Membrane Association and Topology of Citrus Leprosis Virus C2 Movement and Capsid Proteins
Abstract
1. Introduction
2. Materials and Methods
2.1. DNA Manipulation
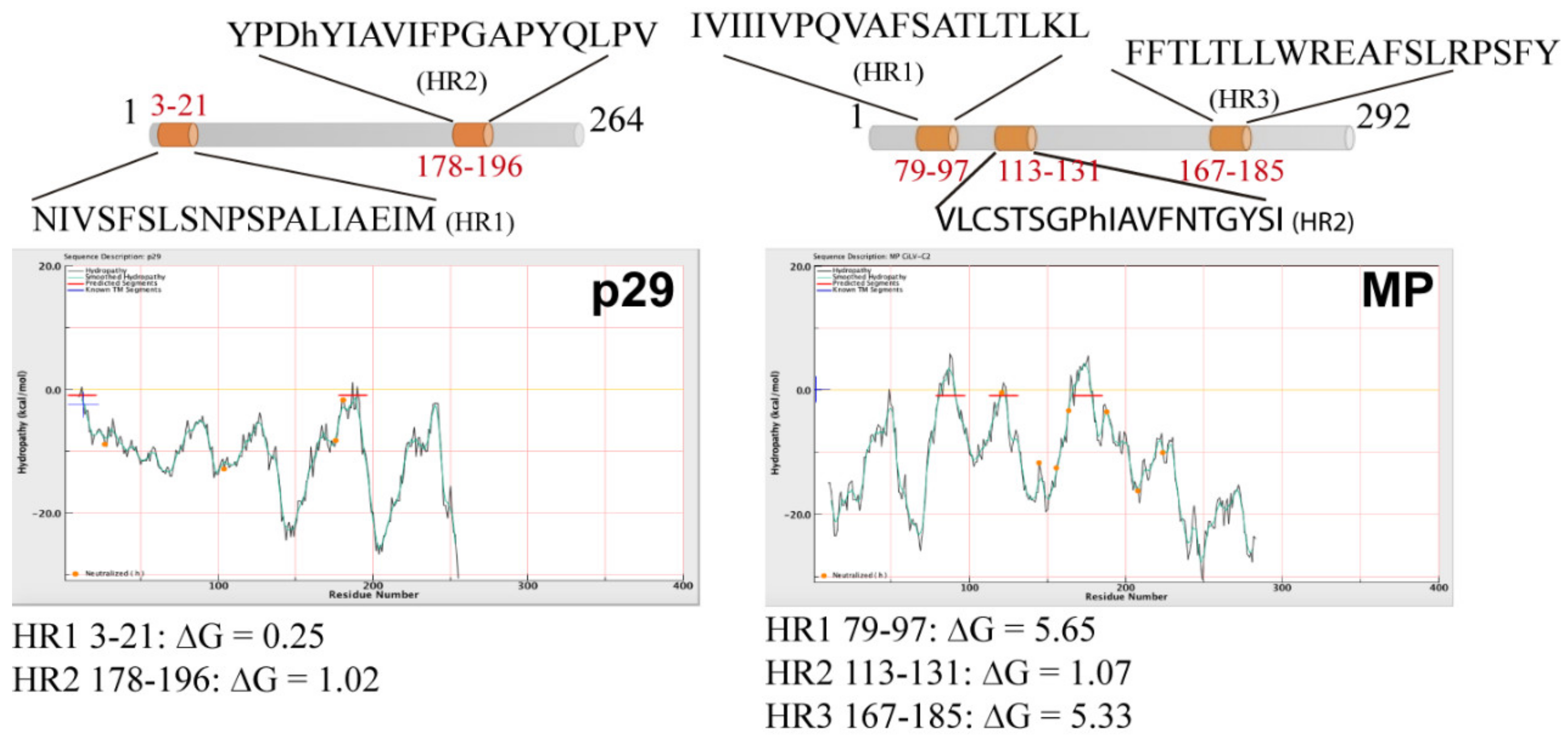
2.2. Computer Analysis
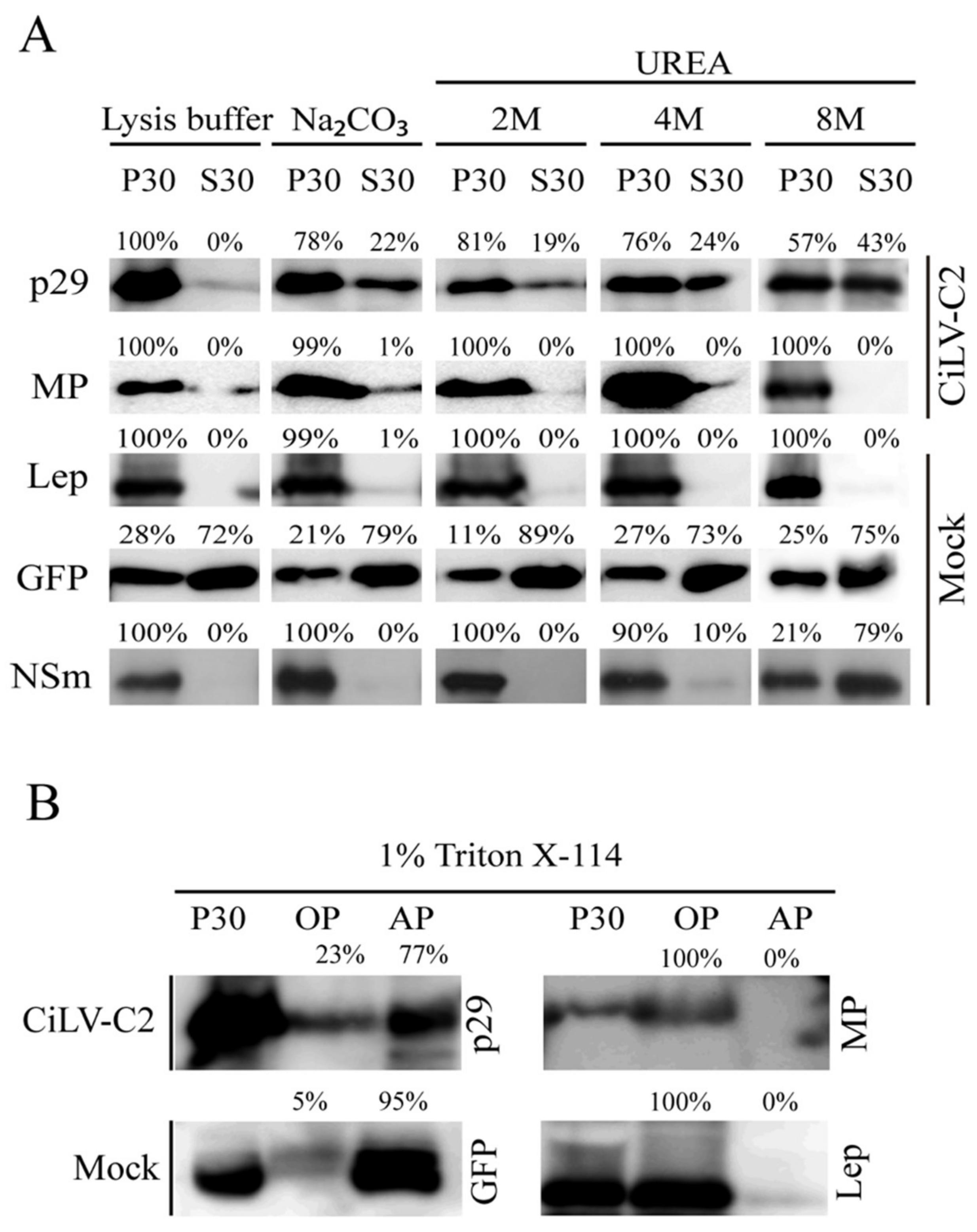
2.3. Subcellular Fractionation, Chemical Treatment, and Western Blot Analyses
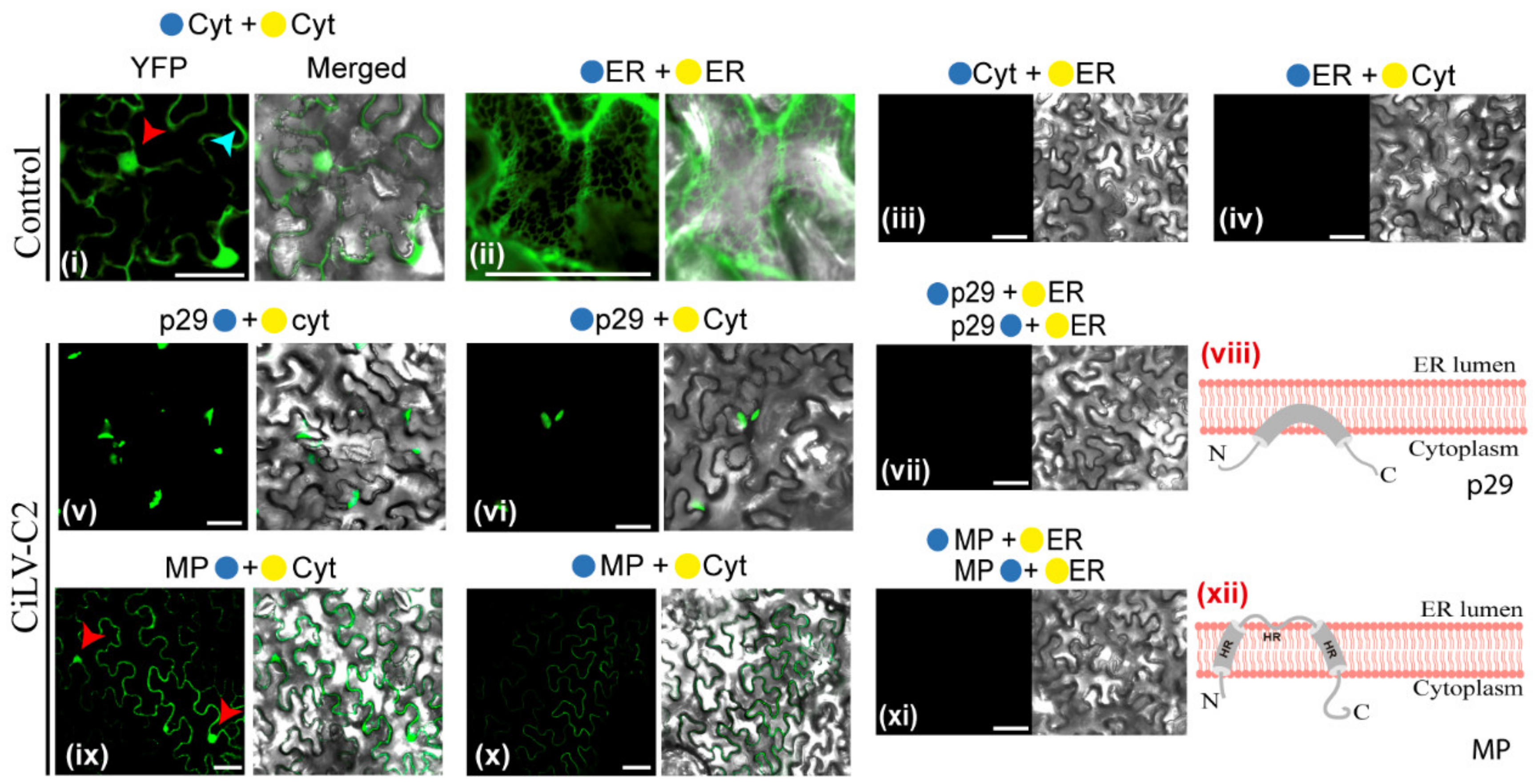
2.4. Bimolecular Fluorescence Complementation Assays
2.5. Confocal Laser Scanning Microscopy
3. Results and Discussion
3.1. The CiLV-C2 p29 and MP Are Membrane-Associated Proteins
3.2. The CiLV-C2 p29 and MP Have the N- and C-Termini Exposed to the Cell Cytoplasmic Compartment
3.3. The MPs of CiLV-C2 and CiLV-C Could Have a Similar Membrane Topology
3.4. Cilevirus MPs Are the First Members of the 30K Superfamily Showing a Transmembrane Association Pattern
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethical Approval
References
- Bastianel, M.; Novelli, V.; Kitajima, E.; Kubo, K.; Bassanezi, R.B. Citrus leprosis: Centennial of an unusual mite virus pathosystem. Plant Dis. 2010, 94, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Choudhary, N.; Guillermo, L.M.; Shao, J.; Govindarajulu, A.; Achor, D.; Wei, G.; Picton, D.D.; Levy, L.; Nakhla, M.K.; et al. A novel virus of the genus Cilevirus causing symptoms similar to citrus leprosis. Phytopathology 2013, 103, 488–500. [Google Scholar] [CrossRef]
- Roy, A.; Hartung, J.S.; Schneider, W.L.; Shao, J.; Leon, G.; Melzer, M.J.; Beard, J.J.; Otero-Colina, G.; Bauchan, G.R.; Ochoa, R.; et al. Role Bending: Complex Relationships between Viruses, Hosts, and Vectors Related to Citrus Leprosis, an Emerging Disease. Phytopathology 2015, 105, 1013–1025. [Google Scholar] [CrossRef] [PubMed]
