Genome-Driven Discovery of Enzymes with Industrial Implications from the Genus Aneurinibacillus
Abstract
1. Introduction
2. Materials and Methods
2.1. Datasets
2.2. Genome Annotation
2.3. Phylogenomic and Comparative Genome Analyses
3. Results and Discussion
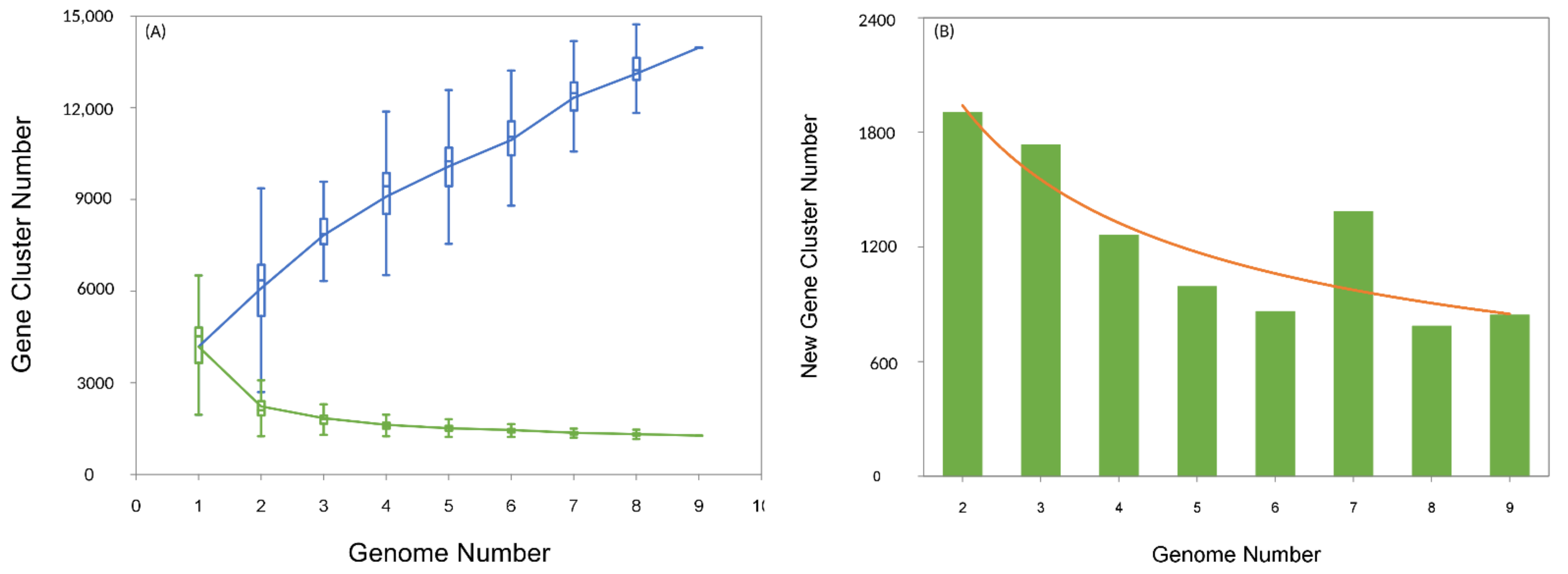
3.1. General Features of Aneurinibacillus Genome
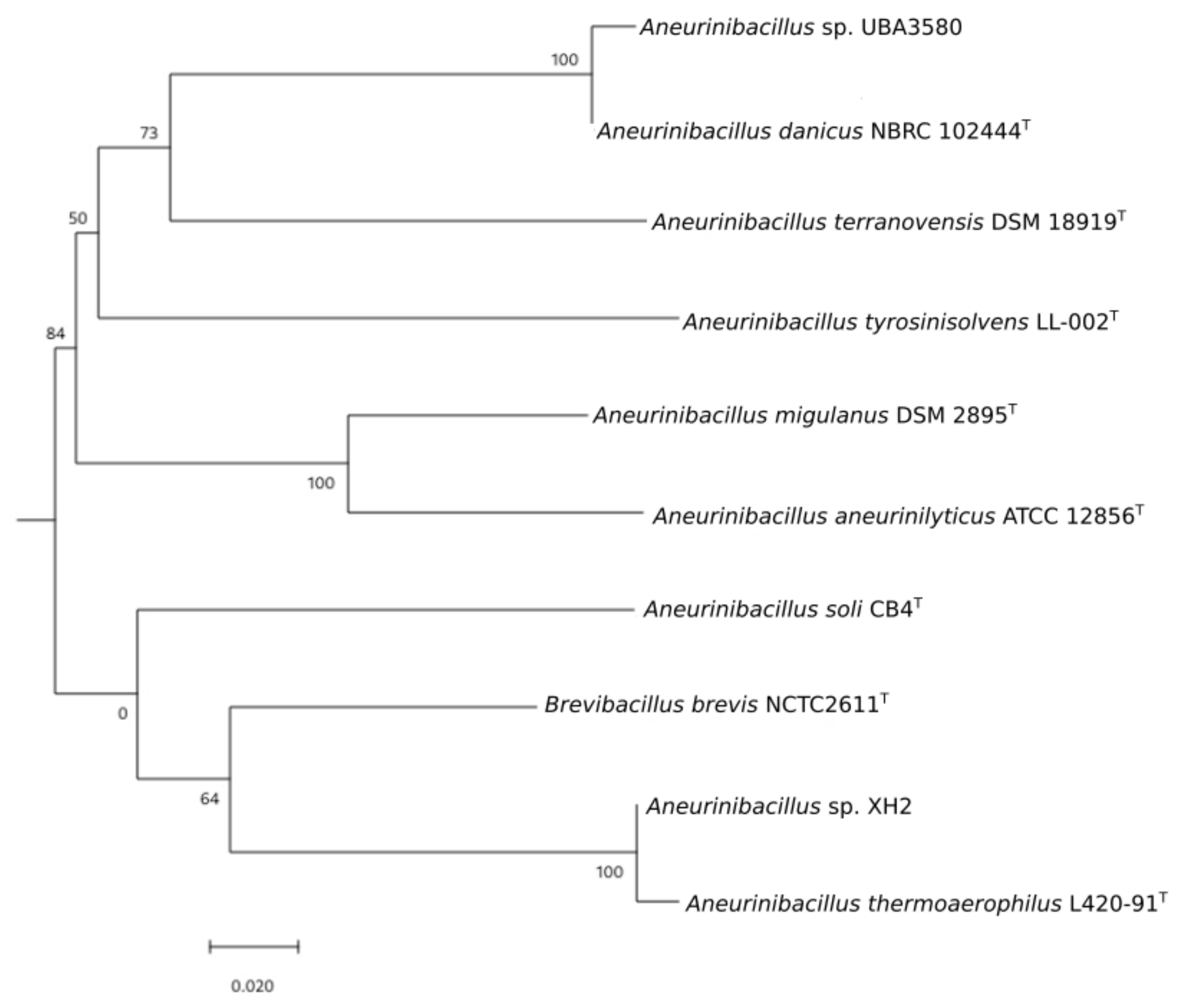
3.2. Phylogenomic Analysis
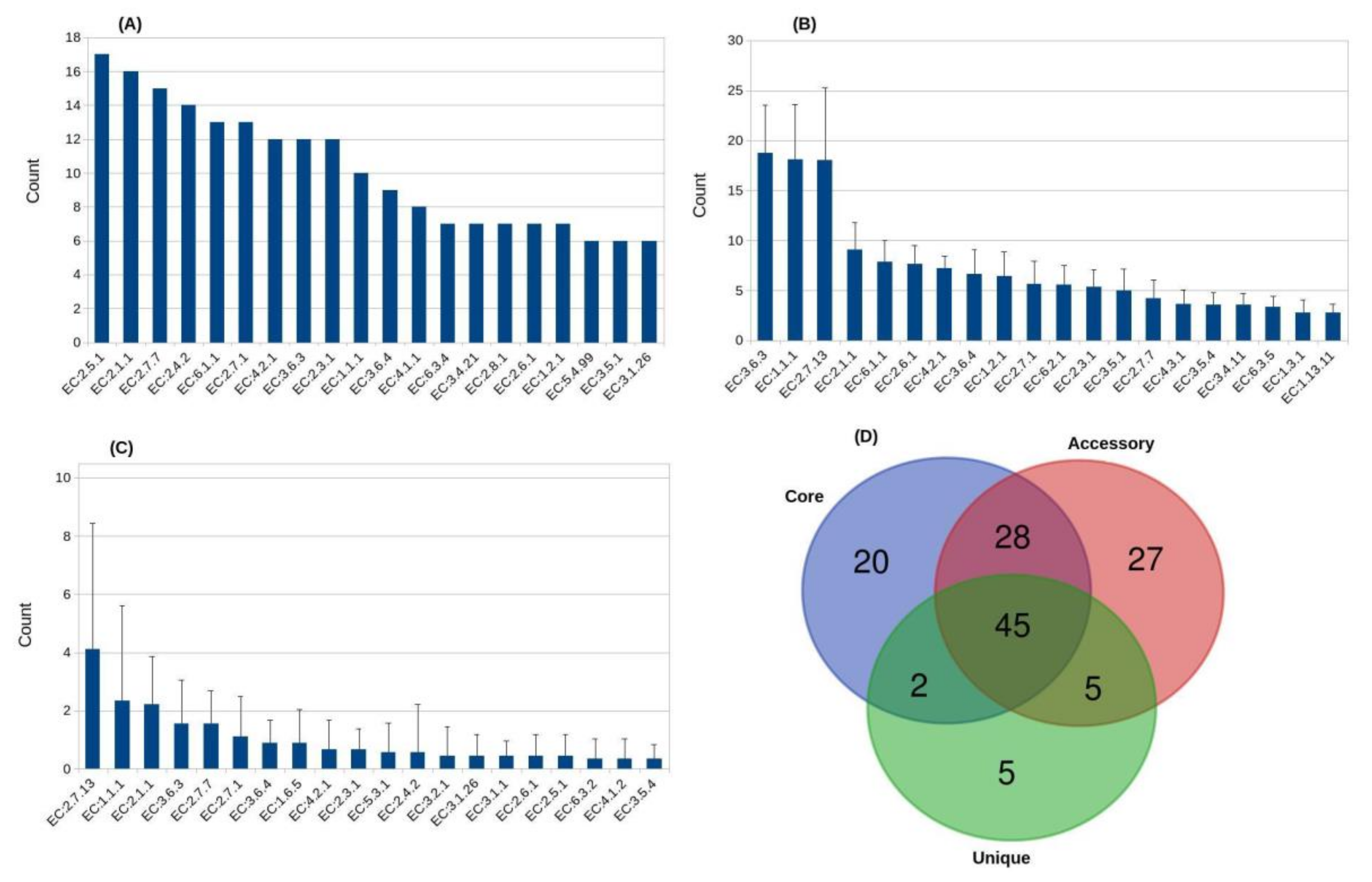
3.3. Abundant Enzyme Classes of the Aneurinibacillus Pan-Genome
3.4. Aneurinibacillus Enzymes with Significant Industrial Implications
