Traditional Bulgarian Dairy Products: Ethnic Foods with Health Benefits
Abstract
1. Introduction
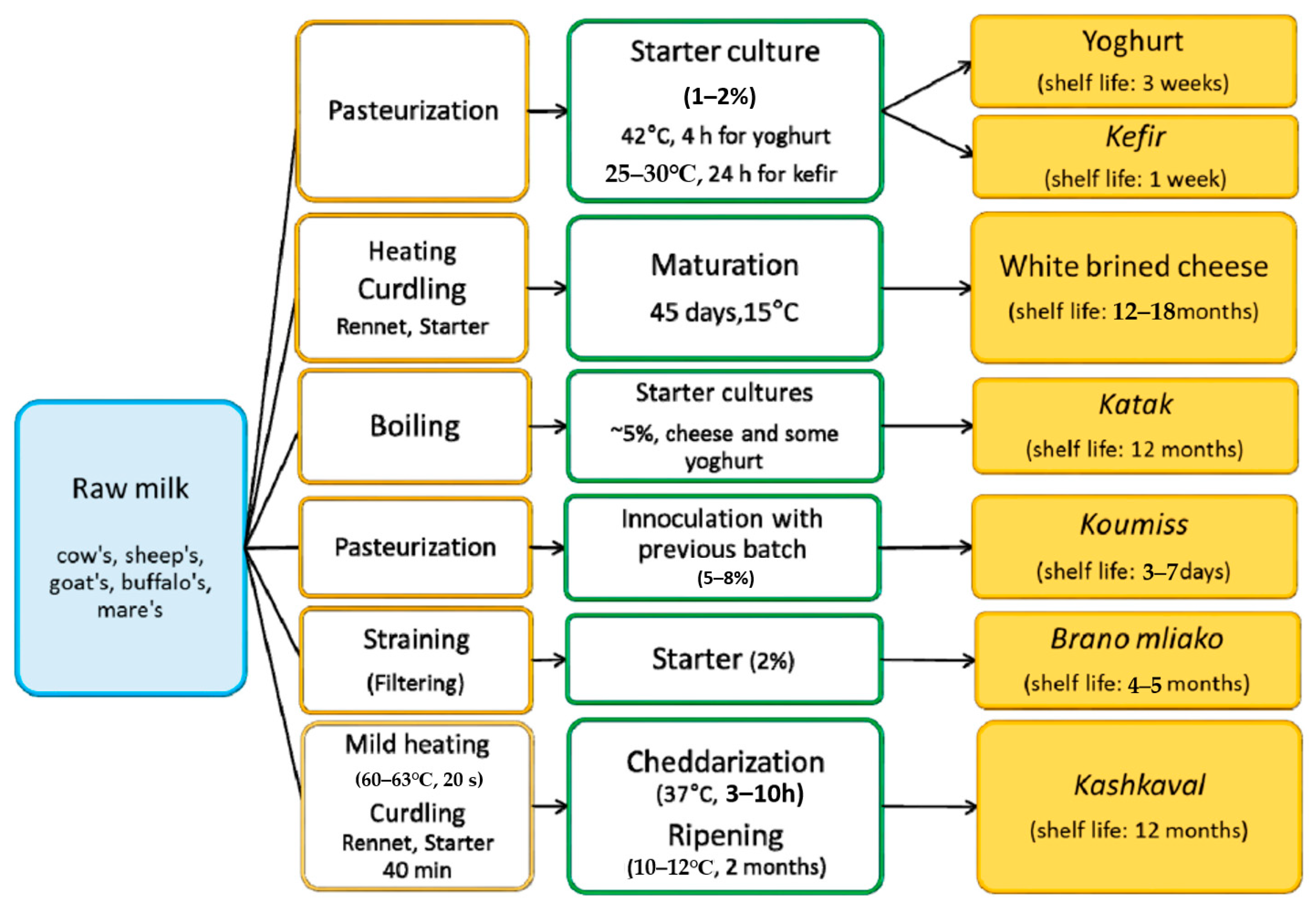
2. Overview of Traditional Bulgarian Dairy Products: Appearance, Nutritional Value, Production Technologies, and Shelf Life
2.1. Yoghurt
2.2. White Brined Cheese
2.3. Kashkaval (Yellow Cheese)
2.4. Katak
2.5. Kefir
2.6. Koumiss
2.7. Rhodope’s Brano Mliako
3. Microbial Content of Traditional Bulgarian Dairy Products
| Product | Starter Strains | Accompanying Microflora | Reference |
|---|---|---|---|
| Yoghurt | L. delbrueckii ssp. bulgaricus, S. thermophilus | L. helveticus, L. paracasei, L. fermentum, Lacticaseibacillus rhamnosus, Leuc. mesenteroides, Leuc. pseudomesenteroides, W. confusa, P. acidilactici, Lc. lactis, Ent. faecium | [10,39,40,41] |
| White brined cheese | Lc. lactis ssp. lactis, L. casei, L. delbrueckii ssp. bulgaricus, S. thermophilus | L. plantarum, L. paraplantarum, L. pentosus, L. paracasei ssp. paracasei, Lentilactobacillus hilgardii, L. brevis, Leuconostoc spp., Ent. faecium, Ent. durans | [49,50,51,52] |
| Kashkaval | Lc. lactis ssp. lactis, Lc. lactis ssp. cremoris, L. delbrueckii ssp. bulgaricus, L. helveticus, S. thermophilus | Leuc. mesenteroides, L. lactis ssp. lactis biovar. diacetylactis, Enterococcus spp. | [53,54,55] |
| Katak | L. delbrueckii ssp. bulgaricus, S. thermophilus | L. delbrueckii ssp. delbrueckii, L. delbrueckii ssp. lactis, P. acidilactici, P. pentosaceus, Enterococcus ssp. | [25,56] |
| Kefir | L. kefiranofaciens, Lentilactobacillus kefiri, Lentilactobacillus buchneri, L. plantarum, L. amylovorus, Levilactobacillus brevis, L. casei, L. paracasei, L. crispatus, Lactobacillus delbrueckii subsp. bulgaricus, L. helveticus, L. parakefiri, L. satsumensis, L. uvarum, S. thermophilus, Lc. lactis ssp. cremoris, Lc. lactis ssp. lactis, Kluyveromyces marxianus, K. lactis | Leuc. lactis, Leuc. mesenteroides, Acetobacter