- Aras, S.; Kabir, M.N.; Chowdhury, S.; Fouladkhah, A.C. Augmenting the pressure-based pasteurization of Listeria monocytogenes by synergism with nisin and mild heat. Int. J. Environ. Res. Public Health 2020, 17, 563. [Google Scholar] [CrossRef] [PubMed]
- Allison, A.; Daniels, E.; Chowdhury, S.; Fouladkhah, A. Effects of elevated hydrostatic pressure against mesophilic background microflora and habituated Salmonella serovars in orange juice. Microorganisms 2018, 6, 23. [Google Scholar] [CrossRef]
- Kabir, M.N.; Aras, S.; Allison, A.; Adhikari, J.; Chowdhury, S.; Fouladkhah, A. Interactions of carvacrol, caprylic acid, habituation, and mild heat for pressure-based inactivation of O157 and non-O157 serogroups of Shiga toxin-producing Escherichia coli in acidic environment. Microorganisms 2019, 7, 145. [Google Scholar] [CrossRef]
- Aras, S.; Kabir, M.N.; Allison, A.; George, J.; Fouladkhah, A. Inactivation of Shiga toxin-producing Escherichia coli O157: H7 and mesophilic background microbiota of meat homogenate using elevated hydrostatic pressure, mild heat, and thymol. J. Food Sci. 2020, 85, 4335–4341. [Google Scholar] [CrossRef]
- Cacace, F.; Bottani, E.; Rizzi, A.; Vignali, G. Evaluation of the economic and environmental sustainability of high pressure processing of foods. Innov. Food Sci. Emerg. Technol. 2020, 60, 102281. [Google Scholar] [CrossRef]
- Huang, H.W.; Hsu, C.P.; Wang, C.Y. Healthy expectations of high hydrostatic pressure treatment in food processing industry. J. Food Drug Anal. 2020, 28, 1–13. [Google Scholar] [CrossRef]
- Torres, J.A.; Velazquez, G. Commercial opportunities and research challenges in the high pressure processing of foods. J. Food Eng. 2005, 67, 95–112. [Google Scholar] [CrossRef]
- Bolumar, T.; Orlien, V.; Sikes, A.; Aganovic, K.; Bak, K.H.; Guyon, C.; Stübler, A.S.; de Lamballerie, M.; Hertel, C.; Brüggemann, D.A. High-pressure processing of meat: Molecular impacts and industrial applications. Compr. Rev. Food Sci. Food Saf. 2020, 20, 332–368. [Google Scholar] [CrossRef]
- Huang, H.W.; Wu, S.J.; Lu, J.K.; Shyu, Y.T.; Wang, C.Y. Current status and future trends of high-pressure processing in food industry. Food Control 2017, 72, 1–8. [Google Scholar] [CrossRef]
- Ahn, J.H.; Balasubramaniam, V.M. Physiological responses of Bacillus amyloliquefaciens spores to high pressure. J. Microbiol. Biotechnol. 2007, 17, 524–529. [Google Scholar] [PubMed]
- Margosch, D.; Ehrmann, M.A.; Buckow, R.; Heinz, V.; Vogel, R.F.; Gänzle, M.G. High-pressure-mediated survival of Clostridium botulinum and Bacillus amyloliquefaciens endospores at high temperature. Appl. Environ. Microbiol. 2006, 72, 3476–3481. [Google Scholar] [CrossRef] [PubMed]
- Margosch, D.; Gänzle, M.G.; Ehrmann, M.A.; Vogel, R.F. Pressure inactivation of Bacillus endospores. Appl. Environ. Microbiol. 2004, 70, 7321–7328. [Google Scholar] [CrossRef]
- López, M.; González, I.; Condón, S.; Bernardo, A. Effect of pH heating medium on the thermal resistance of Bacillus stearothermophilus spores. Int. J. Food Microbiol. 1996, 28, 405–410. [Google Scholar] [CrossRef]
- Park, S.H.; Balasubramaniam, V.M.; Sastry, S.K.; Lee, J. Pressure–ohmic–thermal sterilization: A feasible approach for the inactivation of Bacillus amyloliquefaciens and Geobacillus stearothermophilus spores. Innov. Food Sci. Emerg. Technol. 2013, 19, 115–123. [Google Scholar] [CrossRef]