- Freitas-Astua, J.; Ramos-Gonzalez, P.L.; Arena, G.D.; Tassi, A.D.; Kitajima, E.W. Brevipalpus-transmitted viruses: Parallelism beyond a common vector or convergent evolution of distantly related pathogens? Curr. Opin. Virol. 2018, 33, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.G.; Becerra, C.H.; Freitas-Astua, J.; Salaroli, R.B.; Kitajima, E.W. Natural Infection of Swinglea glutinosa by the Citrus leprosis virus Cytoplasmic Type (CiLV-C) in Colombia. Plant Dis. 2008, 92, 1364. [Google Scholar] [CrossRef] [PubMed]
- Pascon, R.C.; Kitajima, J.P.; Breton, M.C.; Assumpcao, L.; Greggio, C.; Zanca, A.S.; Okura, V.K.; Alegria, M.C.; Camargo, M.E.; Silva, G.G.; et al. The complete nucleotide sequence and genomic organization of Citrus Leprosis associated Virus, Cytoplasmatic type (CiLV-C). Virus Genes 2006, 32, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Leastro, M.O.; Kitajima, E.W.; Silva, M.S.; Resende, R.O.; Freitas-Astua, J. Dissecting the Subcellular Localization, Intracellular Trafficking, Interactions, Membrane Association, and Topology of Citrus Leprosis Virus C Proteins. Front. Plant Sci. 2018, 9, 1299. [Google Scholar] [CrossRef] [PubMed]
- Locali-Fabris, E.C.; Freitas-Astua, J.; Souza, A.A.; Takita, M.A.; Astua-Monge, G.; Antonioli-Luizon, R.; Rodrigues, V.; Targon, M.L.; Machado, M.A. Complete nucleotide sequence, genomic organization and phylogenetic analysis of Citrus leprosis virus cytoplasmic type. J. Gen. Virol. 2006, 87, 2721–2729. [Google Scholar] [CrossRef]