3.4.1. Biosynthetic Potential of Aneurinibacillus
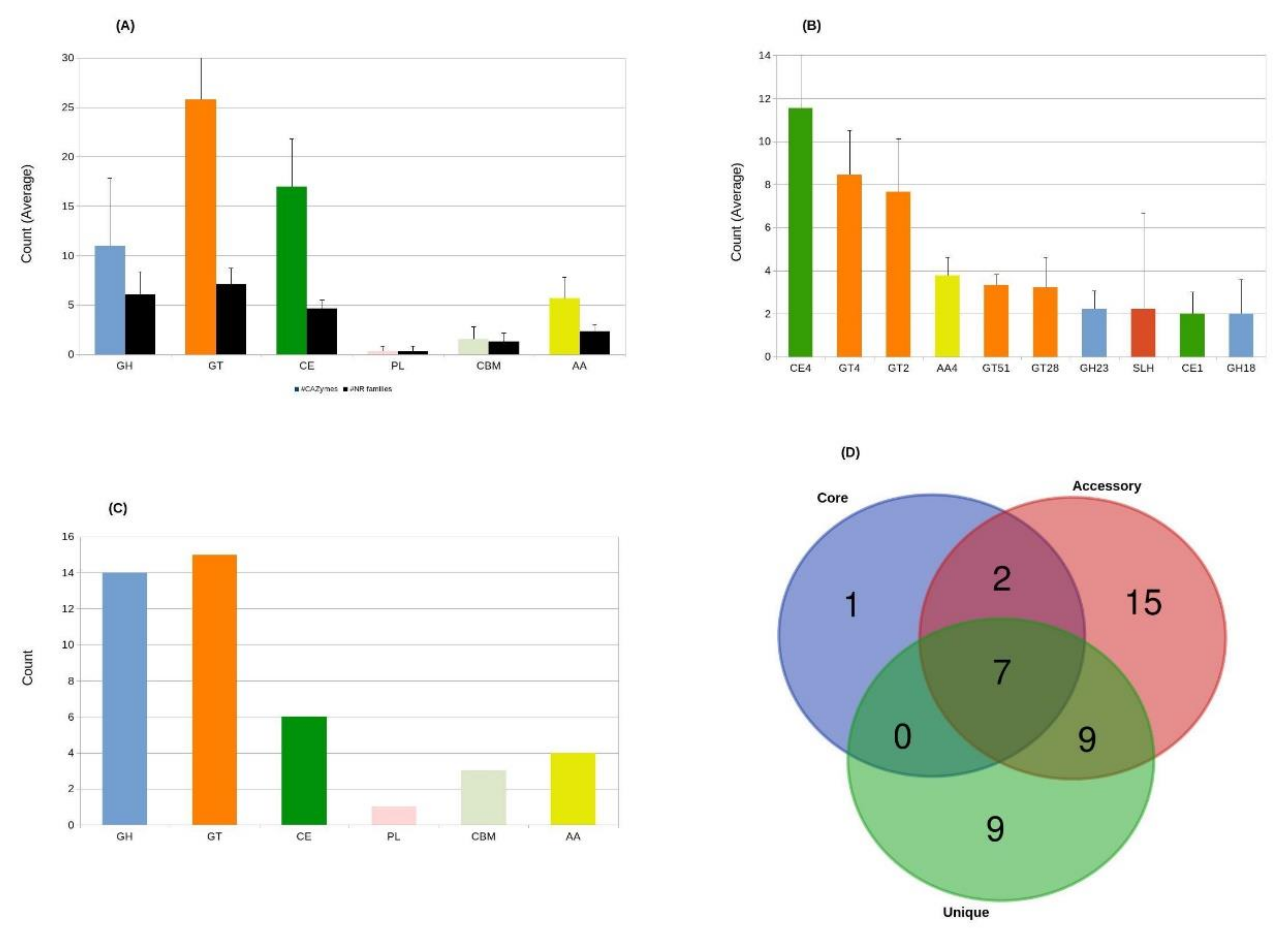
3.4.2. CAZymes of Aneurinibacillus
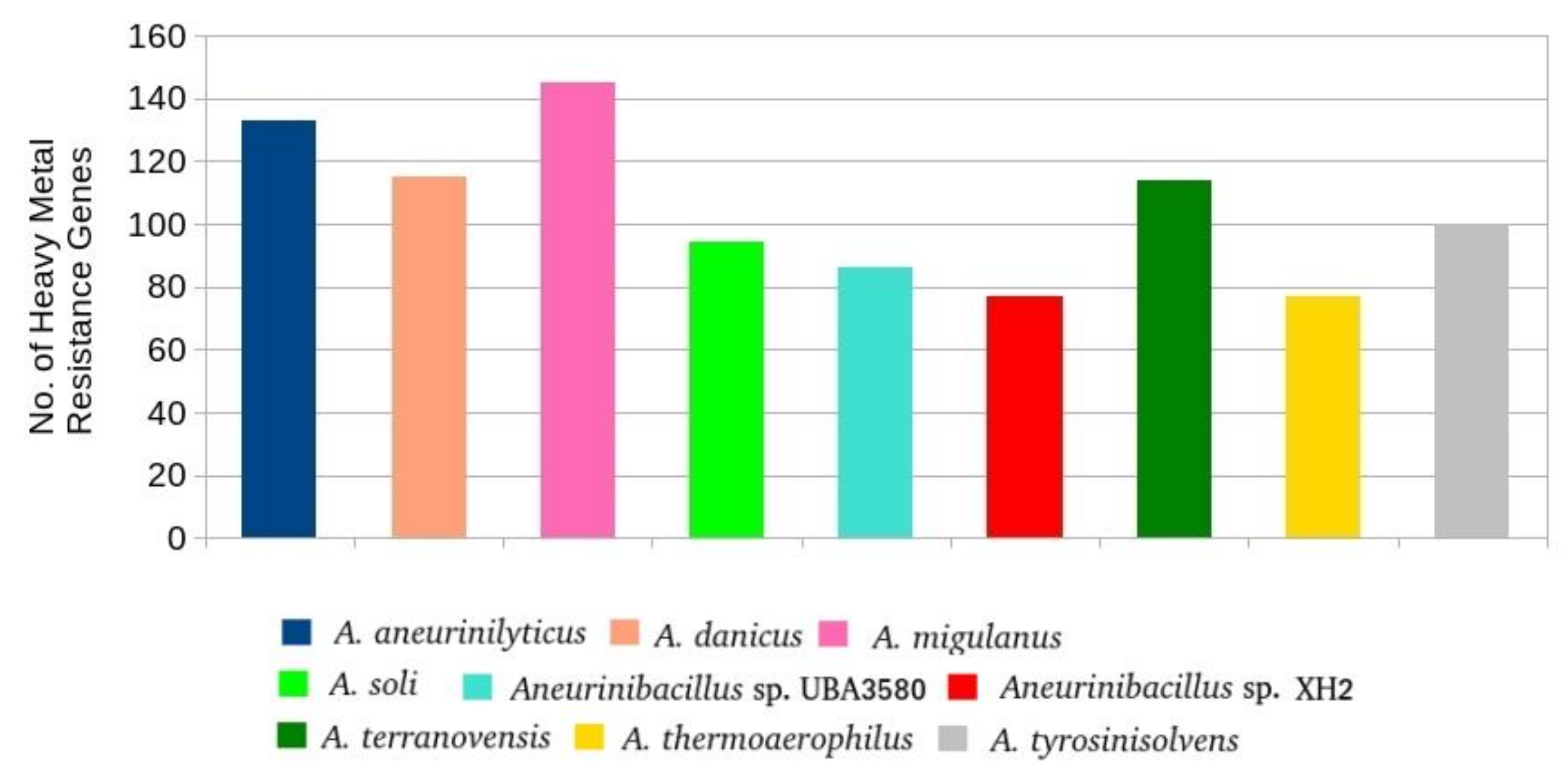
3.4.3. Genes Involved in Heavy Metal and Antibiotic Resistance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Vos, P.; Ludwig, W.; Schleifer, K.H.; Whitman, W.B. Family IV. Paenibacillaceae fam. nov. In Bergey’s Manual of Systematic Bacteriology, 2nd ed.; Springer: New York, NY, USA, 2009; Volume 3. [Google Scholar]
- Paenibacillaceae. Available online: https://lpsn.dsmz.de/family/paenibacillaceae (accessed on 3 December 2020).