- Sikin, A.M.; Walkling-Ribeiro, M.; Rizvi, S.S. Synergistic processing of skim milk with high pressure nitrous oxide, heat, nisin, and lysozyme to inactivate vegetative and spore-forming bacteria. Food Bioprocess. Technol. 2017, 10, 2132–2145. [Google Scholar] [CrossRef]
- Byelashov, O.A.; Kendall, P.A.; Belk, K.E.; Scanga, J.A.; Sofos, J.N. Control of Listeria monocytogenes on vacuum-packaged frankfurters sprayed with lactic acid alone or in combination with sodium lauryl sulfate. J. Food Prot. 2008, 71, 728–734. [Google Scholar] [CrossRef]
- Fouladkhah, A.; Geornaras, I.; Sofos, J.N. Biofilm formation of O157 and non-O157 Shiga toxin-producing Escherichia coli and multidrug-resistant and susceptible Salmonella Typhimurium and Newport and their inactivation by sanitizers. J. Food Sci. 2013, 78, M880–M886. [Google Scholar] [CrossRef]
- Allison, A.; Fouladkhah, A.C. Sensitivity of planktonic cells and biofilm of wild-type and pressure-stressed Cronobacter sakazakii and Salmonella enterica serovars to sodium hypochlorite. Food Prot. Trends 2021, 41, 195–203. [Google Scholar]
- Daryaei, H.; Balasubramaniam, V.M.; Yousef, A.E.; Legan, J.D.; Tay, A. Lethality enhancement of pressure-assisted thermal processing against Bacillus amyloliquefaciens spores in low-acid media using antimicrobial compounds. Food Control. 2016, 59, 234–242. [Google Scholar] [CrossRef]
- Rajan, S.; Ahn, J.; Balasubramaniam, V.M.; Yousef, A.E. Combined pressure-thermal inactivation kinetics of Bacillus amyloliquefaciens spores in egg patty mince. J. Food Prot. 2006, 69, 853–860. [Google Scholar] [CrossRef]
- Margosch, D.; Ehrmann, M.A.; Gänzle, M.G.; Vogel, R.F. Comparison of pressure and heat resistance of Clostridium botulinum and other endospores in mashed carrots. J. Food Prot. 2004, 67, 2530–2537. [Google Scholar] [CrossRef] [PubMed]
- Allison, A.; Chowdhury, S.; Fouladkhah, A. Synergism of mild heat and high-pressure pasteurization against Listeria monocytogenes and natural microflora in phosphate-buffered saline and raw milk. Microorganisms 2018, 6, 102. [Google Scholar] [CrossRef]
- Chowdhury, A.; Chowdhury, S.; Fouladkhah, A. Sensitivity of non-pathogenic LT2 and pathogenic Salmonella enterica serovars to elevated hydrostatic pressure and citricidal under controlled temperature. In International Association for Food Protection 2020 Annual Virtual Meeting; International Association for Food Protection: Des Moines, IA, USA, 2020; Abstract Number. P2–88. [Google Scholar]
- Allison, A.; Kabir, N.; Aras, S.; Chowdhury, S.; Fouladkhah, A. Sensitivity of Bacillus amyloliquefaciens, Geobacillus stearothermophilus, and Bacillus atrophaeus to elevated hydrostatic pressure in presence of mild heat, nisin and lysozyme. In International Association for Food Protection 2019 Annual Meeting, Louisville, KY, USA, 21–24 July 2019; International Association for Food Protection: Des Moines, IA, USA, 2019; Abstract Number. P1–96. [Google Scholar]
- United States Food and Drug Administration. Bacteriological Analytical Methods (FDA BAM). 2001. Aerobic Plate Count. Available online: https://www.fda.gov/food/laboratory-methods-food/bam-chapter-3-aerobic-plate-count (accessed on 28 February 2021).