- Leastro, M.O.; Castro, D.Y.O.; Freitas-Astua, J.; Kitajima, E.W.; Pallas, V.; Sanchez-Navarro, J.A. Citrus Leprosis Virus C Encodes Three Proteins with Gene Silencing Suppression Activity. Front. Microbiol. 2020, 11, 1231. [Google Scholar] [CrossRef]
- Leastro, M.O.; Freitas-Astua, J.; Kitajima, E.W.; Pallas, V.; Sanchez-Navarro, J. Unravelling the involvement of cilevirus p32 protein in the viral transport. Sci. Rep. 2021, 11, 2943. [Google Scholar] [CrossRef]
- Kuchibhatla, D.B.; Sherman, W.A.; Chung, B.Y.; Cook, S.; Schneider, G.; Eisenhaber, B.; Karlin, D.G. Powerful sequence similarity search methods and in-depth manual analyses can identify remote homologs in many apparently “orphan” viral proteins. J. Virol. 2014, 88, 10–20. [Google Scholar] [CrossRef]
- Laliberte, J.F.; Sanfacon, H. Cellular remodeling during plant virus infection. Annu. Rev. Phytopathol. 2010, 48, 69–91. [Google Scholar] [CrossRef]
- Schwartz, M.; Chen, J.; Lee, W.M.; Janda, M.; Ahlquist, P. Alternate, virus-induced membrane rearrangements support positive-strand RNA virus genome replication. Proc. Natl. Acad. Sci. USA 2004, 101, 11263–11268. [Google Scholar] [CrossRef] [PubMed]
- Genovés, A.; Pallás, V.; Navarro, J.A. Contribution of topology determinants of a viral movement protein to its membrane association, intracellular traffic, and viral cell-to-cell movement. J. Virol. 2011, 85, 7797–7809. [Google Scholar] [CrossRef] [PubMed]
- Schepetilnikov, M.V.; Solovyev, A.G.; Gorshkova, E.N.; Schiemann, J.; Prokhnevsky, A.I.; Dolja, V.V.; Morozov, S.Y. Intracellular targeting of a hordeiviral membrane-spanning movement protein: Sequence requirements and involvement of an unconventional mechanism. J. Virol. 2008, 82, 1284–1293. [Google Scholar] [CrossRef][Green Version]
