New Insights into Microbial Diversity of the Traditional Packed Table Olives Aloreña de Málaga through Metataxonomic Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Packed Aloreña de Málaga
2.2. Physicochemical and Microbiological Analyses
2.3. DNA Extraction from Table Olive Samples and Library Preparation
2.4. Bioinformatic Analysis
3. Results
3.1. Physicochemical Analysis
3.2. Microbiological Analysis
3.3. Metataxonomic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Olive Oil Council Economic Affairs & Promotion Unit-International Olive Council. Available online: https://www.internationaloliveoil.org/what-we-do/economic-affairs-promotion-unit/#figures (accessed on 21 October 2020).
- Garrido-Fernández, A.; Fernández Díez, M.J.; Adams, R.M. Table Olives: Production and Processing; Chapman & Hall: London, UK, 1997; pp. 67–109. [Google Scholar]
- Regional Government of Andalusia. Order Approving the Application for Registration of the Protected Designation of Origin “Aceituna Aloreña de Málaga”; Regional Government of Andalusia: Seville, Spain, 2009. Available online: https://www.juntadeandalucia.es/boja/2009/215/44 (accessed on 8 March 2021).
- Official Journal of the European Union (DOUE). Regulation Nº1068/2012. L318/3-L318/4. Available online: https://www.boe.es/buscar/doc.php?id=DOUE-L-2012-82202 (accessed on 8 March 2021).
- Benítez-Cabello, A.; Romero-Gil, V.; Medina, E.; Sánchez, B.; Calero-Delgado, B.; Bautista-Gallego, J.; Jiménez-Díaz, R.; Arroyo-López, F.N. Metataxonomic analysis of the bacterial diversity in table olive dressing components. Food Control 2019, 105, 190–197. [Google Scholar] [CrossRef]
- Arroyo-López, F.N.; Bautista-Gallego, J.; Segovia-Bravo, K.A.; García-García, P.; Durán-Quintana, M.C.; Romero, C.; Garrido-Fernández, A. Instability profile of fresh packed “seasoned” Manzanilla-Aloreña table olives. Food Sci. Technol. 2009, 42, 1629–1639. [Google Scholar] [CrossRef]
- Romero-Gil, V.; Rodríguez-Gómez, F.; Garrido-Fernández, A.; García-García, P.; Arroyo-López, F. Lactobacillus pentosus is the dominant species in spoilt packaged Aloreña de Málaga table olives. LWT 2016, 70, 252–260. [Google Scholar] [CrossRef]
- Romero-Gil, V.; Rodríguez-Gómez, F.J.; Ruiz-Bellido, M.A.; Benítez-Cabello, A.; Garrido Fernández, A.; Arroyo López, F.N. Shelf-life of traditionally-seasoned Aloreña de Málaga table olives based on package appearance and fruit characteris-tics. Grasas Aceites 2019, 70, 10. [Google Scholar] [CrossRef]
- Arroyo-López, F.N.; Medina, E.; Ruiz-Bellido, M.Á.; Romero-Gil, V.; Montes-Borrego, M.; Landa, B.B. Enhancement of the knowledge on fungal communities in directly brined aloreña de málaga green olive fermentations by metabarcoding analysis. PLoS ONE 2016, 11, e0163135. [Google Scholar] [CrossRef] [PubMed]
- Cocolin, L.; Alessandria, V.; Botta, C.; Gorra, R.; De Filippis, F.; Ercolini, D.; Rantsiou, K. NaOH-debittering induces changes in bacterial ecology during table olives fermentation. PLoS ONE 2013, 8, e69074. [Google Scholar] [CrossRef]
