Microbial Succession under Freeze–Thaw Events and Its Potential for Hydrocarbon Degradation in Nutrient-Amended Antarctic Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sampling
2.2. Experimental Microcosms
2.3. Hydrocarbon Quantification
2.4. RNA Extraction and Conversion to cDNA
2.5. ARISA Community Fingerprint
2.6. Quantitative PCR
2.7. cDNA PCR and Sequencing
2.8. Processing Sequencing Data
2.9. Statistical Analysis
3. Results
3.1. Microbial Community Structure
3.2. Sequencing Results and Biodiversity
3.3. Hydrocarbon Removal/Transformation
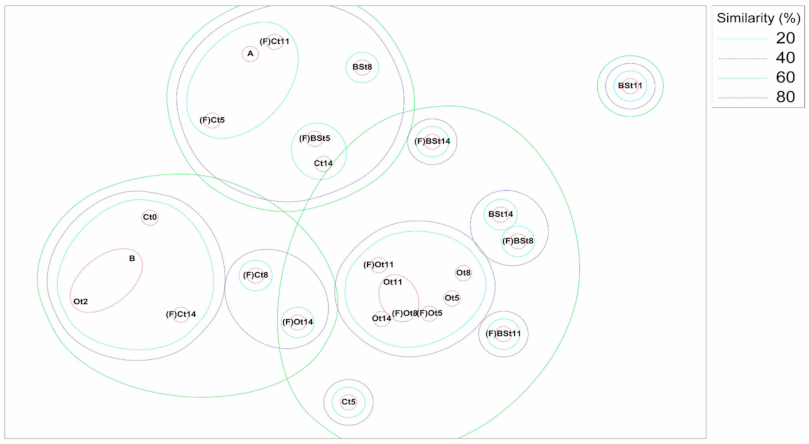
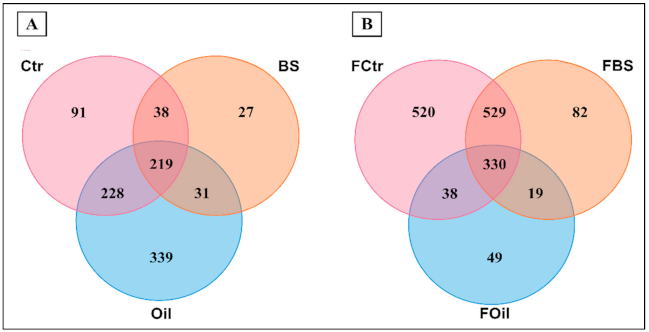
3.4. OTU and Sample Profile Analysis
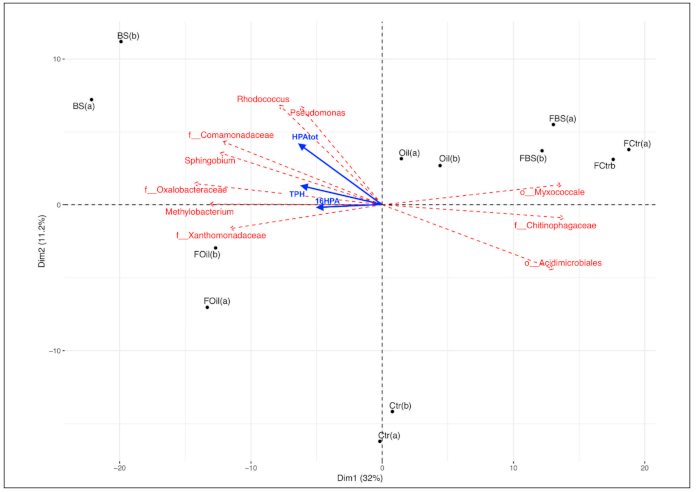
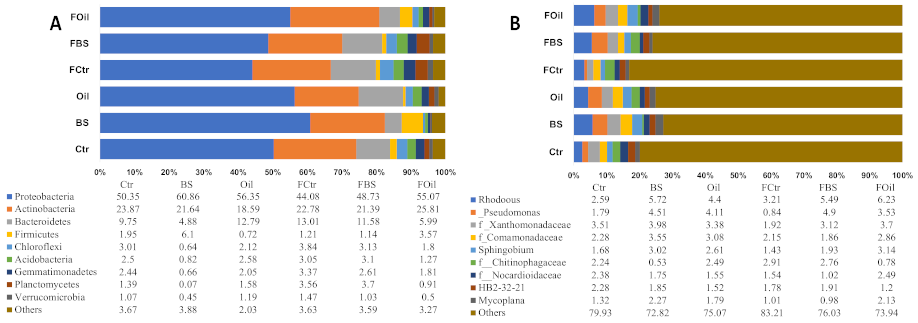
3.5. Taxonomy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aislabie, J.; Balks, M.; Astori, N.; Stevenson, G.; Symons, R. Polycyclic aromatic hydrocarbons in fuel-oil contaminated soils, Antarctica. Science 1999, 39, 2201–2207. [Google Scholar] [CrossRef]
- Smith, R.I.L.; West, H.M.; Avery, L.M. Response of rhizosphere microbial communities associated with Antarctic hairgrass (Deschampsia antarctica) to UV radiation. Polar Biol. 2003, 26, 525–529. [Google Scholar] [CrossRef]
- Coulon, F.; Pelletier, E.; Gourhant, L.; Delille, D. Effects of nutrient and temperature on degradation of petroleum hydrocarbons in contaminated sub-Antarctic soil. Chemosphere 2005, 58, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Stomeo, F.; Makhalanyane, T.P.; Valverde, A.; Pointing, S.B.; Stevens, M.I.; Cary, C.S.; Tuffin, M.I.; Cowan, D.A. Abiotic factors influence microbial diversity in permanently cold soil horizons of a maritime-associated Antarctic Dry Valley. FEMS Microbiol. Ecol. 2012, 82, 326–340. [Google Scholar] [CrossRef] [PubMed]
- Blix, A.S. Adaptations to polar life in mammals and birds. J. Exp. Biol. 2016, 219, 1093–1105. [Google Scholar] [CrossRef]
- Selby, M.J. Antarctica: Soils, weathering processes and environment, I.B. Campbell and G. G. C. Claridge, (developments in soil science 16), Elsevier, Amsterdam, 1987. No. of pages: 368. Earth Surf. Process. Landf. 1989, 14, 753–754. [Google Scholar] [CrossRef]
- Freckman, D.W.; Virginia, R.A. Low-Diversity Antarctic Soil Nematode Communities: Distribution and Response to Disturbance. Ecology 1997, 78, 363–369. [Google Scholar] [CrossRef]
- Arnold, R.J.; Convey, P.; Hughes, K.A.; Wynn-Williams, D.D. Seasonal periodicity of physical factors, inorganic nutrients and microalgae in Antarctic fellfields. Polar Biol. 2003, 26, 396–403. [Google Scholar] [CrossRef]
- Pointing, S.B.; Chan, Y.; Lacap, D.C.; Lau, M.C.Y.; Jurgens, J.A.; Farrell, R.L. Highly specialized microbial diversity in hyper-arid polar desert. Proc. Natl. Acad. Sci. USA 2009, 106, 19964–19969. [Google Scholar] [CrossRef] [PubMed]