- Martinez-Gil, L.; Johnson, A.E.; Mingarro, I. Membrane insertion and biogenesis of the Turnip crinkle virus p9 movement protein. J. Virol. 2010, 84, 5520–5527. [Google Scholar] [CrossRef] [PubMed]
- Pitzalis, N.; Heinlein, M. The roles of membranes and associated cytoskeleton in plant virus replication and cell-to-cell movement. J. Exp. Bot. 2017, 69, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Leastro, M.O.; Pallas, V.; Resende, R.O.; Sanchez-Navarro, J.A. The movement proteins (NSm) of distinct tospoviruses peripherally associate with cellular membranes and interact with homologous and heterologous NSm and nucleocapsid proteins. Virology 2015, 478, 39–49. [Google Scholar] [CrossRef]
- Peiro, A.; Martinez-Gil, L.; Tamborero, S.; Pallas, V.; Sanchez-Navarro, J.A.; Mingarro, I. The Tobacco mosaic virus movement protein associates with but does not integrate into biological membranes. J. Virol. 2014, 88, 3016–3026. [Google Scholar] [CrossRef] [PubMed]
- Peremyslov, V.V.; Pan, Y.W.; Dolja, V.V. Movement protein of a closterovirus is a type III integral transmembrane protein localized to the endoplasmic reticulum. J. Virol. 2004, 78, 3704–3709. [Google Scholar] [CrossRef]
- Martínez-Gil, L.; Sánchez-Navarro, J.A.; Cruz, A.; Pallás, V.; Pérez-Gil, J.; Mingarro, I. Plant virus cell-to-cell movement is not dependent on the transmembrane disposition of its movement protein. J. Virol. 2009, 83, 5535–5543. [Google Scholar] [CrossRef]
- Leastro, M.O.; Pallas, V.; Resende, R.O.; Sanchez-Navarro, J.A. The functional analysis of distinct tospovirus movement proteins (NSM) reveals different capabilities in tubule formation, cell-to-cell and systemic virus movement among the tospovirus species. Virus. Res. 2017, 227, 57–68. [Google Scholar] [CrossRef]