- Daud, N.S.; Mohd Din, A.R.J.; Rosli, M.A.; Azam, Z.M.; Othman, N.Z.; Sarmidi, M.R. Paenibacillus polymyxa bioactive compounds for agricultural and biotechnological applications. Biocatal. Agric. Biotechnol. 2019, 18, 101092. [Google Scholar] [CrossRef]
- Grady, E.N.; MacDonald, J.; Liu, L.; Richman, A.; Yuan, Z.C. Current knowledge and perspectives of Paenibacillus: A review. Microb. Cell Fact. 2016, 15, 203. [Google Scholar] [CrossRef]
- Desjardine, K.; Pereira, A.; Wright, H.; Matainaho, T.; Kelly, M.; Andersen, R.J. Tauramamide, a lipopeptide antibiotic produced in culture by Brevibacillus laterosporus isolated from a marine habitat: Structure elucidation and synthesis. J. Nat. Prod. 2007, 70, 1850–1853. [Google Scholar] [CrossRef]
- Shida, O.; Takagi, H.; Kadowaki, K.; Komagata, K. Proposal for two new genera, Brevibacillus gen. nov. and Aneurinibacillus gen. nov. Int. J. Syst. Bacteriol. 1996, 46, 939–946. [Google Scholar] [CrossRef]
- Lee, K.; Lee, S.S. Aneurinibacillus humi sp. nov., Isolated from Soil Collected in Ukraine. Curr. Microbiol. 2016, 72, 139–144. [Google Scholar] [CrossRef]
- Lee, K.C.; Kim, K.K.; Eom, M.K.; Kim, J.S.; Kim, D.S.; Ko, S.H.; Lee, J.S. Aneurinibacillus soli sp. nov., isolated from mountain soil. Int. J. Syst. Evol. Microbiol. 2014, 64, 3792–3797. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Fujita, R.; Kato, Y.; Asahara, M.; Yokota, A. Reclassification of Brevibacillus brevis strains NCIMB 13288 and DSM 6472 (=NRRL NRS-887) as Aneurinibacillus danicus sp. nov. and Brevibacillus limnophilus sp. nov. Int. J. Syst. Evol. Microbiol. 2004, 54, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Shida, O.; Kadowaki, K.; Komagata, K.; Udaka, S. Characterization of Bacillus brevis with descriptions of Bacillus migulanus sp. nov., Bacillus choshinensis sp. nov., Bacillus parabrevis sp. nov., and Bacillus galactophilus sp. nov. Int. J. Syst. Bacteriol. 1993, 43, 221–231. [Google Scholar] [CrossRef]
- Heyndrickx, M.; Vandemeulebroecke, K.; Scheldeman, P.; Kersters, K.; de Vos, P.; Logan, N.A.; Aziz, A.M.; Ali, N.; Berkeley, R.C. A polyphasic reassessment of the genus Paenibacillus, reclassification of Bacillus lautus (Nakamura 1984) as Paenibacillus lautus comb. nov. and of Bacillus peoriae (Montefusco et al. 1993) as Paenibacillus peoriae comb. nov., and emended descriptions of P. lautus and of P. peoriae. Int. J. Syst. Bacteriol. 1996, 46, 988–1003. [Google Scholar] [CrossRef]
- Tsubouchi, T.; Mori, K.; Miyamoto, N.; Fujiwara, Y.; Kawato, M.; Shimane, Y.; Usui, K.; Tokuda, M.; Uemura, M.; Tame, A.; et al. Aneurinibacillus tyrosinisolvens sp. nov., a tyrosine-dissolving bacterium isolated from organics- and methane-rich seafloor sediment. Int. J. Syst. Evol. Microbiol. 2015, 65, 1999–2005. [Google Scholar] [CrossRef] [PubMed]
- Allan, R.N.; Lebbe, L.; Heyrman, J.; De Vos, P.; Buchanan, C.J.; Logan, N.A. Brevibacillus levickii sp. nov. and Aneurinibacillus terranovensis sp. nov., two novel thermoacidophiles isolated from geothermal soils of northern Victoria Land, Antarctica. Int. J. Syst. Evol. Microbiol. 2005, 55, 1039–1050. [Google Scholar] [CrossRef]
- Parte, A.C.; Sardà Carbasse, J.; Meier-Kolthoff, J.P.; Reimer, L.C.; Göker, M. List of Prokaryotic names with Standing in Nomenclature (LPSN) moves to the DSMZ. Int. J. Syst. Evol. Microbiol. 2020, 70, 5607–5612. [Google Scholar] [CrossRef] [PubMed]
- NCBI Taxonomy. Available online: https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?id=55079 (accessed on 3 December 2020).
- Alenezi, F.N.; Rekik, I.; Chenari Bouket, A.; Luptakova, L.; Weitz, H.J.; Rateb, M.E.; Jaspars, M.; Woodward, S.; Belbahri, L. Increased Biological Activity of Aneurinibacillus migulanus Strains Correlates with the Production of New Gramicidin Secondary Metabolites. Front. Microbiol. 2017, 8, 517. [Google Scholar] [CrossRef] [PubMed]
- Balan, S.S.; Kumar, C.G.; Jayalakshmi, S. Aneurinifactin, a new lipopeptide biosurfactant produced by a marine Aneurinibacillus aneurinilyticus SBP-11 isolated from Gulf of Mannar: Purification, characterization and its biological evaluation. Microbiol. Res. 2017, 194, 1–9. [Google Scholar] [CrossRef]
- Kaur, C.; Selvakumar, G.; Ganeshamurthy, A.N. Exploring the Utility of Aneurinibacillus as a Bioinoculant for Sustainable Crop Production and Environmental Applications. In Bacilli and Agrobiotechnology: Phytostimulation and Biocontrol. Bacilli in Climate Resilient Agriculture and Bioprospecting; Islam, M., Rahman, M., Pandey, P., Boehme, M., Haesaert, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; Volume 2. [Google Scholar]
- Edwards, S.G.; Seddon, B. Mode of antagonism of Brevibacillus brevis against Botrytis cinerea in vitro. J. Appl. Microbiol. 2001, 91, 652–659. [Google Scholar] [CrossRef]
- Belbahri, L.; Alenezi, F.N.; Luptakova, L.; Rateb, M.E.; Woodward, S. Complete Genome Sequence of Aneurinibacillus migulanus E1, a Gramicidin S- and d-Phenylalanyl-l-Propyl Diketopiperazine-Deficient Mutant. Genome Announc. 2015, 3, e01441-15. [Google Scholar] [CrossRef]
- Chauhan, A.; Balgir, P.P.; Shirkot, C.K. Characterization of Aneurinibacillus aneurinilyticus Strain CKMV1 as a Plant Growth Promoting Rhizobacteria. Int. J. Agric. Environ. Biotechnol. 2014, 7, 37–45. [Google Scholar] [CrossRef]
- Sedlacek, P.; Pernicova, I.; Novackova, I.; Kourilova, X.; Kalina, M.; Kovalcik, A.; Koller, M.; Nebesarova, J.; Krzyzanek, V.; Hrubanova, K.; et al. Introducing the Newly Isolated Bacterium Aneurinibacillus sp. H1 as an Auspicious Thermophilic Producer of Various Polyhydroxyalkanoates (PHA) Copolymers-2. Material Study on the Produced Copolymers. Polymers 2020, 12, 1298. [Google Scholar] [CrossRef]
- Asem, D.; Leo, V.V.; Passari, A.K.; Tonsing, M.V.; Joshi, J.B.; Uthandi, S.; Hashem, A.; Abd Allah, E.F.; Singh, B.P. Evaluation of gastrointestinal bacterial population for the production of holocellulose enzymes for biomass deconstruction. PLoS ONE 2017, 12, e0186355. [Google Scholar] [CrossRef]
- Dey, U.; Chatterjee, S.; Mondal, N.K. Isolation and characterization of arsenic-resistant bacteria and possible application in bioremediation. Biotechnol. Rep. 2016, 10, 1–7. [Google Scholar] [CrossRef]
- Godheja, J.; Shekhar, S.K.; Satyanarayan, G.N.V.; Singh, S.P.; Modi, D.R. Antibiotic and Heavy Metal Tolerance of Some Indigenous Bacteria Isolated From Petroleum Contaminated Soil Sediments with A Study of Their Aromatic Hydrocarbon Degradation Potential. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 194–211. [Google Scholar] [CrossRef]