- Aislabie, J.M.; Balks, M.R.; Foght, J.M.; Waterhouse, E.J. Hydrocarbon Spills on Antarctic Soils: Effects and Management. Environ. Sci. Technol. 2004, 38, 1265–1274. [Google Scholar] [CrossRef]
- Röling, W.F.; Van Verseveld, H.W. Natural attenuation: What does the subsurface have in store? Biodegradation 2002, 13, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Bargagli, R. Environmental contamination in Antarctic ecosystems. Sci. Total Environ. 2008, 400, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rosales, C.; Fullana, N.; Musto, H.; Castro-Sowinski, S. Antarctic DNA moving forward: Genomic plasticity and biotechnological potential. FEMS Microbiol. Lett. 2012, 331, 1–9. [Google Scholar] [CrossRef]
- Vázquez, S.; Nogales, B.; Ruberto, L.; Mestre, C.; Christie-Oleza, J.; Ferrero, M.; Bosch, R.; Mac Cormack, W. Characterization of bacterial consortia from diesel-contaminated Antarctic soils: Towards the design of tailored formulas for bioaugmentation. Int. Biodeterior. Biodegrad. 2013, 77, 22–30. [Google Scholar] [CrossRef]
- Cury, J.C.; Jurelevicius, D.A.; Villela, H.D.; Jesus, H.E.; Peixoto, R.S.; Schaefer, C.E.G.R.; Bicego, M.C.; Seldin, L.; Rosado, A.S. Microbial diversity and hydrocarbon depletion in low and high diesel-polluted soil samples from Keller Peninsula, South Shetland Islands. Antarct. Sci. 2015, 27, 263–273. [Google Scholar] [CrossRef][Green Version]
- Sampaio, D.S.; Almeida, J.R.B.; De Jesus, H.E.; Rosado, A.S.; Seldin, L.; Jurelevicius, D. Distribution of Anaerobic Hydrocarbon-Degrading Bacteria in Soils from King George Island, Maritime Antarctica. Microb. Ecol. 2017, 74, 810–820. [Google Scholar] [CrossRef]
- Kim, J.; Lee, A.H.; Chang, W. Enhanced bioremediation of nutrient-amended, petroleum hydrocarbon-contaminated soils over a cold-climate winter: The rate and extent of hydrocarbon biodegradation and microbial response in a pilot-scale biopile subjected to natural seasonal freeze-thaw temperatures. Sci. Total Environ. 2018, 612, 903–913. [Google Scholar] [CrossRef]
- Mohn, W.W.; Stewart, G.R. Limiting factors for hydrocarbon biodegradation at low temperature in Arctic soils. Soil Biol. Biochem. 2000, 32, 1161–1172. [Google Scholar] [CrossRef]
- Lin, X.; Yang, B.; Shen, J.; Du, N. Biodegradation of Crude Oil by an Arctic Psychrotrophic Bacterium Pseudoalteromomas sp. P29. Curr. Microbiol. 2009, 59, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, L.M.M.; Ruberto, L.; Balbo, A.L.; Mac Cormack, W.P. Bioremediation of hydrocarbon-contaminated soils in cold regions: Development of a pre-optimized biostimulation biopile-scale field assay in Antarctica. Sci. Total Environ. 2017, 590–591, 194–203. [Google Scholar] [CrossRef]
- Ferguson, S.H.; Franzmann, P.D.; Snape, I.; Revill, A.T.; Trefry, M.G.; Zappia, L.R. Effects of temperature on mineralisation of petroleum in contaminated Antarctic terrestrial sediments. Chemosphere 2003, 52, 975–987. [Google Scholar] [CrossRef]