fabarum, A. lovaniensis, A. syzygii, Ent. faecium, Gluconobacter japonicus, Weissella spp., Halococcus spp., Candida inconspicua, Dysgonomonas spp., Geotrichum candidum, Kazachstania aerobia, Kz. exigua, Kz. unispora, Lachancea meyersii, Pelomonas spp., Pichia fermentans, P. guilliermondii, P. kudriavzevii, Sacch. cerevisiae, Sacch. martiniae, Sacch. turicensis, Sacch. unisporus, Shewanella spp. | [57,58,59,60,61] |
| Koumiss | L. acidophilus, L. helveticus, Ligilactobacillus salivarius, S. thermophilus, K. lactis | L. buchneri, L. plantarum, Leuconostoc spp., Sacch. lactis, Sacch. unisporus, Torula kumiss | [34,35,36,62] |
4. Health-Promoting Metabolites in Traditional Bulgarian Dairy Products
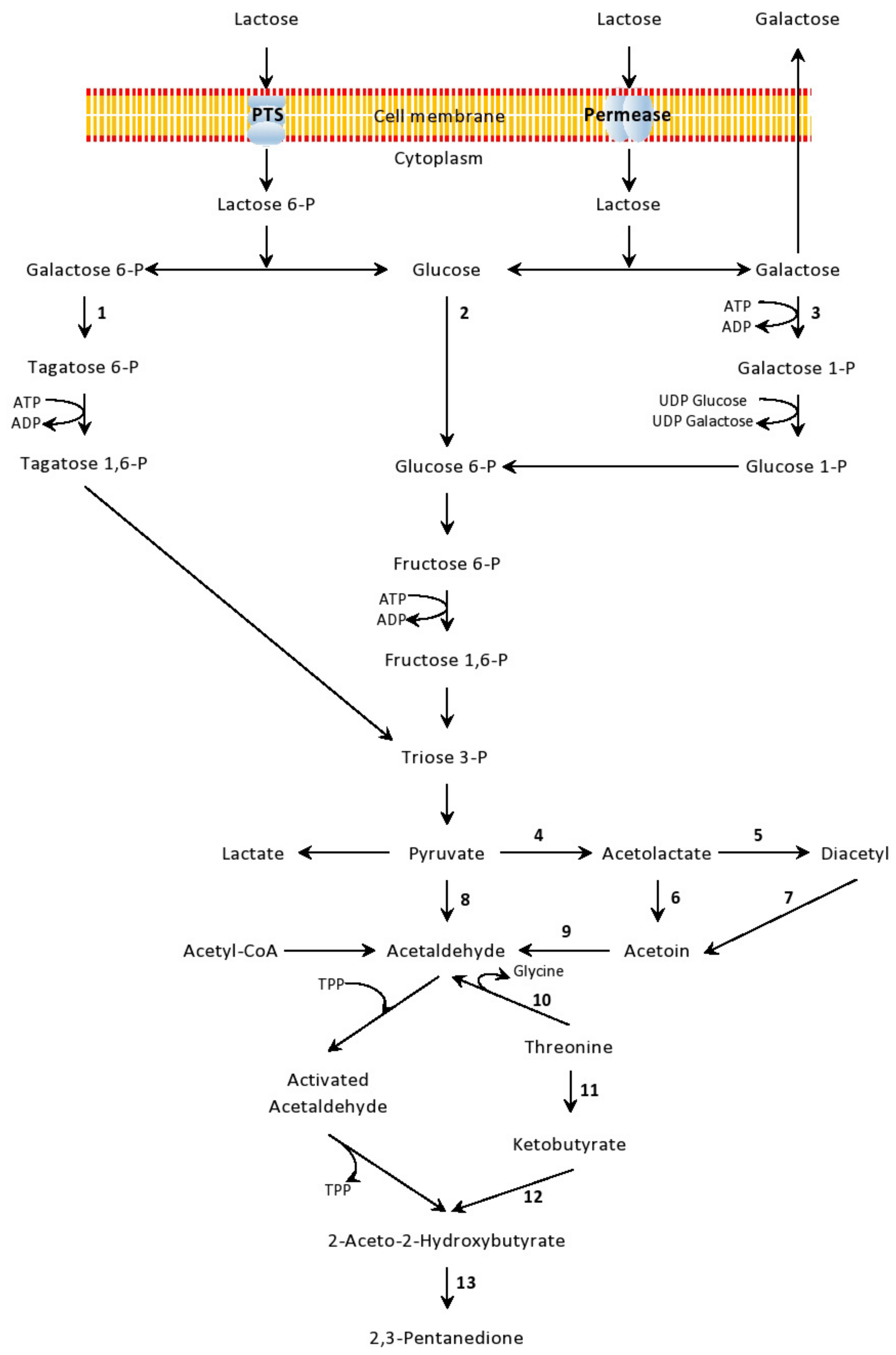
4.1. Free Amino Acids (FAA)
4.2. Bioactive Peptides
4.3. Antioxidants
4.4. Exopolysaccharides (EPS)
4.5. Prebiotics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tribby, D. Yogurt. In The Sensory Evaluation of Dairy Products, 2nd ed.; Clark, S., Costello, M., Drake, M., Bodyfelt, F., Eds.; Springer Science & Business Media: New York, NY, USA, 2009; pp. 191–224. [Google Scholar]
- Rul, F. Yogurt: Microbiology, organoleptic properties and probiotic potential. In Fermented Foods, Part II: Technological Interventions; CRC Press: Boca Raton, FL, USA, 2017; pp. 419–450. [Google Scholar]
- Yang, Y.; Shevchenko, A.; Knaust, A.; Abuduresule, I.; Li, W.; Hu, X.; Wang, C.; Shevchenko, A. Proteomics evidence for kefir dairy in Early Bronze Age China. J. Archaeol. Sci. 2014, 45, 178–186. [Google Scholar] [CrossRef]
- Kostov, D. Domestic and wild animals, from the Neolithic period, in the “Azmashka” settlement hill, near Stara Zagora. Thrakia J. Sci. 2006, 4, 55–60. (In Bulgarian) [Google Scholar]
- LB Bulgaricum, Yoghurt History. Available online: https://lbbulgaricum.bg/en/about-the-company/history/ (accessed on 10 January 2021).