- Allison, A.; Fouladkhah, A. Sensitivity of Salmonella serovars and natural microflora to high-pressure pasteurization: Open access data for risk assessment and practitioners. Data Brief. 2018, 21, 480–484. [Google Scholar] [CrossRef]
- Geeraerd, A.H.; Valdramidis, V.P.; Van Impe, J.F. GInaFiT, A freeware tool to assess non-log-linear microbial survivor curves. Int. J. Food Microbiol. 2005, 102, 95–105. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Aras, S.; Kabir, M.N.; Wadood, S.; Chowdhury, S.; Fouladkhah, A.C. Sensitivity of planktonic cells of Staphylococcus aureus to elevated hydrostatic pressure as affected by mild heat, carvacrol, nisin, and caprylic acid. Int. J. Environ. Res. Public Health 2020, 17, 7033. [Google Scholar] [CrossRef]
- Kabir, M.N.; Aras, S.; George, J.; Wadood, S.; Chowdhury, S.; Fouladkhah, A.C. High-pressure and thermal-assisted pasteurization of habituated, wild-type, and pressure-stressed Listeria monocytogenes, Listeria innocua, and Staphylococcus aureus. LWT 2021, 137, 110445. [Google Scholar] [CrossRef]
- Patazca, E.; Koutchma, T.; Ramaswamy, H.S. Inactivation kinetics of Geobacillus stearothermophilus spores in water using high-pressure processing at elevated temperatures. J. Food Sci. 2006, 71, M110–M116. [Google Scholar] [CrossRef]
- Ratphitagsanti, W.; De Lamo-Castellvi, S.; Balasubramaniam, V.M.; Yousef, A.E. Efficacy of pressure-assisted thermal processing, in combination with organic acids, against Bacillus amyloliquefaciens spores suspended in deionized water and carrot puree. J. Food Sci. 2010, 75, M46–M52. [Google Scholar] [CrossRef] [PubMed]