- Challinor, V.L.; Bode, H.B. Bioactive natural products from novel microbial sources. Ann. N. Y. Acad. Sci. 2015, 1354, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Kim, Y.R.; Jang, I.H.; Hwang, S.; Oh, D.C.; Kim, S.B. Genome-based analysis for the bioactive potential of Streptomyces yeochonensis CN732, an acidophilic filamentous soil actinobacterium. BMC Genom. 2020, 21, 118. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Kim, Y.R.; Kim, S.B. Genome Mining of the Genus Streptacidiphilus for Biosynthetic and Biodegradation Potential. Genes 2020, 11, 1166. [Google Scholar] [CrossRef]
- Yun, B.R.; Malik, A.; Kim, S.B. Genome based characterization of Kitasatospora sp. MMS16-BH015, a multiple heavy metal resistant soil actinobacterium with high antimicrobial potential. Gene 2020, 733, 144379. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.J.; Wattam, A.R.; Aziz, R.K.; Brettin, T.; Butler, R.; Butler, R.M.; Chlenski, P.; Conrad, N.; Dickerman, A.; Dietrich, E.M.; et al. The PATRIC Bioinformatics Resource Center: Expanding data and analysis capabilities. Nucleic Acids Res. 2020, 48, D606–D612. [Google Scholar] [CrossRef]
- Alenezi, F.N.; Weitz, H.J.; Belbahri, L.; Ben Rebah, H.; Luptakova, L.; Jaspars, M.; Woodward, S. Draft Genome Sequence of Aneurinibacillus migulanus Strain Nagano. Genome Announc. 2015, 3, e00232-15. [Google Scholar] [CrossRef]
- Alenezi, F.N.; Weitz, H.J.; Belbahri, L.; Nidhal, J.; Luptakova, L.; Jaspars, M.; Woodward, S. Draft Genome Sequence of Aneurinibacillus migulanus NCTC 7096. Genome Announc. 2015, 3, e00234-15. [Google Scholar] [CrossRef]
- Tsubouchi, T.; Nishi, S.; Maruyama, T.; Hatada, Y. Draft Genome Sequence of Aneurinibacillus tyrosinisolvens LL-002T, Which Possesses Some Pseudouridine Synthases. Genome Announc. 2015, 3, e00529-15. [Google Scholar] [CrossRef]
- Wang, J.P.; Liu, B.; Liu, G.H.; Ge, C.B.; Xiao, R.F.; Zheng, X.F.; Shi, H. High-Quality Draft Genome Sequence of Aneurinibacillus migulanus ATCC 9999T (DSM 2895), a Gramicidin S-Producing Bacterium Isolated from Garden Soil. Genome Announc. 2015, 3, e01227-15. [Google Scholar] [CrossRef]
- Xi, L.; Zhang, Z.; Qiao, N.; Zhang, Y.; Li, J.; Zhao, J.Y.; Xiao, Z. Complete genome sequence of the novel thermophilic polyhydroxyalkanoates producer Aneurinibacillus sp. XH2 isolated from Gudao oilfield in China. J. Biotechnol. 2016, 227, 54–55. [Google Scholar] [CrossRef] [PubMed]
- Alenezi, F.N.; Rekik, I.; Bełka, M.; Ibrahim, A.F.; Luptakova, L.; Jaspars, M.; Woodward, S.; Belbahri, L. Strain-level diversity of secondary metabolism in the biocontrol species Aneurinibacillus migulanus. Microbiol. Res. 2016, 182, 116–124. [Google Scholar] [CrossRef] [PubMed]
- López-Prieto, A.; Rodríguez-López, L.; Rincón-Fontán, M.; Cruz, J.M.; Moldes, A.B. Characterization of extracellular and cell bound biosurfactants produced by Aneurinibacillus aneurinilyticus isolated from commercial corn steep liquor. Microbiol. Res. 2021, 242, 126614. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Cheng, T.; Chu, J.; Li, S.; He, B. Efficient Synthesis of Purine Nucleoside Analogs by a New Trimeric Purine Nucleoside Phosphorylase from Aneurinibacillus migulanus AM007. Molecules 2019, 25, 100. [Google Scholar] [CrossRef]
- Gupta, S.; Pandey, S. ACC Deaminase Producing Bacteria with Multifarious Plant Growth Promoting Traits Alleviates Salinity Stress in French Bean (Phaseolus vulgaris) Plants. Front. Microbiol. 2019, 10, 1506. [Google Scholar] [CrossRef] [PubMed]
- Zottig, X.; Meddeb-Mouelhi, F.; Charbonneau, D.M.; Beauregard, M. Characterization of a Novel Alkalophilic Lipase from Aneurinibacillus thermoaerophilus: Lid Heterogeneity and Assignment to Family I.5. Protein. J. 2017, 36, 478–488. [Google Scholar] [CrossRef]
- NCBI Genome. Available online: https://www.ncbi.nlm.nih.gov/genome (accessed on 3 December 2020).
- Seppey, M.; Manni, M.; Zdobnov, E.M. BUSCO: Assessing Genome Assembly and Annotation Completeness. Methods Mol. Biol. 2019, 1962, 227–245. [Google Scholar]
- Blin, K.; Shaw, S.; Steinke, K.; Villebro, R.; Ziemert, N.; Lee, S.Y.; Medema, M.H.; Weber, T. antiSMASH 5.0: Updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 2019, 47, W81–W87. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.Y.; Kim, H.U.; Lee, S.Y. Deep learning enables high-quality and high-throughput prediction of enzyme commission numbers. Proc. Natl. Acad. Sci. USA 2019, 116, 13996–14001. [Google Scholar] [CrossRef]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef]
- Zhang, H.; Yohe, T.; Huang, L.; Entwistle, S.; Wu, P.; Yang, Z.; Busk, P.K.; Xu, Y.; Yin, Y. dbCAN2: A meta server for automated carbohydrate-active enzyme annotation. Nucleic Acids Res. 2018, 46, W95–W101. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Pal, C.; Bengtsson-Palme, J.; Rensing, C.; Kristiansson, E.; Larsson, D.G. BacMet: Antibacterial biocide and metal resistance genes database. Nucleic Acids Res. 2014, 42, D737–D743. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Göker, M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Chaudhari, N.M.; Gupta, V.K.; Dutta, C. BPGA—An ultra-fast pan-genome analysis pipeline. Sci. Rep. 2016, 6, 24373. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jia, X.; Yang, J.; Ling, Y.; Zhang, Z.; Yu, J.; Wu, J.; Xiao, J. PanGP: A tool for quickly analyzing bacterial pan-genome profile. Bioinformatics 2014, 30, 1297–1299. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Hahnke, R.L.; Petersen, J.; Scheuner, C.; Michael, V.; Fiebig, A.; Rohde, C.; Rohde, M.; Fartmann, B.; Goodwin, L.A.; et al. Complete genome sequence of DSM 30083(T), the type strain (U5/41(T)) of Escherichia coli, and a proposal for delineating subspecies in microbial taxonomy. Stand. Genom. Sci. 2014, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Medini, D.; Donati, C.; Tettelin, H.; Masignani, V.; Rappuoli, R. The microbial pan-genome. Curr. Opin. Genet. Dev. 2005, 15, 589–594. [Google Scholar] [CrossRef] [PubMed]
- De Maayer, P.; Chan, W.Y.; Rubagotti, E.; Venter, S.N.; Toth, I.K.; Birch, P.R.; Coutinho, T.A. Analysis of the Pantoea ananatis pan-genome reveals factors underlying its ability to colonize and interact with plant, insect and vertebrate hosts. BMC Genom. 2014, 15, 404. [Google Scholar] [CrossRef] [PubMed]
- Figshare. Available online: https://figshare.com/articles/dataset/pangenom-fasta_zip/13673473 (accessed on 25 February 2021).