- De Angelis, M.; Campanella, D.; Cosmai, L.; Summo, C.; Rizzello, C.G.; Caponio, F. Microbiota and metabolome of un-started and started Greek-type fermentation of Bella di Cerignola table olives. Food Microbiol. 2015, 52, 18–30. [Google Scholar] [CrossRef]
- Medina, E.; Bellido, M.A.R.; Romero-Gil, V.; Rodríguez-Gómez, F.J.; Montes-Borrego, M.; Landa, B.B.; López, F.N.A. Assessment of the bacterial community in directly brined Aloreña de Málaga table olive fermentations by metagenetic analysis. Int. J. Food Microbiol. 2016, 236, 47–55. [Google Scholar] [CrossRef]
- Medina, E.; Brenes, M.; García-García, P.; Romero, C.; de Castro, A. Microbial ecology along the processing of Spanish olives darkened by oxidation. Food Control 2018, 86, 35–41. [Google Scholar] [CrossRef]
- Rodríguez-Gómez, F.J.; Ruiz-Bellido, M.Á.; Romero-Gil, V.; Benítez-Cabello, A.; Garrido-Fernández, A.; López, F.N.A. Microbiological and physicochemical changes in natural green heat-shocked aloreña de málaga table olives. Front. Microbiol. 2017, 8, 2209. [Google Scholar] [CrossRef]
- Benítez-Cabello, A.; Romero-Gil, V.; Medina-Pradas, E.; Garrido-Fernández, A.; Arroyo-López, F.N. Exploring bacteria diversity in commercialized table olive biofilms by metataxonomic and compositional data analysis. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2012, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Toju, H.; Tanabe, A.S.; Yamamoto, S.; Sato, H. High-coverage ITS primers for the DNA-based identification of as-comycetes and basidiomycetes in environmental samples. PLoS ONE 2012, 7, e40863. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Ramiro-Garcia, J.; Hermes, G.D.; Giatsis, C.; Sipkema, D.; Zoetendal, E.G.; Schaap, P.J.; Smidt, H. NG-Tax, a highly accurate and validated pipeline for analysis of 16S rRNA amplicons from complex biomes. F1000Research 2018, 5, 1791. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Abarenkov, K.; Zirk, A.; Piirmann, T.; Pöhönen, R.; Ivanov, F.; Nilsson, R.; Kõljalg, U. Full UNITE+INSD Dataset for Eukaryotes. Version 04.02. UNITE Community. 2020. Available online: https://search.datacite.org/works/10.15156/bio/786373 (accessed on 8 March 2021).
- McMurdie, P.J.; Holmes, S. Phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Kembel, S.W.; Cowan, P.D.; Helmus, M.R.; Cornwell, W.K.; Morlon, H.; Ackerly, D.D.; Blomberg, S.P.; Webb, C.O. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 2010, 26, 1463–1464. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; p. 213. [Google Scholar]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical analysis of taxonomic and functional pro-files. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate—A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Romero-Gil, V.; Medina, E.; Garrido-Fernández, A.; Arroyo-López, F.N. Foodborne pathogen survival in comercial Aloreña de Málaga table olive packaging. Front. Microbiol. 2018, 9, 2471. [Google Scholar] [CrossRef]