- Ruberto, L.; Dias, R.; Balbo, A.L.; Vazquez, S.; Hernandez, E.; Mac Cormack, W. Influence of nutrients addition and bioaugmentation on the hydrocarbon biodegradation of a chronically contaminated Antarctic soil. J. Appl. Microbiol. 2009, 106, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Henry, H.A. Soil freeze–thaw cycle experiments: Trends, methodological weaknesses and suggested improvements. Soil Biol. Biochem. 2007, 39, 977–986. [Google Scholar] [CrossRef]
- McWatters, R.; Wilkins, D.; Spedding, T.; Hince, G.; Raymond, B.; Lagerewskij, G.; Terry, D.; Wise, L.; Snape, I. On site remediation of a fuel spill and soil reuse in Antarctica. Sci. Total Environ. 2016, 571, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Rike, A.G.; Haugen, K.B.; Børresen, M.; Engene, B.; Kolstad, P. In situ biodegradation of petroleum hydrocarbons in frozen arctic soils. Cold Reg. Sci. Technol. 2003, 37, 97–120. [Google Scholar] [CrossRef]
- Chang, W.; Klemm, S.; Beaulieu, C.; Hawari, J.; Whyte, L.; Ghoshal, S. Petroleum Hydrocarbon Biodegradation un-der Seasonal Freeze-Thaw Soil Temperature Regimes in Contaminated Soils from a Sub-Arctic Site. Environ. Sci. Technol. 2011, 45, 1061–1066. [Google Scholar] [CrossRef]
- Tuorto, S.J.; Darias, P.; McGuinness, L.R.; Panikov, N.; Zhang, T.; Häggblom, M.M.; Kerkhof, L.J. Bacterial genome replication at subzero temperatures in permafrost. ISME J. 2013, 8, 139–149. [Google Scholar] [CrossRef]
- Karppinen, E.M.; Stewart, K.J.; Farrell, R.E.; Siciliano, S.D. Petroleum hydrocarbon remediation in frozen soil using a meat and bonemeal biochar plus fertilizer. Chemosphere 2017, 173, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Bölter, M. Soil development and soil biology on King George Island, Maritime Antarctic. Pol. Polar Res. 2011, 32, 105–116. [Google Scholar] [CrossRef]
- De Jesus, H.E.; Peixoto, R.S.; Cury, J.C.; Van Elsas, J.D.; Rosado, A.S. Evaluation of soil bioremediation techniques in an aged diesel spill at the Antarctic Peninsula. Appl. Microbiol. Biotechnol. 2015, 99, 10815–10827. [Google Scholar] [CrossRef]
- Wagener, A.D.L.; Meniconi, M.D.F.G.; Hamacher, C.; Farias, C.O.; Da Silva, G.C.; Gabardo, I.T.; Scofield, A.D.L. Hydrocarbons in sediments of a chronically contaminated bay: The challenge of source assignment. Mar. Pollut. Bull. 2012, 64, 284–294. [Google Scholar] [CrossRef]
- Fisher, M.M.; Triplett, E.W. Automated Apporach for Ribosomal Intergenic Spacer Analysis of microbial diversity and its application to freshwater bacterial communities. Appl. Environ. Microbiol. 1999, 65, 4630–4636. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, M.; Brusetti, L.; Quatrini, P.; Borin, S.; Puglia, A.M.; Rizzi, A.; Sorlini, C.; Corselli, C.; Zanardini, E.; Daffonchio, D. Comparison of Different Primer Sets for Use in Automated Ribosomal Intergenic Spacer Analysis of Complex Bacterial Communities Comparison of Different Primer Sets for Use in Automated Ribosomal Intergenic Spacer Analysis of Complex Bacterial Communities. Appl. Environ. Microbiol. 2004, 70, 6147–6156. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. Primer V6: User Manual/Tutorial; Primer: Plymouth, UK, 2006. [Google Scholar]