- Haskaraca, G.; Juneja, V.K.; Mukhopadhyay, S.; Kolsarici, N. The effects of grapefruit seed extract on the thermal inactivation of Listeria monocytogenes in sous-vide processed döner kebabs. Food Control 2019, 95, 71–76. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO)/World Health Organization (WHO). Interventions for the Control of Non-Typhoidal Salmonella spp. in Beef and Pork. Microbiological Risk Assessment Series 30. 2016. Available online: http://www.fao.org/3/a-i5317e.pdf (accessed on 28 February 2021).
- United States Food and Drug Administration. Direct Food Substance Affirmed as Generally Recognized as Safe. Code of Federal Regulations Title 21. 2019. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1061 (accessed on 19 March 2021).
- Reverter-Carrión, L.; Sauceda-Gálvez, J.N.; Codina-Torrella, I.; Hernández-Herrero, M.M.; Gervilla, R.; Roig-Sagués, A.X. Inactivation study of Bacillus subtilis, Geobacillus stearothermophilus, Alicyclobacillus acidoterrestris and Aspergillus niger spores under Ultra-High Pressure Homogenization, UV-C light and their combination. Innov. Food Sci. Emerg. Technol. 2018, 48, 258–264. [Google Scholar] [CrossRef]
- Heinz, V.; Knorr, D. Effects of high pressure on spores. In Ultra High Pressure Treatments of Foods; Hendrickx, M.E.G., Knorr, D., Ludikhuyze, L., Van Loey, A., Heinz, V., Eds.; Food Engineering Series; Springer: Boston, MA, USA, 2001. [Google Scholar]
- Paidhungat, M.; Setlow, B.; Daniels, W.B.; Hoover, D.; Papafragkou, E.; Setlow, P. Mechanisms of induction of germination of Bacillus subtilis spores by high pressure. Appl. Environ. Microbiol. 2002, 68, 3172–3175. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.M.; Hoover, D.G. Sensitivity of Bacillus coagulans spores to combinations of high hydrostatic pressure, heat, acidity and nisin. J. Appl. Bacteriol. 1996, 81, 363–368. [Google Scholar]