- Alves, M.; Esteves, E.; Quintas, C. Effect of preservatives and acidifying agents on the shelf life of packed cracked green table olives from Maçanilha cultivar. Food Packag. Shelf Life 2015, 5, 32–40. [Google Scholar] [CrossRef]
- Arroyo-López, F.; Querol, A.; Bautista-Gallego, J.; Garrido-Fernández, A. Role of yeasts in table olive production. Int. J. Food Microbiol. 2008, 128, 189–196. [Google Scholar] [CrossRef]
- López, F.N.A.; Romero, C.; Quintana, M.D.C.D.; López, A.L.; García, P.G.; Fernández, A.G. Kinetic study of the physicochemical and microbiological changes in “Seasoned” olives during the shelf-life period. J. Agric. Food Chem. 2005, 53, 5285–5292. [Google Scholar] [CrossRef]
- López, F.N.A.; Quintana, M.C.D.; Fernández, A.G. Microbial evolution during storage of seasoned olives prepared with organic acids with potassium sorbate, sodium benzoate, and ozone used as preservatives. J. Food Prot. 2006, 69, 1354–1364. [Google Scholar] [CrossRef]
- Casado, F.J.; Sánchez, A.H.; De Castro, A.; Rejano, L.; Beato, V.M.; Montaño, A. Fermented vegetables containing benzoic and ascorbic acids as additives: Benzene formation during storage and impact of additives on quality parameters. J. Agric. Food Chem. 2011, 59, 2403–2409. [Google Scholar] [CrossRef] [PubMed]
- Blana, V.A.; Polymeneas, N.; Tassou, C.C.; Panagou, E.Z. Survival of potential probiotic lactic acid bacteria on fermented green table olives during packaging in polyethylene pouches at 4 and 20 °C. Food Microbiol. 2016, 53, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, A.; Grand, M.; Liniger, M.; Iversen, C. Detection and frequency of Cronobacter spp. (Enterobacter sa-kazakii) in different categories of ready-to-eat foods other than infant formula. Int. J. Food Microbiol. 2009, 136, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Beuchat, L.R.; Komitopoulou, E.; Beckers, H.; Betts, R.P.; Bourdichon, F.; Fanning, S.; Joosten, H.M.; Ter Kuile, B.H. Low–Water activity foods: Increased concern as vehicles of foodborne pathogens. J. Food Prot. 2013, 76, 150–172. [Google Scholar] [CrossRef] [PubMed]
- Vij, V.; Ailes, E.; Wolyniak, C.; Angulo, F.J.; Klontz, K.C. Recalls of spices due to bacterial contamination monitored by the U.S. food and drug administration: The predominance of Salmonellae. J. Food Prot. 2006, 69, 233–237. [Google Scholar] [CrossRef]
- Hurtado, A.; Reguant, C.; Bordons, A.; Rozès, N. Lactic acid bacteria from fermented table olives. Food Microbiol. 2012, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kazou, M.; Tzamourani, A.; Panagou, E.Z.; Tsakalidou, E. Unraveling the microbiota of natural black cv. Kalamata fermented olives through 16S and ITS metataxonomic analysis. Microorganisms 2020, 8, 672. [Google Scholar] [CrossRef] [PubMed]