- Muyzer, G.; de Waal, E.C.; Uitterlinden, A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UCHIME2: Improved chimera prediction for amplicon sequencing. bioRxiv 2016, 074252. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2013. [Google Scholar]
- Chen, H.; Boutros, P.C. VennDiagram: A package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinform. 2011, 12, 1–7. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Paradis, E.; Schliep, K. ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatic 2018, 35, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. The Split-Apply-Combine Strategy for Data Analysis. J. Stat. Softw. 2011, 40, 1–29. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: AnRPackage for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Austral Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Eriksson, M.; Ka, J.; Mohn, W.W. Effects of Low Temperature and Freeze-Thaw Cycles on Hydrocarbon Biodegrada-tion in Arctic Tundra Soil Effects of Low Temperature and Freeze-Thaw Cycles on Hydrocarbon Biodegradation in Arctic Tundra Soil. Appl. Environ. Microbiol. 2001, 67, 5107–5112. [Google Scholar] [CrossRef] [PubMed]
- Børresen, M.; Barnes, D.; Rike, A. Repeated freeze–thaw cycles and their effects on mineralization of hexadecane and phenanthrene in cold climate soils. Cold Reg. Sci. Technol. 2007, 49, 215–225. [Google Scholar] [CrossRef]
- Rivkina, E.M.; Friedmann, E.I.; McKay, C.P.; Gilichinsky, D.A. Metabolic Activity of Permafrost Bacteria below the Freezing Point. Appl. Environ. Microbiol. 2000, 66, 3230–3233. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Gutiérrez, J.; Figueras, A.; Albaigés, J.; Jiménez, N.; Viñas, M.; Solanas, A.M.; Novoa, B. Bacterial Communities from Shoreline Environments (Costa da Morte, Northwestern Spain) Affected by the Prestige Oil Spill. Appl. Environ. Microbiol. 2009, 75, 3407–3418. [Google Scholar] [CrossRef]
- Aburto, A.; Peimbert, M. Degradation of a benzene–toluene mixture by hydrocarbon-adapted bacterial communities. Ann. Microbiol. 2010, 61, 553–562. [Google Scholar] [CrossRef]
- Whyte, L.G.; Smits, T.H.M.; Labbe, D.; Witholt, B.; Greer, C.W.; Beilen, J.B. Van Gene Cloning and Characterization of Multiple Alkane Hydroxylase Systems in Rhodococcus Strains Q15 and NRRL B-16531. Society 2002, 68, 5933–5942. [Google Scholar] [CrossRef]
- Bozal, N.; Montes, M.J.; Tudela, E.; Guinea, J. Characterization of several Psychrobacter strains isolated from Antarctic environments and description of Psychrobacter luti sp. nov. and Psychrobacter fozii sp. nov. Int. J. Syst. Evol. Microbiol. 2003, 53, 1093–1100. [Google Scholar] [CrossRef]