- Fisberg, M.; Machado, R. History of yogurt and current patterns of consumption. Nutr. Rev. 2015, 73, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Stoilova, E.R. Producing Bulgarian Yoghurt: Manufacturing and Exporting Authenticity, 1st ed.; Amsterdam University Press: Amsterdam, The Netherlands, 2014; pp. 38–45. [Google Scholar] [CrossRef]
- Mackowiak, P.A. Recycling Metchnikoff: Probiotics, the Intestinal Microbiome and the Quest for Long Life. Front. Public Health 2013, 1, 52. [Google Scholar] [CrossRef]
- Activity of the Dairies in Bulgaria in 2020: Official Bulletin of Bulgarian Ministry of Agriculture, Foods and Forests. Available online: https://www.mzh.government.bg/en/statistics-and-analyses/ (accessed on 15 January 2021).
- Velikova, P.; Petrov, K.; Lozanov, V.; Tsvetanova, F.; Stoyanov, A.; Wu, Z.; Liu, Z.; Petrova, P. Microbial diversity and health-promoting properties of the traditional Bulgarian yogurt. Biotechnol. Biotechnol. Equip. 2018, 32, 1205–1217. [Google Scholar] [CrossRef]
- European Union. EC Regulation No 853/2004 of the European Parliament. Specific hygiene rules for food of animal origin. Off. J. Eur. Union 2004, 56, 71–131. [Google Scholar]
- International Dairy Federation. FIL-IDF Standard 99A. Sensory Evaluation of Dairy Product; International Dairy Federation: Brussels, Belgium, 1987. [Google Scholar]
- Ivanov, G.; Balabanova, T.; Ivanova, I.; Baltadzhieva, M. Proteolysis in caw and buffalo milk Bulgarian white brined cheese during refrigerated storage. Sci. Works Univ. Food Technol. 2015, 62, 158–161. [Google Scholar]
- Boyanova, P.; Panayotov, P.; Ganchovska, V.; Bosakova–Ardenska, A. Microscopic method for qualification of the cut surface of white brined cheese. J. Agric. Sci. Technol. 2012, 4, 306–310. [Google Scholar]
- Yegin, S.; Dekker, P. Progress in the field of aspartic proteinases in cheese manufacturing: Structures, functions, catalytic mechanism, inhibition, and engineering. Dairy Sci. Technol. 2013, 93, 565–594. [Google Scholar] [CrossRef]
- Emtage, J.S.; Angal, S.; Doel, M.T.; Harris, T.J.; Jenkins, B.; Lilley, G.; Lowe, P.A. Synthesis of calf prochymosin (prorennin) in Escherichia coli. Proc. Natl. Acad. Sci. USA 1983, 80, 3671–3675. [Google Scholar] [CrossRef]
- Ivanova, S.; Angelov, L.; Odjakova, T. Biological active components of ewe’s white brine cheese produced in the Western Rhodopes. JMAB 2015, 18, 629–638. [Google Scholar]
- Ivanov, G.; Balabanova, T.; Baltadzhieva, M.; Ivanova, I. Lipolysis in cold stored caw and buffalo milk white brined cheese. Sci. Works Univ. Food Technol. 2015, 62, 139–144. [Google Scholar]
- Teter, A.; Barłowska, J.; Król, J.; Brodziak, A.; Rutkowska, J.; Litwińczuk, Z. Volatile compounds and amino acid profile of short-ripened farmhouse cheese manufactured from the milk of the White-Backed native cow breed. LWT 2020, 129, 109602. [Google Scholar] [CrossRef]
- Aladjadjiyan, A.; Zheleva, I.; Kartalska, Y. Traditional Bulgarian Dairy Food. In Traditional Foods. Integrating Food Science and Engineering Knowledge into the Food Chain, 1st ed.; Kristbergsson, K., Oliveira, J., Eds.; Springer: Boston, MA, USA, 2016; Volume 10, pp. 115–122. [Google Scholar]
- McSweeney, P.L.H. Cheese manufacture and ripening and their influence on cheese flavour. In Improving the Flavour of Cheese, Woodhead Publishing Series in Food Science, Technology and Nutrition; Weimer, B.C., Ed.; Woodhead Publishing Ltd.: Cambridge, UK, 2007; pp. 1–25. [Google Scholar]
- Markova, A.; Ivanov, G. Effect of refrigeration temperature on the fermentation process in kashkaval cheese. Sci. Works Univ. Food Technol. 2019, 66, 31–36. [Google Scholar]
- Ivanov, G.; Markova, A. Proteolysis in kashkaval cheese stored at different temperatures. Food Sci. Appl. Biotechnol. 2020, 3, 111–117. [Google Scholar] [CrossRef]
- Tserovska, L.; Stefanova, S.; Yordanova, T. Identification of lactic acid bacteria isolated from katyk, goat’s milk and cheese. J. Cult. Collect. 2002, 3, 48–52. [Google Scholar]
- Danova, S.; Nemska, V.; Tropcheva, R. Bulgarian yogurt-like product “Katak”. In Yoghurt in Health and Disease Prevention, 1st ed.; Shah, P.N., Ed.; Academic Press: London, UK, 2017; pp. 307–329. [Google Scholar]
- Farnworth, E.; Mainville, I. Kefir-a fermented milk product. In Handbook of Fermented Functional Foods, 2nd ed.; Farnworth, E.R., Ed.; CRC Press: Boca Raton, FL, USA, 2008; Volume 2, pp. 89–127. [Google Scholar]
- Magalhães, K.T.; de Melo Pereira, G.V.; Campos, C.R.; Dragone, G.; Schwan, R.F. Brazilian kefir: Structure, microbial communities and chemical composition. Braz. J. Microbiol. 2011, 42, 693–702. [Google Scholar] [CrossRef]
- Rea, M.; Lennartsson, T.; Dillon, P.; Drinan, F.; Reville, W.; Heapes, M.; Cogan, T. Irish kefir-like grains: Their structure, microbial composition and fermentation kinetics. J. Appl. Microbiol. 1996, 81, 83–94. [Google Scholar] [CrossRef]