- Argyri, K.; Doulgeraki, A.I.; Manthou, E.; Grounta, A.; Argyri, A.A.; Nychas, G.J.E.; Tassou, C.C. Microbial di-versity of fermented greek table olives of halkidiki and konservolia varieties from different regions as revealed by metagenomic analysis. Microorganisms 2020, 8, 1241. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-López, F.; Romero-Gil, V.; Bautista-Gallego, J.; Rodríguez-Gómez, F.; Jiménez-Díaz, R.; García-García, P.; Querol, A.; Garrido-Fernández, A. Yeasts in table olive processing: Desirable or spoilage microorganisms? Int. J. Food Microbiol. 2012, 160, 42–49. [Google Scholar] [CrossRef]
- Alves, M.; Gonçalves, T.; Quintas, C. Microbial quality and yeast population dynamics in cracked green table olives’ fermentations. Food Control 2012, 23, 363–368. [Google Scholar] [CrossRef]
- Kurtzman, C.; Fell, J.W.; Boekhout, T. The Yeasts: A Taxonomic Study; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Heperkan, D.; Meric, B.E.; Sismanoglu, G.; Dalkiliç, G.; Güler, F.K. Mycobiota, Mycotoxigenic Fungi, and Citrinin Production in Black Olives. In Advances in Food Mycology; Springer: Boston, MA, USA, 2006; pp. 203–210. [Google Scholar]
- Heperkan, D.; Dazkır, G.S.; Kansu, D.Z.; Karbancıoglu Güler, F. Influence of temperature on citrinin accumulation by Penicillium citrinum and Peniccillium verrucosum in black table olives. Toxin Rev. 2009, 28, 180–186. [Google Scholar] [CrossRef]
- Medina-Pradas, E.; Arroyo-López, F.N. Presence of toxic microbial metabolites in table olives. Front. Microbiol. 2015, 6, 873. [Google Scholar] [CrossRef] [PubMed]



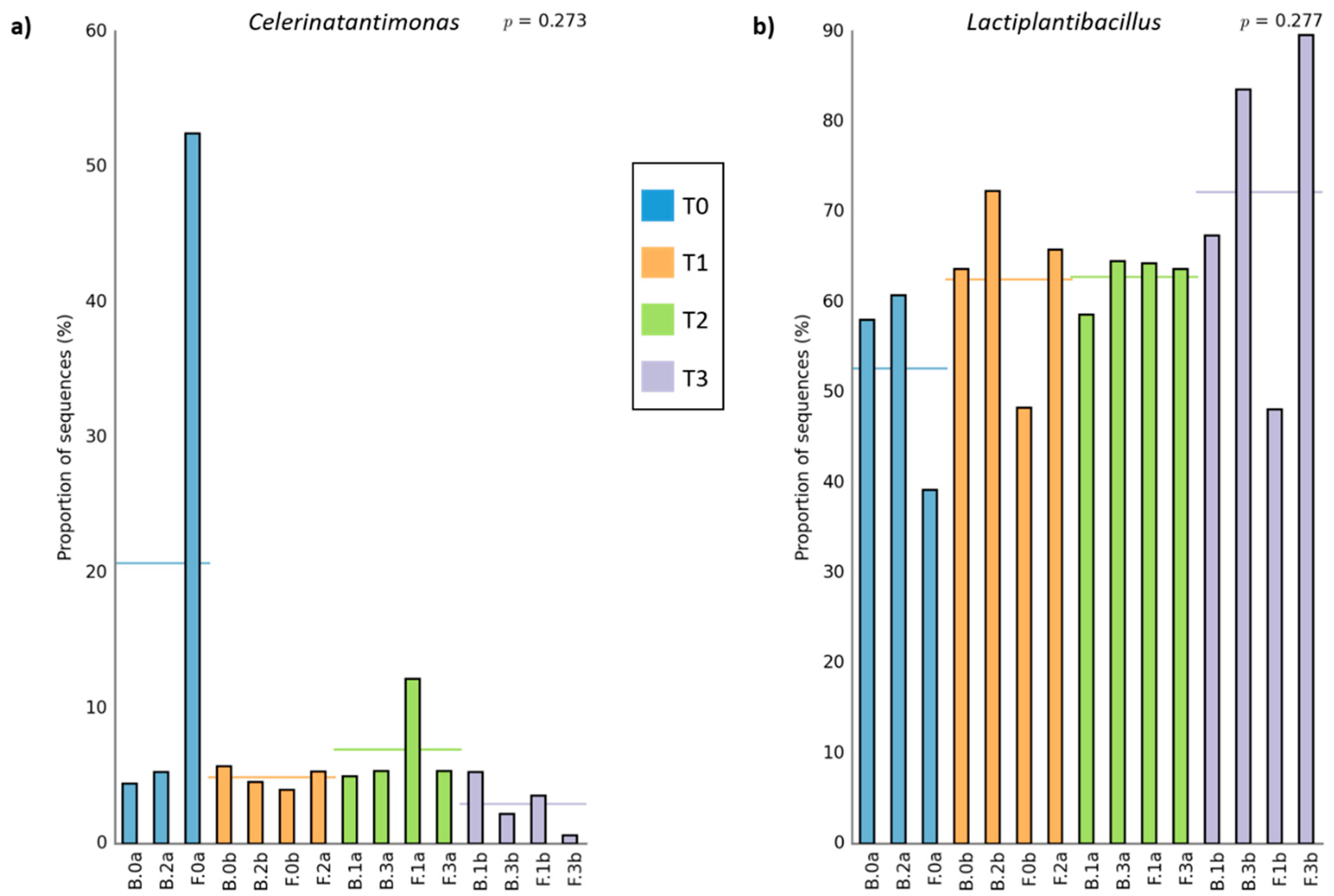
| Bacteria Population | |||||
| Time | Observed | FaithPD | Shannon | Simpson | Clonality |
| T0 (n = 4) | 42.25 ± 1.71 | 1.32 ± 0.09 | 2.09 ± 0.12 | 0.79 ± 0.04 | 0.95 ± 0.00 |
| T1 (n = 4) | 40.67 ± 2.08 | 1.19 ± 0.25 | 2.06 ± 0.06 | 0.78 ± 0.02 | 0.95 ± 0.00 |
| T2 (n = 3) | 45.00 ± 4.83 | 1.36 ± 0.08 | 2.20 ± 0.04 | 0.81 ± 0.01 | 0.95 ± 0.00 |
| T3 (n = 4) | 47.50 ± 15.78 | 0.98 ± 0.11 | 2.00 ± 0.46 | 0.70 ± 0.15 | 0.96 ± 0.01 |
| Matrix | |||||
| Brine (n = 8) | 42.38 ± 4.14 | 1.23 ± 0.19 | 2.11 ± 0.09 | 0.78 ± 0.04 | 0.95 ± 0.00 |
| Fruit (n = 7) | 46.00 ± 11.28 | 1.20 ± 0.22 | 2.07 ± 0.35 | 0.75 ± 0.12 | 0.95 ± 0.01 |
| Fungi Population | |||||
| Time | Observed | FaithPD | Shannon | Simpson | Clonality |
| T0 (n = 4) | 35.50 ± 1.91 | 2.08 ± 0.10 | 2.05 ± 0.09 | 0.70 ± 0.04 | 0.94 ± 0.00 |
| T1 (n = 3) | 34.67 ± 1.53 | 2.01 ± 0.02 | 2.02 ± 0.04 | 0.70 ± 0.01 | 0.94 ± 0.00 |
| T2 (n = 3) | 33.25 ± 3.40 | 2.07 ± 0.18 | 2.03 ± 0.15 | 0.70 ± 0.05 | 0.94 ± 0.00 |
| T3 (n = 4) | 32.00 ± 2.00 | 1.87 ± 0.22 | 2.29 ± 0.30 | 0.78 ± 0.09 | 0.93 ± 0.01 |
| Matrix | |||||
| Brine (n = 8) | 33.63 ± 2.33 | 1.96 ± 0.14 | 2.08 ± 0.07 | 0.71 ± 0.02 | 0.94 ± 0.00 |
| Fruit (n = 6) | 34.33 ± 2.94 | 2.09 ± 0.15 | 2.11 ± 0.28 | 0.72 ± 0.09 | 0.94 ± 0.01 |
| Taxonomic Unit/Sample | * B-T0 | B-T1 | B-T2 | B-T3 | F-T0 | F-T1 | F-T2 | F-T3 |
|---|---|---|---|---|---|---|---|---|
| Lactiplantibacillus | 58.27 | 66.83 | 60.47 | 74.65 | 43.23 | 47.55 | 64.12 | 62.33 |
| Pediococcus | 34.53 | 26.07 | 32.03 | 20.04 | 27.41 | 46.43 | 25.19 | 29.53 |