- Schubert, H.L.; Blumenthal, R.M.; Cheng, X. Many paths to methyltransfer: A chronicle of convergence. Trends Biochem. Sci. 2003, 28, 329–335. [Google Scholar] [CrossRef]
- Ward, L.C.; McCue, H.V.; Carnell, A.J. Carboxyl Methyltransferases: Natural Functions and Potential Applications in Industrial Biotechnology. ChemCatChem 2021, 13, 121. [Google Scholar] [CrossRef]
- Acton, Q.A. Acid Anhydride Hydrolases—Advances in Research and Application; ScholarlyEditions: Atlanta, GA, USA, 2012. [Google Scholar]
- Gianfreda, L.; Xu, F.; Bollag, J. Laccases: A useful group of oxidoreductive enzymes. Bioremediat. J. 1999, 3, 1–26. [Google Scholar] [CrossRef]
- Sheldon, W.M. Applications of oxidoreductases. Curr. Opin. Biotechnol. 1999, 10, 370–375. [Google Scholar]
- Zheng, Y.G.; Yin, H.H.; Yu, D.F.; Chen, X.; Tang, X.L.; Zhang, X.J.; Xue, Y.P.; Wang, Y.J.; Liu, Z.Q. Recent advances in biotechnological applications of alcohol dehydrogenases. Appl. Microbiol. Biotechnol. 2017, 101, 987–1001. [Google Scholar] [CrossRef]
- Stock, A.M.; Robinson, V.L.; Goudreau, P.N. Two-component signal transduction. Annu. Rev. Biochem. 2000, 69, 183–215. [Google Scholar] [CrossRef]
- Bem, A.E.; Velikova, N.; Pellicer, M.T.; Baarlen, P.; Marina, A.; Wells, J.M. Bacterial histidine kinases as novel antibacterial drug targets. ACS Chem. Biol. 2015, 10, 213–224. [Google Scholar] [CrossRef]
- Velikova, N.; Fulle, S.; Manso, A.S.; Mechkarska, M.; Finn, P.; Conlon, J.M.; Oggioni, M.R.; Wells, J.M.; Marina, A. Putative histidine kinase inhibitors with antibacterial effect against multi-drug resistant clinical isolates identified by in vitro and in silico screens. Sci. Rep. 2016, 6, 26085. [Google Scholar] [CrossRef]
- Deschenes, R.J.; Lin, H.; Ault, A.D.; Fassler, J.S. Antifungal properties and target evaluation of three putative bacterial histidine kinase inhibitors. Antimicrob. Agents Chemother. 1999, 43, 1700–1703. [Google Scholar] [CrossRef][Green Version]
- Noinaj, N.; Wattanasak, R.; Lee, D.Y.; Wally, J.L.; Piszczek, G.; Chock, P.B.; Stadtman, T.C.; Buchanan, S.K. Structural insights into the catalytic mechanism of Escherichia coli selenophosphate synthetase. J. Bacteriol. 2012, 194, 499–508. [Google Scholar] [CrossRef]
- Seale, L.A. Selenocysteine β-Lyase: Biochemistry, Regulation and Physiological Role of the Selenocysteine Decomposition Enzyme. Antioxidants (Basel) 2019, 8, 357. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.L.; Simonović, M. Synthesis and decoding of selenocysteine and human health. Croat. Med. J. 2012, 53, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Dumora, C.; Marche, M.; Doignon, F.; Aigle, M.; Cassaigne, A.; Crouzet, M. First characterization of the phosphonoacetaldehyde hydrolase gene of Pseudomonas aeruginosa. Gene 1997, 197, 405–412. [Google Scholar] [CrossRef]
- Morais, M.C.; Zhang, W.; Baker, A.S.; Zhang, G.; Dunaway-Mariano, D.; Allen, K.N. The crystal structure of bacillus cereus phosphonoacetaldehyde hydrolase: Insight into catalysis of phosphorus bond cleavage and catalytic diversification within the HAD enzyme superfamily. Biochemistry 2000, 39, 10385–10396. [Google Scholar] [CrossRef]
- Hilderbrand, R.L. The Role of Phosphonates in Living Systems, 1st ed.; CRC Press: Boca Raton, FL, USA, 1983. [Google Scholar]
- Wu, Z.; Wouters, J.; Poulter, C.D. Isopentenyl diphosphate isomerase. Mechanism-based inhibition by diene analogues of isopentenyl diphosphate and dimethylallyl diphosphate. J. Am. Chem. Soc. 2005, 127, 17433–17438. [Google Scholar] [CrossRef]
- Tetali, S.D. Terpenes and isoprenoids: A wealth of compounds for global use. Planta 2019, 249, 1–8. [Google Scholar] [CrossRef]
- Čorić, I.; Holland, P.L. Insight into the Iron-Molybdenum Cofactor of Nitrogenase from Synthetic Iron Complexes with Sulfur, Carbon, and Hydride Ligands. J. Am. Chem. Soc. 2016, 138, 7200–7211. [Google Scholar] [CrossRef] [PubMed]
- Vey, J.L.; Yang, J.; Li, M.; Broderick, W.E.; Broderick, J.B.; Drennan, C.L. Structural basis for glycyl radical formation by pyruvate formate-lyase activating enzyme. Proc. Natl. Acad. Sci. USA 2008, 105, 16137–16141. [Google Scholar] [CrossRef]
- Boden, R.; Borodina, E.; Wood, A.P.; Kelly, D.P.; Murrell, J.C.; Schäfer, H. Purification and characterization of dimethylsulfide monooxygenase from Hyphomicrobium sulfonivorans. J. Bacteriol. 2011, 193, 1250–1258. [Google Scholar] [CrossRef]
- Yoshida, K.; Yamaguchi, M.; Morinaga, T.; Ikeuchi, M.; Kinehara, M.; Ashida, H. Genetic modification of Bacillus subtilis for production of D-chiro-inositol, an investigational drug candidate for treatment of type 2 diabetes and polycystic ovary syndrome. Appl. Environ. Microbiol. 2006, 72, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, F.; Neubauer, P.; Kurreck, A. The Peculiar Case of the Hyper-thermostable Pyrimidine Nucleoside Phosphorylase from Thermus thermophilus. Chembiochem 2020. [Google Scholar] [CrossRef]
- Kautsar, S.A.; Blin, K.; Shaw, S.; Navarro-Muñoz, J.C.; Terlouw, B.R.; van der Hooft, J.J.J.; van Santen, J.A.; Tracanna, V.; Suarez Duran, H.G.; Pascal Andreu, V.; et al. MIBiG 2.0: A repository for biosynthetic gene clusters of known function. Nucleic Acids Res. 2020, 48, D454–D458. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Tang, W.; Goto, Y.; Nair, S.K.; van der Donk, W.A. Lantibiotics from Geobacillus thermodenitrificans. Proc. Natl. Acad. Sci. USA 2012, 109, 5241–5246. [Google Scholar] [CrossRef]
- Maksimov, M.O.; Pan, S.J.; James Link, A. Lasso peptides: Structure, function, biosynthesis, and engineering. Nat. Prod. Rep. 2012, 29, 996–1006. [Google Scholar] [CrossRef]
- Zhu, S.; Hegemann, J.D.; Fage, C.D.; Zimmermann, M.; Xie, X.; Linne, U.; Marahiel, M.A. Insights into the Unique Phosphorylation of the Lasso Peptide Paeninodin. J. Biol. Chem. 2016, 291, 13662–13678. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Fage, C.D.; Hegemann, J.D.; Mielcarek, A.; Yan, D.; Linne, U.; Marahiel, M.A. The B1 Protein Guides the Biosynthesis of a Lasso Peptide. Sci. Rep. 2016, 6, 35604. [Google Scholar] [CrossRef]
- Ejaz, U.; Muhammad, S.; Hashmi, I.A.; Ali, F.I.; Sohail, M. Utilization of methyltrioctylammonium chloride as new ionic liquid in pretreatment of sugarcane bagasse for production of cellulase by novel thermophilic bacteria. J. Biotechnol. 2020, 317, 34–38. [Google Scholar] [CrossRef]
- Lairson, L.L.; Henrissat, B.; Davies, G.J.; Withers, S.G. Glycosyltransferases: Structures, functions, and mechanisms. Ann. Rev. Biochem. 2008, 77, 521–555. [Google Scholar] [CrossRef]
- Nakamura, A.M.; Nascimento, A.S.; Polikarpov, I. Structural diversity of carbohydrate esterases. Biotechnol. Res. Innov. 2017, 1, 35–51. [Google Scholar] [CrossRef]
- Fukushima, T.; Kitajima, T.; Sekiguchi, J. A polysaccharide deacetylase homologue, PdaA, in Bacillus subtilis acts as an N-acetylmuramic acid deacetylase in vitro. J. Bacteriol. 2005, 187, 1287–1292. [Google Scholar] [CrossRef] [PubMed]
- Biely, P.; Côté, G.L.; Kremnický, L.; Greene, R.V.; Dupont, C.; Kluepfel, D. Substrate specificity and mode of action of acetylxylan esterase from Streptomyces lividans. FEBS Lett. 1996, 396, 257–260. [Google Scholar] [CrossRef]
- Biely, P.; Côté, G.L.; Kremnický, L.; Weisleder, D.; Greene, R.V. Substrate specificity of acetylxylan esterase from Schizophyllum commune: Mode of action on acetylated carbohydrates. Biochim. Biophys. Acta 1996, 1298, 209–222. [Google Scholar] [CrossRef]
- Blair, D.E.; Schüttelkopf, A.W.; MacRae, J.I.; van Aalten, D.M. Structure and metal-dependent mechanism of peptidoglycan deacetylase, a streptococcal virulence factor. Proc. Natl. Acad. Sci. USA 2005, 102, 15429–15434. [Google Scholar] [CrossRef] [PubMed]
- Mesnage, S.; Fontaine, T.; Mignot, T.; Delepierre, M.; Mock, M.; Fouet, A. Bacterial SLH domain proteins are non-covalently anchored to the cell surface via a conserved mechanism involving wall polysaccharide pyruvylation. EMBO J. 2000, 19, 4473–4484. [Google Scholar] [CrossRef] [PubMed]
- Janesch, B.; Messner, P.; Schäffer, C. Are the surface layer homology domains essential for cell surface display and glycosylation of the S-layer protein from Paenibacillus alvei CCM 2051T? J. Bacteriol. 2013, 195, 565–575. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gadd, G.M. Microbial control of heavy metal pollution. In Microbial Control of Environmental Pollution; Fry, J.C., Gadd, G.M., Herbert, R.A., Jones, C.W., Watson-Craik, I., Eds.; Cambridge University Press: Cambridge, UK, 1992; pp. 59–88. [Google Scholar]
- Gadd, G.M. Metals and microorganisms: A problem of definition. FEMS Microbiol. Lett. 1992, 100, 197–203. [Google Scholar] [CrossRef]
- Mohammed, A.S.; Kapri, A.; Goel, R. Heavy metal pollution: Source, impact, and remedies. In Biomanagement of Metal Contaminated Soils; Khan, M., Zaidi, A., Goel, R., Musarrat, J., Eds.; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar] [CrossRef]
- Fernandes, C.C.; Kishi, L.T.; Lopes, E.M.; Omori, W.P.; Souza, J.A.M.; Alves, L.M.C.; Lemos, E.G.M. Bacterial communities in mining soils and surrounding areas under regeneration process in a former ore mine. Braz. J. Microbiol. 2018, 49, 489–502. [Google Scholar] [CrossRef]
- Colak, F.; Olgun, A.; Atar, N.; Yazıcıoglu, D. Heavy metal resistances and biosorptive behaviors of Paenibacillus polymyxa: Batch and column studies. J. Ind. Eng. Chem. 2013, 19, 863–869. [Google Scholar] [CrossRef]
- Govarthanan, M.; Mythili, R.; Selvankumar, T.; Kamala-Kannan, S.; Rajasekar, A.; Chang, Y.C. Bioremediation of heavy metals using an endophytic bacterium Paenibacillus sp. RM isolated from the roots of Tridax procumbens. 3 Biotech 2016, 6, 242. [Google Scholar] [CrossRef]
- Cervantes, C.; Ji, G.; Ramírez, J.L.; Silver, S. Resistance to arsenic compounds in microorganisms. FEMS Microbiol. Rev. 1994, 15, 355–367. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry (ATSDR). Available online: https://www.atsdr.cdc.gov/toxprofiles/tp2.pdf (accessed on 2 February 2021).