- Frengova, G.I.; Simova, E.D.; Beshkova, D.M.; Simov, Z.I. Exopolysaccharides Produced by Lactic Acid Bacteria of Kefir Grains. Z. Nat. C 2002, 57, 805–810. [Google Scholar] [CrossRef]
- Assadi, M.; Pourahmad, R.; Moazami, N. Use of isolated kefir starter cultures in kefir production. World J. Microbiol. Biotechnol. 2000, 16, 541–543. [Google Scholar] [CrossRef]
- Otles, S.; Cagindi, O. Kefir: A Probiotic Dairy-Composition, Nutritional and Therapeutic Aspects. Pakistan J. Nutr. 2003, 2, 54–59. [Google Scholar]
- Glaeser, H.; Hangst, E.; Ziegler, K. Biochemische charakterisierung der in molkereikefir und kefirkulturen vorkommende hefen. Dtsch. Molk. Ztg. 1986, 16, 483–490. [Google Scholar]
- Uniacke-Lowe, T.; Huppertz, T.; Fox, P.F. Equine milk proteins: Chemistry, structure and nutritional significance. Int. Dairy J. 2010, 20, 609–629. [Google Scholar] [CrossRef]
- Danova, S.; Petrov, K.; Pavlov, P.; Petrova, P. Isolation and characterization of Lactobacillus strains involved in koumiss fermentation. Int. J. Dairy Technol. 2005, 58, 100–105. [Google Scholar] [CrossRef]
- Montanari, G.; Zambonelli, C.; Grazia, L.; Kamesheva, G.K.; Shigaeva, M.K. Saccharomyces unispora as the principle alcoholic fermentation microorganism of traditional koumiss. J. Dairy Res. 1996, 63, 327–331. [Google Scholar]
- Behera, S.K.; Panda, S.K.; Kayitesi, E.; Mulaba-Bafubiandi, A.F. Kefir and Koumiss: Origin, Health Benefits and Current Status of Knowledge. In Fermented Food—Part II: Technological Interventions, 1st ed.; Ray, R.C., Montet, D., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 400–417. [Google Scholar]
- Montanari, G.; Zambonelli, C.; Fiori, G. The koumiss, a fermented milk drink. Ind. Alim. 1997, 36, 5–9. (In Italian) [Google Scholar]
- Gruev, P. Microbiological basis of industrial production of Rodopi mountains Brano mliako cultured milk. Nauchni Trudove VIHVP 1970, 17, 283–292. (In Bulgarian) [Google Scholar]
- Hao, P.; Zheng, H.; Yu, Y.; Ding, G.; Gu, W.; Chen, S.; Yu, Z.; Ren, S.; Oda, M.; Konno, T.; et al. Complete Sequencing and Pan-Genomic Analysis of Lactobacillus delbrueckii subsp. bulgaricus Reveal Its Genetic Basis for Industrial Yogurt Production. PLoS ONE 2011, 6, e15964. [Google Scholar] [CrossRef]
- Liu, M.; Siezen, R.J.; Nauta, A. In Silico Prediction of Horizontal Gene Transfer Events in Lactobacillus bulgaricus and Streptococcus thermophilus Reveals Protocooperation in Yogurt Manufacturing. Appl. Environ. Microbiol. 2009, 75, 4120–4129. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.; Petrov, K.; Lozanov, V.; Hristov, I.; Wu, Z.; Liu, Z.; Petrova, P. Bioactive Compounds Produced by the Accompanying Microflora in Bulgarian Yoghurt. Processes 2021, 9, 114. [Google Scholar] [CrossRef]
- Solopova, A.; Bachmann, H.; Teusink, B.; Kok, J.; Neves, A.R.; Kuipers, O.P. A Specific Mutation in the Promoter Region of the Silent cel Cluster Accounts for the Appearance of Lactose-Utilizing Lactococcus lactis MG1363. Appl. Environ. Microbiol. 2012, 78, 5612–5621. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrzak-Piekarczyk, T. Lactose and β-Glucosides metabolism and its regulation in Lactococcus lactis: A Review. In Lactic Acid Bacteria—R & D for Food, Health and Livestock Purposes; Kongo, J.M., Ed.; InTechOpen: London, UK, 2013; pp. 467–486. [Google Scholar]
- Vaughan, E.E.; van den Bogaard, P.T.; Catzeddu, P.; Kuipers, O.P.; de Vos, W.M. Activation of silent gal genes in the lac-gal regulon of Streptococcus thermophilus. J. Bacteriol. 2001, 183, 1184–1194. [Google Scholar] [CrossRef]
- Tenea, G.N.; Suárez, J. Probiotic Potential and Technological Properties of Bacteriocinogenic Lactococcus lactis Subsp. lactis UTNGt28 from a Native Amazonian Fruit as a Yogurt Starter Culture. Microorganisms 2020, 8, 733. [Google Scholar] [CrossRef]
- Cheng, H. Volatile Flavor Compounds in Yogurt: A Review. Crit. Rev. Food Sci. Nutr. 2010, 50, 938–950. [Google Scholar] [CrossRef]
- Pastink, M.I.; Teusink, B.; Hols, P.; Visser, S.; De Vos, W.M.; Hugenholtz, J. Genome-Scale Model of Streptococcus thermophilus LMG18311 for Metabolic Comparison of Lactic Acid Bacteria. Appl. Environ. Microbiol. 2009, 75, 3627–3633. [Google Scholar] [CrossRef]
- Ott, A.; Hugi, A.; Baumgartner, M.; Chaintreau, A. Sensory investigation of yogurt flavor perception: Mutual influence of volatiles and acidity. J. Agric. Food Chem. 2000, 48, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Özer, B. Cheese: Microflora of White-Brined Cheeses. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.-L., Eds.; Academic Press: London, UK, 2014; pp. 402–408. [Google Scholar]
- Nemska, V.; Lazarova, N.; Georgieva, N.; Danova, S. Lactobacillus spp. from traditional Bulgarian dairy products. J. Univ. Chem. Technol. Metall. 2016, 51, 693–704. [Google Scholar]