- Hurtado-Ortiz, R.; Nazimoudine, A.; Criscuolo, A.; Hugon, P.; Mornico, D.; Brisse, S.; Bizet, C.; Clermont, D. Psychrobacter pasteurii and Psychrobacter piechaudii sp. nov., two novel species within the genus Psychrobacter. Int. J. Syst. Evol. Microbiol. 2017, 67, 3192–3197. [Google Scholar] [CrossRef] [PubMed]
- Lasek, R.; Dziewit, L.; Ciok, A.; Decewicz, P.; Romaniuk, K.; Jedrys, Z.; Wibberg, D.; Schlüter, A.; Pühler, A.; Bartosik, D. Genome content, metabolic pathways and biotechnological potential of the psychrophilic Arctic bacterium Psychrobacter sp. DAB_AL43B, a source and a host of novel Psychrobacter -specific vectors. J. Biotechnol. 2017, 263, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Singh, S.M.; Singh, R.N.; Naik, S.; Roy, U.; Srivastava, A.; Bölter, M. Bacterial communities in ancient permafrost profiles of Svalbard, Arctic. J. Basic Microbiol. 2017, 57, 1018–1036. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, L.; Zhang, J.; Yin, J.; Huang, S. Bacterial Community Structure after Long-term Organic and Inorganic Fertilization Reveals Important Associations between Soil Nutrients and Specific Taxa Involved in Nutrient Transformations. Front. Microbiol. 2017, 8, 187. [Google Scholar] [CrossRef] [PubMed]
- Mishamandani, S.; Gutierrez, T.; Berry, D.; Aitken, M.D. Response of the bacterial community associated with a cosmopolitan marine diatom to crude oil shows a preference for the biodegradation of aromatic hydrocarbons. Environ. Microbiol. 2016, 18, 1817–1833. [Google Scholar] [CrossRef]
- Li, A.-Z.; Lin, L.-Z.; Zhang, M.-X.; Zhu, H.-H. Arenibacter antarcticus sp. nov., isolated from marine sediment. Int. J. Syst. Evol. Microbiol. 2017, 67, 4601–4605. [Google Scholar] [CrossRef]
- Doronina, N.V.; Kaparullina, E.N.; Trotsenko, Y.A. Methyloversatilis thermotolerans sp. nov., a novel thermotolerant facultative methylotroph isolated from a hot spring. Int. J. Syst. Evol. Microbiol. 2014, 64, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Rochman, F.F.; Sheremet, A.; Tamas, I.; Saidi-Mehrabad, A.; Kim, J.-J.; Dong, X.; Sensen, C.W.; Gieg, L.M.; Dunfield, P.F. Benzene and Naphthalene Degrading Bacterial Communities in an Oil Sands Tailings Pond. Front. Microbiol. 2017, 8, 1845. [Google Scholar] [CrossRef] [PubMed]
- Al-Jawasim, M.; Yu, K.; Park, J.-W. Synergistic effect of crude oil plus dispersant on bacterial community in a louisiana salt marsh sediment. FEMS Microbiol. Lett. 2015, 362, fnv144. [Google Scholar] [CrossRef]
- Tsuboi, S.; Yamamura, S.; Nakajima-Kambe, T.; Iwasaki, K. Diversity of alkane hydroxylase genes on the rhizoplane of grasses planted in petroleum-contaminated soils. SpringerPlus 2015, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rosario-Passapera, R.; Keddis, R.; Wong, R.; Lutz, R.A.; Starovoytov, V.; Vetriani, C. Parvibaculum hydrocarboniclasticum sp. nov., a mesophilic, alkane-oxidizing alphaproteobacterium isolated from a deep-sea hydrothermal vent on the East Pacific Rise. Int. J. Syst. Evol. Microbiol. 2012, 62, 2921–2926. [Google Scholar] [CrossRef] [PubMed]