- Ramaroson, M.; Guillou, S.; Rossero, A.; Rezé, S.; Anthoine, V.; Moriceau, N.; Martin, J.L.; Duranton, F.; Zagorec, M. Selection procedure of bioprotective cultures for their combined use with high pressure processing to control spore-forming bacteria in cooked ham. Int. J. Food Microbiol. 2018, 276, 28–38. [Google Scholar] [CrossRef] [PubMed]
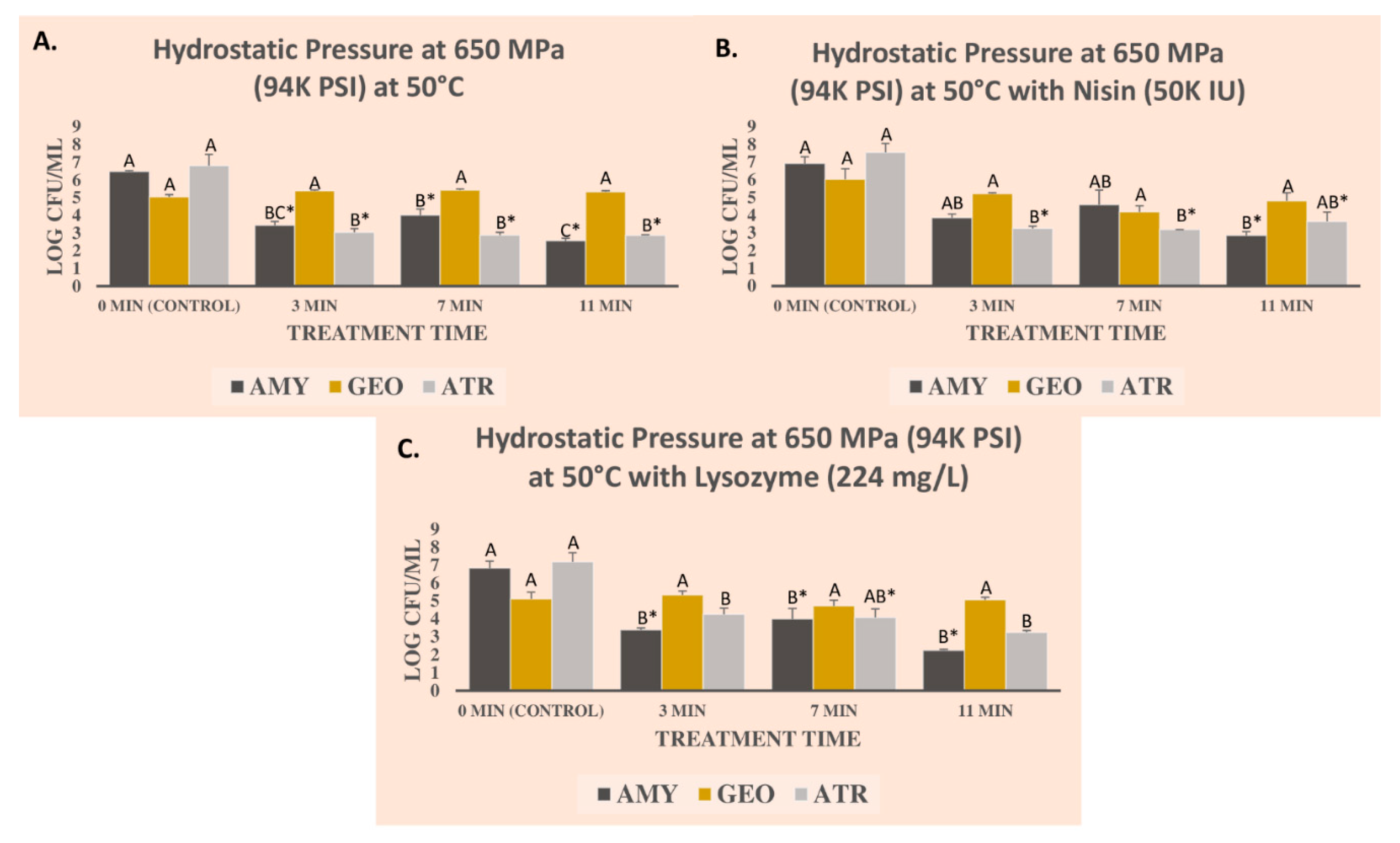
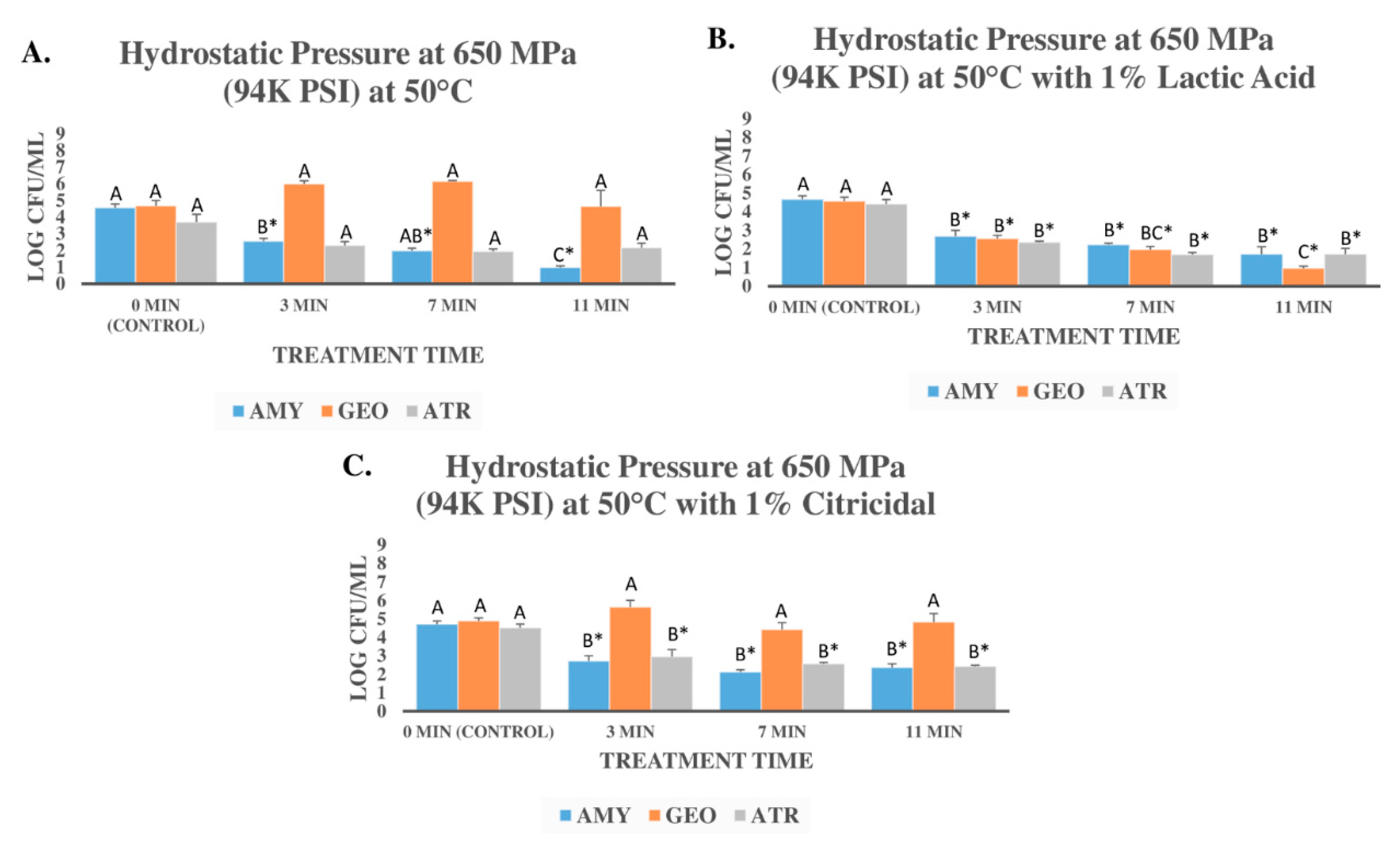
| Pathogen | Treatement a | Medium | D-Value b | Kmax c | R2 | Std Error |
|---|---|---|---|---|---|---|
| B. amyloliquefaciens | HPP | Carrot Juice | 3.45 | 0.67 | 0.57 | 0.15 |
| HPP + Nisin | Carrot Juice | 3.34 | 0.83 | 0.48 | 0.23 | |
| HPP + Lysozyme | Carrot Juice | 2.85 | 1.01 | 0.62 | 0.21 | |
| G. stearothermophilus | HPP | Carrot Juice | - d | - | - | - |
| HPP + Nisin | Carrot Juice | 8.18 | 0.28 | 0.11 | 0.16 | |
| HPP + Lysozyme | Carrot Juice | 41.84 | 0.51 | 0.36 | 0.31 | |
| B. atrophaeus | HPP | Carrot Juice | 2.82 | 0.98 | 0.62 | 0.21 |
| HPP + Nisin | Carrot Juice | 3.61 | 0.56 | 0.26 | 0.40 | |
| HPP + Lysozyme | Carrot Juice | 3.06 | 0.93 | 0.50 | 0.29 | |
| B. amyloliquefaciens | HPP | Distilled Water | 3.32 | 0.69 | 0.81 | 0.25 |
| HPP + Lactic Acid | Distilled Water | 4.11 | 0.56 | 0.12 | 0.57 | |
| HPP + CitricidalTM | Distilled Water | 5.07 | 0.45 | 0.48 | 0.12 | |
| G. stearothermophilus | HPP | Distilled Water | 131.58 | 0.02 | <0.1 | 0.21 |
| HPP + Lactic Acid | Distilled Water | 14.33 | 0.03 | <0.1 | 0.20 | |
| HPP + CitricidalTM | Distilled Water | 24.33 | 0.04 | <0.1 | 0.12 | |
| B. atrophaeus | HPP | Distilled Water | 7.88 | 0.29 | 0.21 | 0.13 |