- Georgieva, R.; Iliev, I.; Chipeva, V.; Dimitonova, S.; Samelis, J.; Danova, S. Identification and in vitro characterization of Lactobacillus plantarum strains from artisanal Bulgarian white brined cheeses. J. Basic Microbiol. 2008, 48, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Favaro, L.; Basaglia, M.; Casella, S.; Hue, I.; Dousset, X.; de Melo Franco, B.D.G.; Todorov, S. Bacteriocinogenic potential and safety evaluation of non-starter Enterococcus faecium strains isolated from homemade white brined cheese. Food Microbiol. 2014, 38, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Teneva-Angelova, T.; Balabanova, T.; Boyanova, P.; Beshkova, D. Traditional Balkan fermented milk products. Eng. Life Sci. 2018, 18, 807–819. [Google Scholar] [CrossRef]
- Begovic, J.; Brandsma, J.; Jovcic, B.; Tolinacki, M.; Veljovic, K.; Meijer, W.; Topisirovic, L. Analysis of dominant lactic acid bacteria from artisanal raw milk cheeses produced on the mountain Stara Planina, Serbia. Arch. Biol. Sci. 2011, 63, 11–20. [Google Scholar] [CrossRef]
- Dimitrov, D.; Simov, Z.; Dimitrov, Z.; Ospanov, A. Improving of the microbiological and proteolytic profile of kashkaval cheese by modification in heat treatments of cow’s milk and cheddared curd. J. Microbiol. Biotechnol. Food Sci. 2015, 4, 546–549. [Google Scholar] [CrossRef]
- Kirilov, N.; Dimov, S.; Dalgalarrondo, M.; Ignatova, T.; Kambarev, S.; Stoyanovski, S.; Danova, S.; Iliev, I.; Haertle, T.; Chobert, J.; et al. Characterization of enterococci isolated from homemade Bulgarian cheeses and katuk. Eur. Food Res. Technol. 2011, 233, 1029–1040. [Google Scholar] [CrossRef]
- Chen, H.; Wang, S.; Chen, M. Microbiological study of lactic acid bacteria in kefir grains by culture-dependent and culture-independent methods. Food Microbiol. 2008, 25, 492–501. [Google Scholar] [CrossRef]
- Garofalo, C.; Osimani, A.; Milanović, V.; Aquilanti, L.; De Filippis, F.; Stellato, G.; Di Mauro, S.; Turchetti, B.; Buzzini, P.; Ercolini, D.; et al. Bacteria and yeast microbiota in milk kefir grains from different Italian regions. Food Microbiol. 2015, 49, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, X.; Tian, Z.; Yang, Y.; Yang, Z. Characterization of an exopolysaccharide produced by Lactobacillus plantarum YW11 isolated from Tibet Kefir. Carbohydr. Polym. 2015, 125, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Ho, C.; Mao, C.; Barham, N.; Huang, Y.; Ho, F.; Wu, Y.; Hou, Y.; Shih, M.; Li, W.; et al. A thermo- and toxin-tolerant kefir yeast for biorefinery and biofuel production. Appl. Energy 2014, 132, 465–474. [Google Scholar] [CrossRef]
- Pogačić, T.; Šinko, S.; Zamberlin, S.; Samaržija, D. Microbiota of kefir grains. Mljekarstvo 2013, 63, 3–14. [Google Scholar]
- Guzel-Seydim, Z.; Koktas, T.; Greene, A.K. Kefir and Koumiss: Microbiology and Technology. In Development and Manufacture of Yogurt and Other Functional Dairy Products; Yildiz, F., Ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 143–163. [Google Scholar]
- Savijoki, K.; Ingmer, H.; Varmanen, P. Proteolytic systems of lactic acid bacteria. Appl. Microbiol. Biotechnol. 2006, 71, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.J.; Platt, D.H.; Caldwell, R.W. Therapeutic Use of Citrulline in Cardiovascular Disease. Cardiovasc. Drug Rev. 2006, 24, 275–290. [Google Scholar] [CrossRef]
- Balabanova, T.; Ivanova, M.; Vlaseva, R. Effect of rennet type and ripening period on chemical properties of Bulgarian white brined cheese. Int. Food Res. J. 2017, 24, 2414–2418. [Google Scholar]
- Simova, E.; Simov, Z.; Beshkova, D.; Frengova, G.; Dimitrov, Z.; Spasov, Z. Amino acid profiles of lactic acid bacteria, isolated from kefir grains and kefir starter made from them. Int. J. Food Microbiol. 2006, 107, 112–123. [Google Scholar] [CrossRef]
- Dimitrov, Z.; Chorbadjiyska, E.; Gotova, I.; Pashova, K.; Ilieva, S. Selected adjunct cultures remarkably increase the content of bioactive peptides in Bulgarian white brined cheese. Biotechnol. Biotechnol. Equip. 2014, 29, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R. Dairy products, yogurts, and bone health. Am. J. Clin. Nutr. 2014, 99, 1256S–1262S. [Google Scholar] [CrossRef] [PubMed]
- Simova, E.; Beshkova, D.; Dimitrov, Z.; Simov, Z. In vitro and in situ bacteriocin activity of lactic acid bacteria from Bulgarian dairy products and methods for making of Lactobacillus protective fermented milks with bacteriocin inhibitory substances. Bulg. J. Agric. Sci. 2008, 14, 28–42. [Google Scholar]
- Simova, E.; Beshkova, D.; Dimitrov, Z. Characterization and antimicrobial spectrum of bacteriocins produced by lactic acid bacteria isolated from traditional Bulgarian dairy products. J. Appl. Microbiol. 2009, 106, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Tropcheva, R.; Nikolova, D.; Evstatieva, Y.; Danova, S. Antifungal activity and identification of Lactobacilli, isolated from traditional dairy product “katak”. Anaerobe 2014, 28, 78–84. [Google Scholar] [CrossRef]