- Looper, J.K.; Cotto, A.; Kim, B.-Y.; Lee, M.-K.; Liles, M.R.; Chadhain, S.M.N.; Son, A. Microbial community analysis of Deepwater Horizon oil-spill impacted sites along the Gulf coast using functional and phylogenetic markers. Environ. Sci. Process. Impacts 2013, 15, 2068–2079. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, Y.; Zhang, W.; Wang, F.; Bian, Y.; Boughner, L.A.; Jiang, X. Novel Biochar-Plant Tandem Approach for Remediating Hexachlorobenzene Contaminated Soils: Proof-of-Concept and New Insight into the Rhizosphere. J. Agric. Food Chem. 2016, 64, 5464–5471. [Google Scholar] [CrossRef]
- Zhou, H.; Li, X.; Chu, Z.; Zhang, J. Effect of temperature downshifts on a bench-scale hybrid A/O system: Process performance and microbial community dynamics. Chemosphere 2016, 153, 500–507. [Google Scholar] [CrossRef]
- Hou, J.; Liu, W.; Wang, B.; Wang, Q.; Luo, Y.; Franks, A.E. PGPR enhanced phytoremediation of petroleum contaminated soil and rhizosphere microbial community response. Chemosphere 2015, 138, 592–598. [Google Scholar] [CrossRef]
- Salam, L.B.; Obayori, S.O.; Nwaokorie, F.O.; Suleiman, A.; Mustapha, R. Metagenomic insights into effects of spent engine oil perturbation on the microbial community composition and function in a tropical agricultural soil. Environ. Sci. Pollut. Res. 2017, 24, 7139–7159. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, X.; Wu, X.; Chen, G.; Bakken, L.R.; Zhao, L.; Frostegård, Å. Carbon-driven enrichment of the crucial nitrate-reducing bacteria in limed peat soil microcosms. Lett. Appl. Microbiol. 2017, 65, 159–164. [Google Scholar] [CrossRef]
- Blazewicz, S.J.; Barnard, R.L.; Daly, R.A.; Firestone, M.K. Evaluating rRNA as an indicator of microbial activity in environmental communities: Limitations and uses. ISME J. 2013, 7, 2061–2068. [Google Scholar] [CrossRef]
- Edwards, A.C.; Cresser, M.S. Freezing and Its Effect on Chemical and Biological Properties of Soil. Adv. Soil Sci. 1992, 18, 59–79. [Google Scholar]
- Brooks, P.D.; McKnight, D.; Elder, K. Carbon limitation of soil respiration under winter snowpacks: Potential feedbacks between growing season and winter carbon fluxes. Glob. Chang. Biol. 2005, 11, 231–238. [Google Scholar] [CrossRef]
- Yergeau, E.; Kowalchuk, G.A. Responses of Antarctic soil microbial communities and associated functions to temperature and freeze-thaw cycle frequency. Environ. Microbiol. 2008, 10, 2223–2235. [Google Scholar] [CrossRef]
- Jroundi, F.; Martinez-Ruiz, F.; Merroun, M.L.; Gonzalez-Muñoz, M.T. Exploring bacterial community composition in Mediterranean deep-sea sediments and their role in heavy metal accumulation. Sci. Total Environ. 2020, 712, 135660. [Google Scholar] [CrossRef]