- Smedley, P.L.; Kinniburgh, D.G. A Review of the Source, Behaviour and Distribution of Arsenic in Natural Waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Ben Fekih, I.; Zhang, C.; Li, Y.P.; Zhao, Y.; Alwathnani, H.A.; Saquib, Q.; Rensing, C.; Cervantes, C. Distribution of Arsenic Resistance Genes in Prokaryotes. Front. Microbiol. 2018, 9, 2473. [Google Scholar] [CrossRef] [PubMed]
- Maizel, D.; Blum, J.S.; Ferrero, M.A.; Utturkar, S.M.; Brown, S.D.; Rosen, B.P.; Oremland, R.S. Characterization of the extremely arsenic-resistant Brevibacterium linens strain AE038-8 isolated from contaminated groundwater in Tucumán, Argentina. Int. Biodeterior. Biodegrad. 2016, 107, 147–153. [Google Scholar] [CrossRef]
- Rosen, B.P. Families of arsenic transporters. Trends Microbiol. 1999, 7, 207–212. [Google Scholar] [CrossRef]
- Rosen, B.P. Biochemistry of arsenic detoxification. FEBS Lett. 2002, 529, 86–92. [Google Scholar] [CrossRef]
- Kaur, S.; Kamli, M.R.; Ali, A. Diversity of arsenate reductase genes (arsC Genes) from arsenic-resistant environmental isolates of E. coli. Curr. Microbiol. 2009, 59, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.C.; Rosen, B.P. New mechanisms of bacterial arsenic resistance. Biomed. J. 2016, 39, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Leonhartsberger, S.; Huber, A.; Lottspeich, F.; Böck, A. The hydH/G Genes from Escherichia coli code for a zinc and lead responsive two-component regulatory system. J. Mol. Biol. 2001, 307, 93–105. [Google Scholar] [CrossRef]
- Hu, Y.H.; Wang, H.L.; Zhang, M.; Sun, L. Molecular analysis of the copper-responsive CopRSCD of a pathogenic Pseudomonas fluorescens strain. J. Microbiol. 2009, 47, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Sutil, M.C.; Pérez, J.; Gómez-Santos, N.; Shimkets, L.J.; Moraleda-Muñoz, A.; Muñoz-Dorado, J. The Myxococcus xanthus two-component system CorSR regulates expression of a gene cluster involved in maintaining copper tolerance during growth and development. PLoS ONE 2013, 8, e68240. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.T.; González, M.V.; González, G.; Vargas, E.; Campos-García, J.; Cervantes, C. Involvement of DNA helicases in chromate resistance by Pseudomonas aeruginosa PAO1. Mutat. Res. 2005, 578, 202–209. [Google Scholar] [CrossRef]
- Kormutakova, R.; Klucar, L.; Turna, J. DNA sequence analysis of the tellurite-resistance determinant from clinical strain of Escherichia coli and identification of essential genes. Biometals 2000, 13, 135–139. [Google Scholar] [CrossRef]
- Willsky, G.R.; Malamy, M.H. Effect of arsenate on inorganic phosphate transport in Escherichia coli. J. Bacteriol. 1980, 144, 366–374. [Google Scholar] [CrossRef]
- Surin, B.P.; Rosenberg, H.; Cox, G.B. Phosphate-specific transport system of Escherichia coli: Nucleotide sequence and gene-polypeptide relationships. J. Bacteriol. 1985, 161, 189–198. [Google Scholar] [CrossRef]
- Anderson, D.S.; Adhikari, P.; Nowalk, A.J.; Chen, C.Y.; Mietzner, T.A. The hFbpABC transporter from Haemophilus influenzae functions as a binding-protein-dependent ABC transporter with high specificity and affinity for ferric iron. J. Bacteriol. 2004, 186, 6220–6229. [Google Scholar] [CrossRef]
- Nishino, K.; Yamaguchi, A. Analysis of a complete library of putative drug transporter genes in Escherichia coli. J. Bacteriol. 2001, 183, 5803–5812. [Google Scholar] [CrossRef]
- McMurry, L.M.; Oethinger, M.; Levy, S.B. Triclosan targets lipid synthesis. Nature 1998, 394, 531–532. [Google Scholar] [CrossRef] [PubMed]
- Huda, N.; Lee, E.W.; Chen, J.; Morita, Y.; Kuroda, T.; Mizushima, T.; Tsuchiya, T. Molecular cloning and characterization of an ABC multidrug efflux pump, VcaM, in Non-O1 Vibrio cholerae. Antimicrob. Agents Chemother. 2003, 47, 2413–2417. [Google Scholar] [CrossRef]
- Bentley, J.; Hyatt, L.S.; Ainley, K.; Parish, J.H.; Herbert, R.B.; White, G.R. Cloning and sequence analysis of an Escherichia coli gene conferring bicyclomycin resistance. Gene 1993, 127, 117–120. [Google Scholar] [CrossRef]
| Genome Name | BioProject Accession | No. of Contigs | Genome Size (Mb) | GC Content | Patric CDS | Pseudogenes | tRNA (rRNA) | No. of Accessory Genes | No. of Unique Genes | Genome Completeness (%) | Isolation Source |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aneurinibacillus aneurinilyticus ATCC 12856T | PRJNA38313 | 395 | 5.30 | 43.2 | 5594 | 184 | 39/4 | 2781 | 1545 | 99.1 | Gastrointestinal tract |
| Aneurinibacillus danicus NBRC 102444T | PRJDB6144 | 297 | 4.42 | 46.67 | 4912 | 123 | 30/3 | 2505 | 743 | 99.3 | NA |
| Aneurinibacillus migulanus DSM 2895T | PRJNA291108 | 28 | 6.35 | 42.94 | 7259 | 504 | 85/12 | 2850 | 969 | 99.5 | Soil |
| Aneurinibacillus soli CB4T | PRJDB4401 | 1 | 4.12 | 45.89 | 4221 | 54 | 169/51 | 1479 | 982 | 98.0 | NA |
| Aneurinibacillus sp. UBA3580 | PRJNA348753 | 137 | 3.56 | 47.15 | 3971 | NA | 40/NA | 2066 | 326 | 91.5 | Topsoil |
| Aneurinibacillus sp. XH2 | PRJNA287204 | 1 | 3.67 | 44.86 | 4051 | 85 | 114/34 | 1865 | 88 | 98.7 | Oil produced water |
| Aneurinibacillus terranovensis DSM 18919T | PRJNA188818 | 118 | 4.24 | 44.9 | 4500 | 186 | 75/9 | 1353 | 1049 | 98.7 | Geothermal soil |
| Aneurinibacillus thermoaerophilus L 420-91T | PRJEB15926 | 103 | 3.66 | 44.51 | 4176 | 152 | 61/13 | 1903 | 182 | 99.1 | NA |
| Aneurinibacillus tyrosinisolvens LL-002T | PRJDB3821 | 136 | 5.69 | 44.54 | 6607 | 368 | 123/3 | 1806 | 1725 | 95.1 | Organics- and methane-rich seafloor sediment |
| Query Strain | Subject Strain | dDDH (d0, in %) | dDDH (d4, in %) | dDDH (d6, in %) |
|---|---|---|---|---|
| Aneurinibacillus sp. XH2 | Aneurinibacillus thermoaerophilus L 420-91T | 94 | 98.4 | 96.5 |
| Aneurinibacillus danicus NBRC 102444T | Aneurinibacillus sp. UBA3580 | 68.5 | 95.5 | 75.3 |
| Aneurinibacillus aneurinilyticus ATCC 12856T | Aneurinibacillus migulanus DSM 2895T | 44.9 | 35.8 | 42.3 |
| Aneurinibacillus sp. XH2 | Brevibacillus brevis NCTC 2611T | 12.8 | 30.9 | 13.2 |
| Aneurinibacillus danicus NBRC 102444T | Brevibacillus brevis NCTC 2611T | 12.7 | 28.3 | 13.1 |
| Aneurinibacillus soli CB4T | Brevibacillus brevis NCTC 2611T | 12.9 | 27.5 | 13.3 |
| Aneurinibacillus migulanus DSM 2895T | Brevibacillus brevis NCTC 2611T | 12.7 | 26.4 | 13.1 |
| Aneurinibacillus tyrosinisolvens LL-002T | Brevibacillus brevis NCTC 2611T | 12.6 | 24.9 | 13 |