- Snider, C.; Jayasinghe, S.; Hristova, K.; White, S.H. MPEx: A tool for exploring membrane proteins. Protein Sci. Publ. Protein Soc. 2009, 18, 2624–2628. [Google Scholar] [CrossRef]
- Leastro, M.O.; Freitas-Astua, J.; Kitajima, E.W.; Pallas, V.; Sanchez-Navarro, J.A. Dichorhaviruses Movement Protein and Nucleoprotein form a Protein Complex That May be Required for Virus Spread and Interacts in vivo with Viral Movement-Related Cilevirus Proteins. Front. Microbiol. 2020, 11, 571807. [Google Scholar] [CrossRef] [PubMed]
- Bordier, C. Phase separation of integral membrane proteins in Triton X-114 solution. J. Biol. Chem. 1981, 256, 1604–1607. [Google Scholar] [CrossRef]
- Baeza-Delgado, C.; Marti-Renom, M.A.; Mingarro, I. Structure-based statistical analysis of transmembrane helices. Eur. Biophys. J. EBJ 2013, 42, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Ojemalm, K.; Higuchi, T.; Lara, P.; Lindahl, E.; Suga, H.; von Heijne, G. Energetics of side-chain snorkeling in transmembrane helices probed by nonproteinogenic amino acids. Proc. Natl. Acad. Sci. USA 2016, 113, 10559–10564. [Google Scholar] [CrossRef]
- Sauri, A.; Tamborero, S.; Martinez-Gil, L.; Johnson, A.E.; Mingarro, I. Viral membrane protein topology is dictated by multiple determinants in its sequence. J. Mol. Biol. 2009, 387, 113–128. [Google Scholar] [CrossRef]
- Nilsson, I.; von Heijne, G. Fine-tuning the topology of a polytopic membrane protein: Role of positively and negatively charged amino acids. Cell 1990, 62, 1135–1141. [Google Scholar] [CrossRef]