- Atlas, R.; Bragg, J. Bioremediation of marine oil spills: When and when not—The Exxon Valdez experience. Microb. Biotechnol. 2009, 2, 213–221. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Wooller, M.J.; Pohlman, J.W.; Quensen, J.; Tiedje, J.M.; Leigh, M.B. Shifts in Identity and Activity of Methanotrophs in Arctic Lake Sediments in Response to Temperature Changes. Appl. Environ. Microbiol. 2012, 78, 4715–4723. [Google Scholar] [CrossRef] [PubMed]
- Nikrad, M.P.; Kerkhof, L.J.; Häggblom, M.M. The subzero microbiome: Microbial activity in frozen and thawing soils. FEMS Microbiol. Ecol. 2016, 92, fiw081. [Google Scholar] [CrossRef] [PubMed]
| Treatments | OTUs | Chao Estimator | Shannon Index | qPCR (Gene Copies g−1) | TPH (μg g−1) | PAHtot (μg g−1) | 16PAH (μg g−1) |
|---|---|---|---|---|---|---|---|
| FCtr | 1417 ± 40.31 | 484 ± 14.14 | 5.27 ± 0.12 | 1.2 × 108 | 49 ± 8.50 a | 507 ± 137.78 a | 123 ± 34.56 a |
| FBS | 960 ± 83.44 | 431 ± 13.44 | 5.11 ± 0.17 | 1.5 × 104 | 566 ± 38.69 bc | 19681 ± 248.03 bc | 616 ± 59.32 b |
| FOil | 436 ± 7.78 | 303 ± 4.31 | 4.86 ± 0.89 | 6.2 × 108 | 1262 ± 280.05 b | 28667 ± 4590.36 b | 1370 ± 330.52 c |
| Ctr | 576 ± 24.75 | 364 ± 40.73 | 5.13 ± 0.57 | 1.1 × 103 | 68 ± 24.50 a | 715 ± 258.08 a | 189 ± 39.37 ab |
| BS | 315 ± 4.95 | 234 ± 1.56 | 4.68 ± 0.34 | 1.1 × 102 | 381 ± 23.64 ac | 13904 ± 2043.34 c | 398 ± 43.84 ab |
| Oil | 817 ± 35.72 | 372 ± 25.54 | 4.96 ± 0.37 | 4.5 × 102 | 409 ± 167.70 bc | 14495 ± 2679.73 bc | 1310 ± 227.32 c |
| Temperature Condition | Taxon | Contrib. % | Cumulative % | Mean Ctr | Mean FCtr | Mean BS | Mean FBS | Mean Oil | Mean FOil |
|---|---|---|---|---|---|---|---|---|---|
| Both conditions (4 °C and freeze/thaw) | Pseudomonas | 3.232 | 3.232 | 0.0179 | 0.0084 | 0.0451 | 0.0490 | 0.0411 | 0.0353 |
| Rhodococcus | 2.979 | 6.211 | 0.0259 | 0.0321 | 0.0572 | 0.0549 | 0.0440 | 0.0623 | |
| Methylobacterium | 2.076 | 8.287 | 0.0128 | 0.0039 | 0.0280 | 0.0027 | 0.0012 | 0.0173 | |
| f__Chitinophagaceae | 2.009 | 10.3 | 0.0224 | 0.0291 | 0.0053 | 0.0276 | 0.0249 | 0.0078 | |
| o__Acidimicrobiales | 1.888 | 12.18 | 0.0202 | 0.0267 | 0.0020 | 0.0178 | 0.0108 | 0.0071 | |
| f__Oxalobacteraceae | 1.793 | 13.98 | 0.0137 | 0.0053 | 0.0290 | 0.0063 | 0.0111 | 0.0197 | |
| g__Sphingobium | 1.473 | 15.45 | 0.0168 | 0.0143 | 0.0302 | 0.0193 | 0.0261 | 0.0314 | |
| f__Xanthomonadaceae | 1.357 | 16.81 | 0.0351 | 0.0192 | 0.0398 | 0.0312 | 0.0338 | 0.0370 | |
| f__Comamonadaceae | 1.313 | 18.12 | 0.0228 | 0.0215 | 0.0355 | 0.0186 | 0.0308 | 0.0286 | |
| o__Myxococcales | 1.289 | 19.41 | 0.0092 | 0.0149 | 0.0020 | 0.0170 | 0.0084 | 0.0018 | |
| 4 °C | Rhodococcus | 3.031 | 3.031 | 0.0259 | - | 0.0572 | - | 0.0440 | - |
| Pseudomonas | 2.842 | 5.873 | 0.0179 | - | 0.0451 | - | 0.0411 | - | |
| Methylobacterium | 2.6 | 8.474 | 0.0128 | - | 0.0280 | - | 0.0012 | - | |