| Aneurinibacillus danicus NBRC 102444T | Aneurinibacillus terranovensis DSM 18919T | 13.4 | 24 | 13.7 |
| Aneurinibacillus aneurinilyticus ATCC 12856T | Brevibacillus brevis NCTC 2611T | 12.7 | 23.3 | 13.1 |
| Aneurinibacillus sp. UBA3580 | Aneurinibacillus terranovensis DSM 18919T | 13.2 | 23.1 | 13.6 |
| Aneurinibacillus thermoaerophilus L 420-91T | Brevibacillus brevis NCTC 2611T | 12.5 | 23.1 | 12.9 |
| Aneurinibacillus terranovensis DSM 18919T | Brevibacillus brevis NCTC 2611T | 12.5 | 23 | 12.9 |
| Aneurinibacillus soli CB4T | Aneurinibacillus tyrosinisolvens LL-002T | 13.5 | 21.8 | 13.8 |
| Aneurinibacillus danicus NBRC 102444T | Aneurinibacillus migulanus DSM 2895T | 18 | 21.7 | 17.7 |
| Aneurinibacillus danicus NBRC 102444T | Aneurinibacillus sp. XH2 | 17.2 | 21.6 | 17.1 |
| Aneurinibacillus migulanus DSM 2895T | Aneurinibacillus soli CB4T | 13.8 | 21.5 | 14.1 |
| Aneurinibacillus migulanus DSM 2895T | Aneurinibacillus sp. UBA3580 | 17.6 | 21.4 | 17.4 |
| Aneurinibacillus aneurinilyticus ATCC 12856T | Aneurinibacillus danicus NBRC 102444T | 17.8 | 21.4 | 17.5 |
| Aneurinibacillus aneurinilyticus ATCC 12856T | Aneurinibacillus sp. UBA3580 | 17.3 | 21.4 | 17.1 |
| Aneurinibacillus sp. UBA3580 | Aneurinibacillus sp. XH2 | 16.5 | 21.3 | 16.4 |
| Aneurinibacillus danicus NBRC 102444T | Aneurinibacillus thermoaerophilus L 420-91T | 16.8 | 21.2 | 16.7 |
| Aneurinibacillus soli CB4T | Aneurinibacillus sp. XH2 | 14.3 | 21 | 14.5 |
| Aneurinibacillus sp. UBA3580 | Aneurinibacillus thermoaerophilus L 420-91T | 16.1 | 20.7 | 16.1 |
| Aneurinibacillus sp. XH2 | Aneurinibacillus tyrosinisolvens LL-002T | 13.4 | 20.6 | 13.7 |
| Aneurinibacillus terranovensis DSM 18919T | Aneurinibacillus tyrosinisolvens LL-002T | 13.5 | 20.4 | 13.8 |
| Aneurinibacillus migulanus DSM 2895T | Aneurinibacillus sp. XH2 | 15.6 | 20.3 | 15.6 |
| Aneurinibacillus aneurinilyticus ATCC 12856T | Aneurinibacillus soli CB4T | 14.1 | 20.2 | 14.3 |
| Aneurinibacillus sp. XH2 | Aneurinibacillus terranovensis DSM 18919T | 13.3 | 20.1 | 13.6 |
| Aneurinibacillus soli CB4T | Aneurinibacillus terranovensis DSM 18919T | 13.1 | 20.1 | 13.4 |
| Aneurinibacillus aneurinilyticus ATCC 12856T | Aneurinibacillus sp. XH2 | 15.6 | 19.9 | 15.6 |
| Aneurinibacillus migulanus DSM 2895T | Aneurinibacillus tyrosinisolvens LL-002T | 13.3 | 19.8 | 13.6 |
| Aneurinibacillus migulanus DSM 2895T | Aneurinibacillus thermoaerophilus L 420-91T | 15.2 | 19.7 | 15.3 |
| Aneurinibacillus terranovensis DSM 18919T | Aneurinibacillus thermoaerophilus L 420-91T | 13.3 | 19.5 | 13.6 |
| Aneurinibacillus danicus NBRC 102444T | Aneurinibacillus tyrosinisolvens LL-002T | 13.6 | 19.5 | 13.9 |
| Aneurinibacillus danicus NBRC 102444T | Aneurinibacillus soli CB4T | 14.3 | 19.4 | 14.5 |
| Aneurinibacillus aneurinilyticus ATCC 12856T | Aneurinibacillus thermoaerophilus L 420-91T | 15.4 | 19.3 | 15.4 |
| Aneurinibacillus migulanus DSM 2895T | Aneurinibacillus terranovensis DSM 18919T | 13 | 19.1 | 13.4 |
| Aneurinibacillus sp. UBA3580 | Aneurinibacillus tyrosinisolvens LL-002T | 13.5 | 18.8 | 13.8 |
| Aneurinibacillus thermoaerophilus L 420-91T | Aneurinibacillus tyrosinisolvens LL-002T | 13.2 | 18.7 | 13.5 |
| Aneurinibacillus aneurinilyticus ATCC 12856T | Aneurinibacillus tyrosinisolvens LL-002T | 13.5 | 18.5 | 13.8 |
| Aneurinibacillus aneurinilyticus ATCC 12856T | Aneurinibacillus terranovensis DSM 18919T | 13 | 18.4 | 13.4 |
| Aneurinibacillus sp. UBA3580 | Brevibacillus brevis NCTC 2611T | 12.5 | 18 | 12.9 |
| Aneurinibacillus soli CB4T | Aneurinibacillus sp. UBA3580 | 13.9 | 18 | 14.1 |
| Aneurinibacillus soli CB4T | Aneurinibacillus thermoaerophilus L 420-91T | 13.9 | 17.9 | 14.1 |
| S. No. | Cluster Type | A. aneurinilyticus ATCC 12856T | A. danicus NBRC 102444T | A. migulanus DSM 2895T | A. soli CB4T | Aneurinibacillus sp. UBA3580 | Aneurinibacillus sp. XH2 | A. terranovensis DSM 18919T | A. thermoaerophilus L 420-91T | A. tyrosinisolvens LL-002T | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Arylpolyene | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 2 |
| 2. | Bacteriocin | 2 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 2 | 9 |
| 3. | Betalactone | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 6 |
| 4. | Hserlactone | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 2 |
| 5. | Lanthipeptide | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 3 |
| 6. | LAP/bacteriocin | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 4 |
| 7. | Lassopeptide | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 8. | NRPS | 4 | 0 | 2 | 2 | 0 | 0 | 1 | 0 | 1 | 10 |
| 9. | Phosphonate | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 10. | Sactipeptide | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 2 |
| 11. | Siderophore | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 5 |
| 12. | T3PKS | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 7 |
| 13. | Terpene | 1 | 1 | 2 | 2 | 1 | 0 | 0 | 0 | 1 | 8 |
| Total | 10 | 6 | 10 | 5 | 4 | 8 | 2 | 8 | 7 | 60 |
| Pan-Genome | CAZy Family | Main Activity | A. aneurinilyticus ATCC 12856T | A. danicus NBRC 102444T | A. migulanus DSM 2895T | A. soli CB4T | Aneurinibacillus sp. UBA3580 | Aneurinibacillus sp. XH2 | A. terranovensis DSM 18919T | A. thermoaerophilus L 420-91T | A. tyrosinisolvens LL-002T |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Accessory | CBM48 | Glycogen-binding function, Beta subunit (glycogen-binding) of AMP-activated protein kinases | - | 1 | - | - | 1 | - | 1 | - | 1 |
| CBM54 | Binding to xylan yeast cell wall | 1 | - | - | 1 | - | - | 1 | - | - | |
| CE7 | Acetyl xylan esterase Cephalosporin-C deacetylase | 1 | - | 1 | - | - | - | - | - | - | |
| GH2 | Β-Galactosidase, β-mannosidase, β-glucuronidase | 1 | 1 | 1 | 1 | 1 | - | - | - | 1 | |
| GH13 | α-amylase pullulanase, Cyclomaltodextrin glucanotransferase | - | 2 | - | - | 2 | - | 3 | - | 2 | |
| GH25 | Lysozyme | 2 | - | - | - | - | - | - | - | 2 | |
| GH31 | α-glucosidase, α-galactosidase, α-mannosidase | 1 | - | - | - | 1 | - | - | - | 1 | |
| GH73 | Lysozyme, mannosyl-glycoprotein endo-β-N acetylglucosaminidase peptidoglycan hydrolase with endo-β-N-acetylglucosaminidase specificity | 2 | 1 | - | - | 1 | 1 | - | 1 | 1 | |
| GH88 | d-4,5-unsaturated β-glucuronyl hydrolase | - | 1 | - | - | 1 | - | - | - | - | |