- Mushegian, A.R.; Elena, S.F. Evolution of plant virus movement proteins from the 30K superfamily and of their homologs integrated in plant genomes. Virology 2015, 476, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Melcher, U. The ‘30K’ superfamily of viral movement proteins. J. Gen. Virol. 2000, 81, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Lin, W.; Sun, K.; Wang, S.; Zhou, X.; Jackson, A.O.; Li, Z. Specificity of Plant Rhabdovirus Cell-to-Cell Movement. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leastro, M.O.; Freitas-Astúa, J.; Kitajima, E.W.; Pallás, V.; Sánchez-Navarro, J.Á. Membrane Association and Topology of Citrus Leprosis Virus C2 Movement and Capsid Proteins. Microorganisms 2021, 9, 418. https://doi.org/10.3390/microorganisms9020418
Leastro MO, Freitas-Astúa J, Kitajima EW, Pallás V, Sánchez-Navarro JÁ. Membrane Association and Topology of Citrus Leprosis Virus C2 Movement and Capsid Proteins. Microorganisms. 2021; 9(2):418. https://doi.org/10.3390/microorganisms9020418
Chicago/Turabian StyleLeastro, Mikhail Oliveira, Juliana Freitas-Astúa, Elliot Watanabe Kitajima, Vicente Pallás, and Jesús Á. Sánchez-Navarro. 2021. "Membrane Association and Topology of Citrus Leprosis Virus C2 Movement and Capsid Proteins" Microorganisms 9, no. 2: 418. https://doi.org/10.3390/microorganisms9020418
APA StyleLeastro, M. O., Freitas-Astúa, J., Kitajima, E. W., Pallás, V., & Sánchez-Navarro, J. Á. (2021). Membrane Association and Topology of Citrus Leprosis Virus C2 Movement and Capsid Proteins. Microorganisms, 9(2), 418. https://doi.org/10.3390/microorganisms9020418