| f__Chitinophagaceae | 1.942 | 10.42 | 0.0224 | - | 0.0053 | - | 0.0249 | - | |
| o__Acidimicrobiales | 1.827 | 12.24 | 0.0202 | - | 0.0020 | - | 0.0108 | - | |
| f__Oxalobacteraceae | 1.714 | 13.96 | 0.0137 | - | 0.0290 | - | 0.0111 | - | |
| g__Corynebacterium | 1.606 | 15.56 | 0.0045 | - | 0.0168 | - | 0.0000 | - | |
| g__Staphylococcus | 1.485 | 17.05 | 0.0059 | - | 0.0155 | - | 0.0000 | - | |
| Unassigned | 1.475 | 18.52 | 0.0075 | - | 0.0165 | - | 0.0010 | - | |
| Sphingobium | 1.369 | 19.89 | 0.0168 | - | 0.0302 | - | 0.0261 | - | |
| Freeze/Thaw | Pseudomonas | 4.116 | 4.116 | - | 0.0084 | - | 0.0490 | - | 0.0353 |
| Rhodococcus | 3.283 | 7.399 | - | 0.0321 | - | 0.0549 | - | 0.0623 | |
| f__Chitinophagaceae | 2.376 | 9.776 | - | 0.0291 | - | 0.0276 | - | 0.0078 | |
| o__Acidimicrobiales | 2.066 | 11.84 | - | 0.0267 | - | 0.0178 | - | 0.0071 | |
| f__Xanthomonadaceae | 1.822 | 13.66 | - | 0.0192 | - | 0.0312 | - | 0.0370 | |
| Sphingobium | 1.817 | 15.48 | - | 0.0143 | - | 0.0193 | - | 0.0314 | |
| o__Myxococcales | 1.716 | 17.2 | - | 0.0149 | - | 0.0170 | - | 0.0018 | |
| f__Oxalobacteraceae | 1.688 | 18.88 | - | 0.0053 | - | 0.0063 | - | 0.0197 | |
| g__Methylobacterium | 1.679 | 20.56 | - | 0.0039 | - | 0.0027 | - | 0.0173 | |
| f__Nocardioidaceae | 1.544 | 22.11 | - | 0.0154 | - | 0.0102 | - | 0.0249 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jesus, H.E.d.; Carreira, R.S.; Paiva, S.S.M.; Massone, C.; Enrich-Prast, A.; Peixoto, R.S.; Rodrigues, J.L.M.; Lee, C.K.; Cary, C.; Rosado, A.S. Microbial Succession under Freeze–Thaw Events and Its Potential for Hydrocarbon Degradation in Nutrient-Amended Antarctic Soil. Microorganisms 2021, 9, 609. https://doi.org/10.3390/microorganisms9030609
Jesus HEd, Carreira RS, Paiva SSM, Massone C, Enrich-Prast A, Peixoto RS, Rodrigues JLM, Lee CK, Cary C, Rosado AS. Microbial Succession under Freeze–Thaw Events and Its Potential for Hydrocarbon Degradation in Nutrient-Amended Antarctic Soil. Microorganisms. 2021; 9(3):609. https://doi.org/10.3390/microorganisms9030609
Chicago/Turabian StyleJesus, Hugo Emiliano de, Renato S. Carreira, Simone S. M. Paiva, Carlos Massone, Alex Enrich-Prast, Raquel S. Peixoto, Jorge L. Mazza Rodrigues, Charles K. Lee, Craig Cary, and Alexandre S. Rosado. 2021. "Microbial Succession under Freeze–Thaw Events and Its Potential for Hydrocarbon Degradation in Nutrient-Amended Antarctic Soil" Microorganisms 9, no. 3: 609. https://doi.org/10.3390/microorganisms9030609
APA StyleJesus, H. E. d., Carreira, R. S., Paiva, S. S. M., Massone, C., Enrich-Prast, A., Peixoto, R. S., Rodrigues, J. L. M., Lee, C. K., Cary, C., & Rosado, A. S. (2021). Microbial Succession under Freeze–Thaw Events and Its Potential for Hydrocarbon Degradation in Nutrient-Amended Antarctic Soil. Microorganisms, 9(3), 609. https://doi.org/10.3390/microorganisms9030609