- Donkor, O.; Henriksson, A.; Singh, T.; Vasiljevic, T.; Shah, N. ACE-inhibitory activity of probiotic yoghurt. Int. Dairy J. 2007, 17, 1321–1331. [Google Scholar] [CrossRef]
- Stefanova, T.; Urshev, Z.; Minkova, S.; Dimitrov, Z. Development of Prototype Fermented Milk Products with Angiotensin-Converting Enzyme Inhibitory Activity. Biotechnol. Biotechnol. Equip. 2009, 23, 1368–1371. [Google Scholar] [CrossRef][Green Version]
- Solieri, L.; Rutella, G.S.; Tagliazucchi, D. Impact of non-starter lactobacilli on release of peptides with angiotensin-converting enzyme inhibitory and antioxidant activities during bovine milk fermentation. Food Microbiol. 2015, 51, 108–116. [Google Scholar] [CrossRef][Green Version]
- Fardet, A.; Rock, E. In vitro and in vivo antioxidant potential of milks, yoghurts, fermented milks and cheeses: A narrative review of evidence. Nutr. Res. Rev. 2018, 31, 52–70. [Google Scholar] [CrossRef] [PubMed]
- Lehnen, T.E.; Da Silva, M.R.; Camacho, A.; Marcadenti, A.; Lehnen, A.M. A review on effects of conjugated linoleic fatty acid (CLA) upon body composition and energetic metabolism. J. Int. Soc. Sports Nutr. 2015, 12, 1–11. [Google Scholar] [CrossRef]
- Bosakova-Ardenska, A.; Danev, A.; Kostadinova-Georgieva, L. Application of computer vison for cheese quality evaluation: A review. Sci. Works Union Sci. Bul. 2019, 62, 68–75. (In Bulgarian) [Google Scholar]
- Dimitrova, T.; Stoycheva, S.; Ivanova, S. Fatty acid profile and qualitative evaluation of the fat fraction in goat white brined cheese on the 45-th day of the ripening process. Sci. Papers Ser. D Anim. Sci. 2020, 63, 394–400. [Google Scholar]
- Dimitrova, T.; Ivanova, S.; Stoycheva, S.; Zunev, P.; Angelov, L.; Naydenova, N. Trans fatty acids and quality assessment of fatty acid composition in white brined cheese from goat’s milk. JMAB 2017, 20, 29–42. [Google Scholar]
- Pallister, T.; Haller, T.; Thorand, B.; Altmaier, E.; Cassidy, A.; Martin, T.; Jennings, A.; Mohney, R.P.; Gieger, C.; MacGregor, A.; et al. Metabolites of milk intake: A metabolomic approach in UK twins with findings replicated in two European cohorts. Eur. J. Nutr. 2017, 56, 2379–2391. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.-P.J.; Sprenger, N.; Montoliu, I.; Rezzi, S.; Kochhar, S.; Nicholson, J.K. Dietary Modulation of Gut Functional Ecology Studied by Fecal Metabonomics. J. Proteome Res. 2010, 9, 5284–5295. [Google Scholar] [CrossRef]
- Kokkinidis, D.G.; Bosdelekidou, E.E.; Iliopoulou, S.M.; Tassos, A.G.; Texakalidis, P.T.; Economopoulos, K.P.; Kousoulis, A.A. Emerging treatments for ulcerative colitis: A systematic review. Scand. J. Gastroenterol. 2017, 19, 1–9. [Google Scholar] [CrossRef]
- Jana, A.; Pahan, K. Sphingolipids in Multiple Sclerosis. NeuroMol. Med. 2010, 12, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Mangino, M.; Cecelja, M.; Psatha, M.; Brosnan, M.J.; Trimmer, J.; Mohney, R.P.; Chowienczyk, P.; Padmanabhan, S.; Spector, T.D.; et al. Metabolomic study of carotid–femoral pulse-wave velocity in women. J. Hypertens. 2015, 33, 791–796. [Google Scholar] [CrossRef]
- Da Silva, C.G.; Specht, A.; Wegiel, B.; Ferran, C.; Kaczmarek, E. Mechanism of Purinergic Activation of Endothelial Nitric Oxide Synthase in Endothelial Cells. Circualtion 2009, 119, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ji, J.; Chen, X.; Jiang, M.; Rui, X.; Dong, M. Structural elucidation and antioxidant activities of exopolysaccharides from Lactobacillus helveticus MB2-1. Carbohydr. Polym. 2014, 102, 351–359. [Google Scholar] [CrossRef]
- Wang, K.; Li, W.; Rui, X.; Chen, X.; Jiang, M.; Dong, M. Characterization of a novel exopolysaccharide with antitumor activity from Lactobacillus plantarum 70810. Int. J. Biol. Macromol. 2014, 63, 133–139. [Google Scholar] [CrossRef]
- Popović, N.; Brdarić, E.; Đokić, J.; Dinić, M.; Veljović, K.; Golić, N.; Terzić-Vidojević, A. Yogurt produced by novel natural starter cultures improves gut epithelial barrier in vitro. Microorganisms 2020, 8, 1586. [Google Scholar] [CrossRef] [PubMed]
- Terzieva, M.; Urshev, Z. Comparison of the Expression Levels of Exopolysaccharide Genes in Streptococcus thermophilus LBB.T54 Grown in Semi-Defined Media and Milk. Acta Microbiol. Bulg. 2018, 34, 116–120. [Google Scholar]
- Sánchez-Medina, I.; Gerwig, G.J.; Urshev, Z.L.; Kamerling, J.P. Structural determination of a neutral exopolysaccharide produced by Lactobacillus delbrueckii ssp. bulgaricus LBB.B332. Carbohydr. Res. 2007, 342, 2735–2744. [Google Scholar] [CrossRef]
- Sánchez-Medina, I.; Gerwig, G.J.; Urshev, Z.L.; Kamerling, J.P. Structure of a neutral exopolysaccharide produced by Lactobacillus delbrueckii ssp. bulgaricus LBB.B26. Carbohydr. Res. 2007, 342, 2430–2439. [Google Scholar] [CrossRef]
- Makino, S.; Ikegami, S.; Kano, H.; Sashihara, T.; Sugano, H.; Horiuchi, H.; Saito, T.; Oda, M. Immunomodulatory Effects of Polysaccharides Produced by Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. J. Dairy Sci. 2006, 89, 2873–2881. [Google Scholar] [CrossRef]