| Celerinatantimonas | 4.77 | 5.05 | 5.07 | 3.68 | 27.89 | 3.91 | 8.62 | 5.23 |
| Unassigned taxa | 0.23 | 0.11 | 0.19 | 0.15 | 0.09 | 0.12 | 0.19 | 0.19 |
| Salinicola | 0.17 | 0.09 | 0.19 | 0.16 | 0.00 | 0.13 | 0.18 | 0.23 |
| Alkalibacterium | 0.17 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Marinilactibacillus | 0.00 | 0.14 | 0.08 | 0.08 | 0.06 | 0.17 | 0.15 | 0.19 |
| Bacillaceae family | 0.00 | 0.00 | 0.12 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Enterobacter | 0.00 | 0.00 | 0.00 | 0.00 | 0.18 | 0.00 | 0.00 | 0.00 |
| Pseudomonas | 0.00 | 0.00 | 0.00 | 0.00 | 0.12 | 0.00 | 0.00 | 0.00 |
| Suttonella | 0.00 | 0.00 | 0.06 | 0.08 | 0.00 | 0.00 | 0.14 | 0.17 |
| Carnimonas | 0.00 | 0.00 | 0.00 | 0.00 | 0.11 | 0.00 | 0.00 | 0.00 |
| Lactobacillaceae family | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.15 | 0.00 | 0.00 |
| Genus/Sample | * B-T0 | B-T1 | B-T2 | B-T3 | F-T0 | F-T1 | F-T2 | F-T3 |
|---|---|---|---|---|---|---|---|---|
| Citeromyces | 53.63 | 56.46 | 56.05 | 53.95 | 57.95 | 57.63 | 56.88 | 19.25 |
| Unassigned taxa | 27.24 | 27.06 | 26.13 | 34.53 | 24.03 | 24.53 | 24.14 | 32.01 |
| Candida | 10.31 | 9.17 | 9.92 | 6.36 | 10.17 | 10.01 | 10.17 | 29.20 |
| Penicillium | 7.89 | 6.57 | 7.07 | 4.79 | 7.00 | 6.46 | 7.76 | 17.01 |
| Wickerhamomyces | 0.52 | 0.31 | 0.46 | 0.18 | 0.48 | 0.53 | 0.45 | 1.86 |
| Aureobasidium | 0.23 | 0.18 | 0.21 | 0.12 | 0.22 | 0.21 | 0.28 | 0.00 |
| Debaryomyces | 0.18 | 0.08 | 0.16 | 0.08 | 0.16 | 0.17 | 0.19 | 0.46 |
| Cladosporium | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.13 | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-García, E.; Benítez-Cabello, A.; Ramiro-García, J.; Romero-Gil, V.; Rodríguez-Gómez, F.; Arroyo-López, F.N. New Insights into Microbial Diversity of the Traditional Packed Table Olives Aloreña de Málaga through Metataxonomic Analysis. Microorganisms 2021, 9, 561. https://doi.org/10.3390/microorganisms9030561
López-García E, Benítez-Cabello A, Ramiro-García J, Romero-Gil V, Rodríguez-Gómez F, Arroyo-López FN. New Insights into Microbial Diversity of the Traditional Packed Table Olives Aloreña de Málaga through Metataxonomic Analysis. Microorganisms. 2021; 9(3):561. https://doi.org/10.3390/microorganisms9030561
Chicago/Turabian StyleLópez-García, Elio, Antonio Benítez-Cabello, Javier Ramiro-García, Verónica Romero-Gil, Francisco Rodríguez-Gómez, and Francisco Noé Arroyo-López. 2021. "New Insights into Microbial Diversity of the Traditional Packed Table Olives Aloreña de Málaga through Metataxonomic Analysis" Microorganisms 9, no. 3: 561. https://doi.org/10.3390/microorganisms9030561
APA StyleLópez-García, E., Benítez-Cabello, A., Ramiro-García, J., Romero-Gil, V., Rodríguez-Gómez, F., & Arroyo-López, F. N. (2021). New Insights into Microbial Diversity of the Traditional Packed Table Olives Aloreña de Málaga through Metataxonomic Analysis. Microorganisms, 9(3), 561. https://doi.org/10.3390/microorganisms9030561