| HPP + Lactic Acid | Distilled Water | 4.39 | 0.52 | 0.57 | 0.11 | |
| HPP + CitricidalTM | Distilled Water | 5.79 | 0.40 | 0.48 | 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aras, S.; Kabir, N.; Wadood, S.; George, J.; Chowdhury, S.; Fouladkhah, A.C. Synergistic Effects of Nisin, Lysozyme, Lactic Acid, and CitricidalTM for Enhancing Pressure-Based Inactivation of Bacillus amyloliquefaciens, Geobacillus stearothermophilus, and Bacillus atrophaeus Endospores. Microorganisms 2021, 9, 653. https://doi.org/10.3390/microorganisms9030653
Aras S, Kabir N, Wadood S, George J, Chowdhury S, Fouladkhah AC. Synergistic Effects of Nisin, Lysozyme, Lactic Acid, and CitricidalTM for Enhancing Pressure-Based Inactivation of Bacillus amyloliquefaciens, Geobacillus stearothermophilus, and Bacillus atrophaeus Endospores. Microorganisms. 2021; 9(3):653. https://doi.org/10.3390/microorganisms9030653
Chicago/Turabian StyleAras, Sadiye, Niamul Kabir, Sabrina Wadood, Jyothi George, Shahid Chowdhury, and Aliyar Cyrus Fouladkhah. 2021. "Synergistic Effects of Nisin, Lysozyme, Lactic Acid, and CitricidalTM for Enhancing Pressure-Based Inactivation of Bacillus amyloliquefaciens, Geobacillus stearothermophilus, and Bacillus atrophaeus Endospores" Microorganisms 9, no. 3: 653. https://doi.org/10.3390/microorganisms9030653
APA StyleAras, S., Kabir, N., Wadood, S., George, J., Chowdhury, S., & Fouladkhah, A. C. (2021). Synergistic Effects of Nisin, Lysozyme, Lactic Acid, and CitricidalTM for Enhancing Pressure-Based Inactivation of Bacillus amyloliquefaciens, Geobacillus stearothermophilus, and Bacillus atrophaeus Endospores. Microorganisms, 9(3), 653. https://doi.org/10.3390/microorganisms9030653