- Makino, S.; Ikegami, S.; Kume, A.; Horiuchi, H.; Sasaki, H.; Orii, N. Reducing the risk of infection in the elderly by dietary intake of yoghurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. Br. J. Nutr. 2010, 104, 998–1006. [Google Scholar] [CrossRef]
- Gotova, I.; Dimitrov, Z.; Najdenski, H. Selected Lactobacillus bulgaricus and Streptococcus thermophilus Strains from Bulgarian Yogurt Demonstrate Significant Anti-Inflammatory Potential. Acta Microbiol. Bulg. 2017, 33, 3. [Google Scholar]
- Liu, C.-F.; Tseng, K.-C.; Chiang, S.-S.; Lee, B.-H.; Hsu, W.-H.; Pan, T.-M. Immunomodulatory and antioxidant potential of Lactobacillus exopolysaccharides. J. Sci. Food Agric. 2011, 91, 2284–2291. [Google Scholar] [CrossRef]
- Shao, L.; Wu, Z.; Zhang, H.; Chen, W.; Ai, L.; Guo, B. Partial characterization and immunostimulatory activity of exopolysaccharides from Lactobacillus rhamnosus KF5. Carbohydr. Polym. 2014, 107, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Surayot, U.; Wang, J.; Seesuriyachan, P.; Kuntiya, A.; Tabarsa, M.; Lee, Y.; Kim, J.-K.; Park, W.; You, S. Exopolysaccharides from lactic acid bacteria: Structural analysis, molecular weight effect on immunomodulation. Int. J. Biol. Macromol. 2014, 68, 233–240. [Google Scholar] [CrossRef]
- Slattery, C.; Cotter, P.D.; O’Toole, P.W. Analysis of Health Benefits Conferred by Lactobacillus Species from Kefir. Nutritiens 2019, 11, 1252. [Google Scholar] [CrossRef]
- Petrova, P.; Petrov, K. Lactic Acid Fermentation of Cereals and Pseudocereals: Ancient Nutritional Biotechnologies with Modern Applications. Nutritiens 2020, 12, 1118. [Google Scholar] [CrossRef] [PubMed]
- Petrova, P.; Petrov, K. Traditional cereal beverage Boza—Fermentation technology; microbial content and healthy effects. In Fermented Food—Part II: Technological Interventions, 1st ed.; Ray, R., Montet, D., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 284–305. [Google Scholar]
- Gibson, G.R.; Scott, K.P.; Rastall, R.A.; Tuohy, K.M.; Hotchkiss, A.; Dubert-Ferrandon, A.; Gareau, M.; Murphy, E.F.; Saulnier, D.; Loh, G.; et al. Dietary prebiotics: Current status and new definition. Food Sci. Technol. Bull. Funct. Foods 2010, 7, 1–19. [Google Scholar] [CrossRef]
- Alander, M.; Matto, J.; Kneifel, W.; Johansson, M.; Kogler, B.; Crittenden, R.; Mattila-Sandholm, T.; Saarela, M. Effect of galacto-oligosaccharide supplementation on human faecal microfora and on survival and persistence of Bifidobacterium lactis Bb-12 in the gastrointestinal tract. Int. Dairy J. 2001, 11, 817–825. [Google Scholar] [CrossRef]
- Gänzle, M.G.; Haase, G.; Jelen, P. Lactose: Crystallization, hydrolysis and value-added derivatives. Int. Dairy J. 2008, 18, 685–694. [Google Scholar] [CrossRef]
- Petrova, P.; Petrov, K. Prebiotic–probiotic relationship: The genetic fundamentals of polysaccharides conversion by Bifidobacterium and Lactobacillus genera. In Handbook of Food Bioengineering, 1st ed.; Grumezescu, A.M., Holban, A.M., Eds.; Elsevier Inc.: San Diego, CA, USA, 2017; Volume 2, pp. 237–278. [Google Scholar]
- Wiciński, M.; Sawicka, E.; Gębalski, J.; Kubiak, K.; Malinowski, B. Human Milk Oligosaccharides: Health Benefits, Potential Applications in Infant Formulas, and Pharmacology. Nutritiens 2020, 12, 266. [Google Scholar] [CrossRef]
- Van Leeuwen, S.S.; Poele, E.M.T.; Chatziioannou, A.C.; Benjamins, E.; Haandrikman, A.; Dijkhuizen, L. Goat Milk Oligosaccharides: Their Diversity, Quantity, and Functional Properties in Comparison to Human Milk Oligosaccharides. J. Agric. Food Chem. 2020, 68, 13469–13485. [Google Scholar] [CrossRef] [PubMed]
- Goh, Y.J.; Klaenhammer, T.R. Genetic Mechanisms of Prebiotic Oligosaccharide Metabolism in Probiotic Microbes. Annu. Rev. Food Sci. Technol. 2015, 6, 137–156. [Google Scholar] [CrossRef]
- Kostopoulos, I.; Elzinga, J.; Ottman, N.; Klievink, J.T.; Blijenberg, B.; Aalvink, S.; Boeren, S.; Mank, M.; Knol, J.; De Vos, W.M.; et al. Akkermansia muciniphila uses human milk oligosaccharides to thrive in the early life conditions in vitro. Sci. Rep. 2020, 10, 14330. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Wu, J.H.Y. Flavonoids, Dairy Foods, and Cardiovascular and Metabolic Health: A Review of Emerging Biologic Pathways. Circ. Res. 2018, 122, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Quinn, E.M.; Slattery, H.; Walsh, D.; Joshi, L.; Hickey, R.M. Bifidobacterium longum subsp. infantis ATCC 15697 and Goat Milk Oligosaccharides Show Synergism In Vitro as Anti-Infectives against Campylobacter jejuni. Foods 2020, 9, 348. [Google Scholar] [CrossRef]