| GT1 | UDP-glucuronosyltransferase zeatin O-β-xylosyltransferase, Hydroxyacylsphingosine 1-β-galactosyltransferase | 1 | - | - | - | - | - | - | - | 1 | |
| GT5 | UDP-Glc, glycogen glucosyltransferase ADP-Glc, Starch glucosyltransferase NDP-Glc, Starch glucosyltransferase | - | 1 | - | - | 1 | - | 1 | - | 1 | |
| GT35 | Glycogen, Starch phosphorylase | - | 1 | - | - | 1 | - | 1 | - | 1 | |
| GT89 | β-D-arabinofuranosyl-1-monophosphoryldecaprenol arabinan β-1,2-arabinofuranosyltransferase | 1 | - | 1 | - | - | - | - | - | - | |
| GT94 | GDP-Man: GlcA-β-1,2-Man-α-1,3-Glc-β-1, 4-Glc-α-1-PP-undecaprenol β-1, 4-mannosyltransferase | - | - | - | - | - | 1 | - | 1 | - | |
| PL17 | Alginate lyase, Oligoalginate lyase | - | 1 | - | - | 1 | - | 1 | - | - | |
| Core | GT26 | UDP-ManNAcA: β-N-acetyl mannosaminuronyltransferase, UDP-ManNAc: β-N-acetyl-mannosaminyltransferase UDP-Glc: β-1,4-glucosyltransferase | 1 | ||||||||
| Unique | AA7 | Glucooligosaccharide oxidase, Chitooligosaccharide oxidase | - | - | - | - | 1 | - | - | - | - |
| GH8 | d-4,5-unsaturated β-glucuronyl hydrolase | - | - | - | - | - | - | - | - | 1 | |
| GH32 | Invertase, endo-inulinase, β-2,6-fructan 6 levanbiohydrolase | - | - | - | - | - | - | 1 | - | - | |
| GH94 | Cellobiose phosphorylase, laminaribiose phosphorylase, Cellodextrin phosphorylase | - | - | - | - | - | - | 1 | - | - | |
| GH126 | α-amylase | - | - | - | 1 | - | - | - | - | - | |
| GT27 | Polypeptide α-N-acetylgalactosaminyl transferase | - | 1 | - | - | - | - | - | - | - | |
| GT32 | α-1,6-mannosyltransferase, α-1,4-N-acetylglucosaminyltransferase, α-1,4-N-acetylgalactosaminyltransferase | - | 1 | - | - | - | - | - | - | - | |
| GT83 | Undecaprenyl phosphate-α-L-Ara4N, 4-amino-4-deoxy-β-L-arabinosyltransferase, Dodecaprenyl phosphate-β-galacturonic acid, lipopolysaccharide core α-galacturonosyl transferase | - | - | - | - | - | - | 1 | - | - | |
| GT84 | Cyclic β-1,2-glucan synthase | - | - | - | - | - | - | 1 | - | - | |
| Gene | Description | Type | Resistance | A. aneurinilyticus ATCC 12856T | A. danicus NBRC 102444T | A. migulanus DSM 2895T | A. soli CB4T | Aneurinibacillus sp. UBA3580 | Aneurinibacillus sp. XH2 | A. terranovensis DSM 18919T | A. thermoaerophilus L 420-91T | A. tyrosinisolvens LL-002T |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| zraR/hydH | Transcriptional regulatory protein ZraR | Regulator | Zinc (Zn) | 21 | 16 | 20 | 6 | 9 | 8 | 14 | 8 | 14 |
| corR | Sigma-54 dependent DNA-binding response regulator | Regulator | Copper (Cu) | 8 | 12 | 16 | 2 | 9 | 8 | 12 | 6 | 10 |
| copR | Transcriptional activator protein | Regulator | Copper (Cu) | 5 | 5 | 5 | 5 | 4 | 2 | 7 | 2 | 4 |
| terD | Tellurium resistance protein | Unknown | Tellurium (Te) | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 |
| mdeA | Methionine gamma-lyase | Enzyme | Cetrimide (CTM), Benzylkonium Chloride (BAC), Hoechst 33342, Ethidium Bromide, Rhodamine 6G, Acriflavine, Tetraphenylphosphonium (TPP), Chlorhexidine, Crystal Violet, Dequalinium, Pentamidine, Pyronin Y | 4 | 5 | 5 | 4 | 5 | 2 | 2 | 2 | 3 |
| chtR | DNA-binding response regulator | Regulator | Chlorhexidine | 5 | 0 | 8 | 1 | 0 | 1 | 0 | 1 | 0 |
| fecD | Fe(3+) dicitrate transport system permease protein | Enzyme | Nickel (Ni), Cobalt (Co) | 2 | 3 | 5 | 0 | 2 | 3 | 0 | 3 | 0 |
| merE | Mercuric resistance protein | Membrane Transporter | Mercury (Hg) | 3 | 3 | 2 | 5 | 1 | 0 | 5 | 0 | 2 |
| mntR | Manganese transport regulator | Regulator | Manganese (Mn), Magnesium (Mg) | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 4 |
| ruvB | ATP-dependent DNA helicase | Enzyme | Chromium (Cr), Tellurium (Te), Selenium (Se) | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| fecE | Fe(3+) dicitrate transport ATP-binding protein | Membrane Transporter | Nickel (Ni), Cobalt (Co) | 5 | 4 | 4 | 1 | 2 | 3 | 1 | 3 | 1 |
| nikD | Nickel import ATP-binding protein | Binding protein | Nickel (Ni) | 2 | 0 | 4 | 2 | 0 | 0 | 2 | 0 | 0 |
| baeR | Member of the two-component regulatory system BaeS/BaeR | Regulator | Zinc (Zn), Tungsten (W), Sodium Deoxycholate (SDC) | 2 | 1 | 3 | 4 | 1 | 2 | 4 | 2 | 2 |
| cpxR | Response regulator of stress-related two-component regulatory system. | Regulator | Hydrogen Peroxide (H2O2), Benzylkonium Chloride (BAC), Chlorhexidine | 2 | 2 | 4 | 3 | 2 | 2 | 2 | 2 | 2 |
| soda | Mn-dependent superoxide dismutase | Enzyme | Selenium (Se), Hydrogen Peroxide (H2O2) | 3 | 2 | 2 | 3 | 1 | 2 | 2 | 3 | 1 |
| Can | Aconitate hydratase | Enzyme | Iron (Fe) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| arsT | Arsenic (As) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | ||
| irlR | Transcriptional activator protein | Regulator | Cadmium (Cd), Zinc (Zn) | 2 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 1 |
| lmrS | LmrS is an efflux protein capable of extruding multiple and structurally unrelated antimicrobial compounds. | Efflux | Tetraphenylphosphonium (TPP), Sodium Dodecyl Sulfate (SDS), Ethidium Bromide, Cetrimide (CTM) | 2 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 3 |
| vcaM | ABC transporter | Binding protein | Ethidium Bromide, Rhodamine 6G, 4,6-diamidino-2-phenylindole (DAPI), Tetraphenylphosphonium (TPP), Acridine Orange | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamli, M.R.; Alzahrani, N.A.Y.; Hajrah, N.H.; Sabir, J.S.M.; Malik, A. Genome-Driven Discovery of Enzymes with Industrial Implications from the Genus Aneurinibacillus. Microorganisms 2021, 9, 499. https://doi.org/10.3390/microorganisms9030499
Kamli MR, Alzahrani NAY, Hajrah NH, Sabir JSM, Malik A. Genome-Driven Discovery of Enzymes with Industrial Implications from the Genus Aneurinibacillus. Microorganisms. 2021; 9(3):499. https://doi.org/10.3390/microorganisms9030499
Chicago/Turabian StyleKamli, Majid Rasool, Nada A. Y. Alzahrani, Nahid H. Hajrah, Jamal S. M. Sabir, and Adeel Malik. 2021. "Genome-Driven Discovery of Enzymes with Industrial Implications from the Genus Aneurinibacillus" Microorganisms 9, no. 3: 499. https://doi.org/10.3390/microorganisms9030499
APA StyleKamli, M. R., Alzahrani, N. A. Y., Hajrah, N. H., Sabir, J. S. M., & Malik, A. (2021). Genome-Driven Discovery of Enzymes with Industrial Implications from the Genus Aneurinibacillus. Microorganisms, 9(3), 499. https://doi.org/10.3390/microorganisms9030499