- Leong, A.; Liu, Z.; Almshawit, H.; Zisu, B.; Pillidge, C.; Rochfort, S.; Gill, H. Oligosaccharides in goats’ milk-based infant formula and their prebiotic and anti-infection properties. Br. J. Nutr. 2019, 122, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.Q.; Li, S.; Li, Y.P.; Wu, W.J.; Lee, S.M.; Yan, R. The Presystemic Interplay between Gut Microbiota and Orally Administered Calycosin-7-O-beta-D-Glucoside. Drug Metab. Dispos. 2015, 43, 1601–1611. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T.; et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 1829. [Google Scholar] [CrossRef]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.P.; Anastasovska, J.; Ghourab, S.; Hankir, M.K.; Zhang, S.; et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef]
- Besten, G.D.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
| Properties | Kind of Milk | ||||
|---|---|---|---|---|---|
| Cow’s | Sheep’s | Buffalo’s | Goat’s | Mixed | |
| Dry matter (%) | |||||
| Full-fat milk | 11.8 | 16.5 | 16.0 | 11.0 | 13.0 |
| Low-fat milk | 10.3 | - | - | - | - |
| Milk protein, % | 3.2 | 5.2 | 4.2 | 3.0 | 4.0 |
| Fat (%) | |||||
| Full-fat milk | 3.6 | 6.5 | 7.0 | 3.0 | 5.0 |
| Low-fat milk | 2.0 | - | - | - | - |
| Total (Thörner) acidity (°T) | 90.0–150.0 | ||||
| Storage temperature (°C) | 2.0–6.0 | ||||
| Properties | Type of Bulgarian White Brined Cheese | ||||
|---|---|---|---|---|---|
| Cow’s | Sheep’s | Buffalo’s | Goat’s | Mixed | |
| Dry matter (%) 1 | 46.0 | 48.0 | 48.0 | 48.0 | 46.0 |
| Fat in dry matter (%) 1 | 44.0 | 48.0 | 48.0 | 44.0 | 45.0 |
| Total (Thörner) acidity (°T) | |||||
| Cheese | 200–270 | ||||
| Brine | 160–180 | ||||
| Salt (%) | |||||
| Cheese | 3.5 ± 0.5 | ||||
| Brine | 6–10 | ||||
| Ripening (%) 1,2 | 14.0 | 16.0 | 14.0 | 14.0 | 14.0 |
| Energy value (kcal/100 g) 1 | 264 | 287 | 287 | 264 | 269 |
| Properties | Kind of milk | ||||
|---|---|---|---|---|---|
| Cow’s | Sheep’s | Buffalo’s | Goat’s | Mixed | |
| Dry matter (%) 1 | 56.0 | 58.0 | 56.0 | 58.0 | 57.0 |
| Fat in dry matter (%) 1 | 45.0 | 50.0 | 45.0 | 50.0 | 46.0 |
| Preservatives | Not present | ||||
| Emulsifiers and stabilizers | Not present | ||||
| Salt (%) | 1.8–3.0 | ||||
| Ripening (%) 1,2 | 20.0 | 22.0 | 20.0 | 22.0 | 20.0 |
| Energy value, kcal/100g | 335 | 363 | 335 | 363 | 344 |
| Energy value, kJ/100 g | 1402 | 1519 | 1402 | 1519 | 1439 |
| Parameter | Month 1 | Month 12 after Storage | |||
|---|---|---|---|---|---|
| 4.0 °C | 1.0 °C | −7.5 °C | −18.0 °C | ||
| Moisture content (%) | 41.9 | 41.4 | 41.3 | 41.4 | 41.2 |
| Protein content (%) | 21.8 | 21.9 | 21.9 | 22.0 | 22.2 |
| Fat in dry matter (%) | Without significant change, in the range 34.5–35.1 | ||||
| NaCl (%) | 2.10 | 2.20 | 2.10 | 2.20 | 2.20 |
| pH | 5.55 | 5.4 | 5.55 | 5.55 | 5.55 |
| Total acidity (°T) | 175.0 | 212.0 | 178.0 | 176.0 | 176.0 |
| WSN/TN (%) 1 | 13.88 | 29.55 | 21.28 | 16.13 | 14.44 |
| NPN/TN (%) 1 | 9.97 | 16.02 | 12.26 | 10.36 | 10.08 |
| Parameter | Kefir | Koumiss |
|---|---|---|
| Milk protein | 2.7% | ~3% |
| Milk fat | <10% | ~2% |
| Lactic acid | <0.6% | 0.7–1.8% |
| Ethanol | – | 0.6–2.5% (v/w) |
| Starter culture (CFU/g) | 1 × 107 | 1 × 107 |
| Yeasts (CFU/g) | 1 × 104 | 1 × 104 |
| Product | Free Amino Acids | Bioactive Peptides | Antioxidants | EPS | Prebiotics | Reference |
|---|---|---|---|---|---|---|
| Yoghurt | L. bulgaricus L. helveticus L. fermentum L. paracasei P. acidilactici | L. bulgaricus L. helveticus L. casei L. acidophilus S. thermophilus | L. helveticus Leuc. mesenteroides P. acidilactici | L. bulgaricus L. helveticus L. fermentum L. rhamnosus S. thermophilus W. confusa | L. bulgaricus | [10,41,62,72,73,74,75,86,96,97] |
| Cheese | L. bulgaricus L. helveticus | L. rhamnosus L. bulgaricus Lc. lactis ssp. lactis Ent. faecalis Ent. faecium L. plantarum L. casei | L. helveticus | [44,69,70] | ||
| Katak | L. bulgaricus L. helveticus | L. brevis | L. bulgaricus L. helveticus | [71] | ||
| Kefir | L. helveticus | L. bulgaricus | L. kefiranofaciens | [66,71] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrova, P.; Ivanov, I.; Tsigoriyna, L.; Valcheva, N.; Vasileva, E.; Parvanova-Mancheva, T.; Arsov, A.; Petrov, K. Traditional Bulgarian Dairy Products: Ethnic Foods with Health Benefits. Microorganisms 2021, 9, 480. https://doi.org/10.3390/microorganisms9030480
Petrova P, Ivanov I, Tsigoriyna L, Valcheva N, Vasileva E, Parvanova-Mancheva T, Arsov A, Petrov K. Traditional Bulgarian Dairy Products: Ethnic Foods with Health Benefits. Microorganisms. 2021; 9(3):480. https://doi.org/10.3390/microorganisms9030480
Chicago/Turabian StylePetrova, Penka, Ivan Ivanov, Lidia Tsigoriyna, Nadezhda Valcheva, Evgenia Vasileva, Tsvetomila Parvanova-Mancheva, Alexander Arsov, and Kaloyan Petrov. 2021. "Traditional Bulgarian Dairy Products: Ethnic Foods with Health Benefits" Microorganisms 9, no. 3: 480. https://doi.org/10.3390/microorganisms9030480
APA StylePetrova, P., Ivanov, I., Tsigoriyna, L., Valcheva, N., Vasileva, E., Parvanova-Mancheva, T., Arsov, A., & Petrov, K. (2021). Traditional Bulgarian Dairy Products: Ethnic Foods with Health Benefits. Microorganisms, 9(3), 480. https://doi.org/10.3390/microorganisms9030480








