Identification of Trypanosoma cruzi Growth Inhibitors with Activity In Vivo within a Collection of Licensed Drugs
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Drugs
2.3. Host Cells Cultures
2.4. Culture of Parasites
2.5. T. cruzi Growth Inhibition Assay
2.6. Anti-Amastigote Specific Assay
2.7. NIH-3T3 Cells-Based Assays
2.8. Vero and HepG2 Toxicity Assays
2.9. T. cruzi In Vivo Inhibition Assay
2.10. Data Analysis
3. Results
3.1. Anti-T. cruzi Activity on Vero Cells
3.2. Identification of Drugs with Specific Activity against the Parasite
3.3. Anti-T. cruzi Growth Inhibition of Active Drugs Confirmed
3.4. HepG2 Toxicity Assay
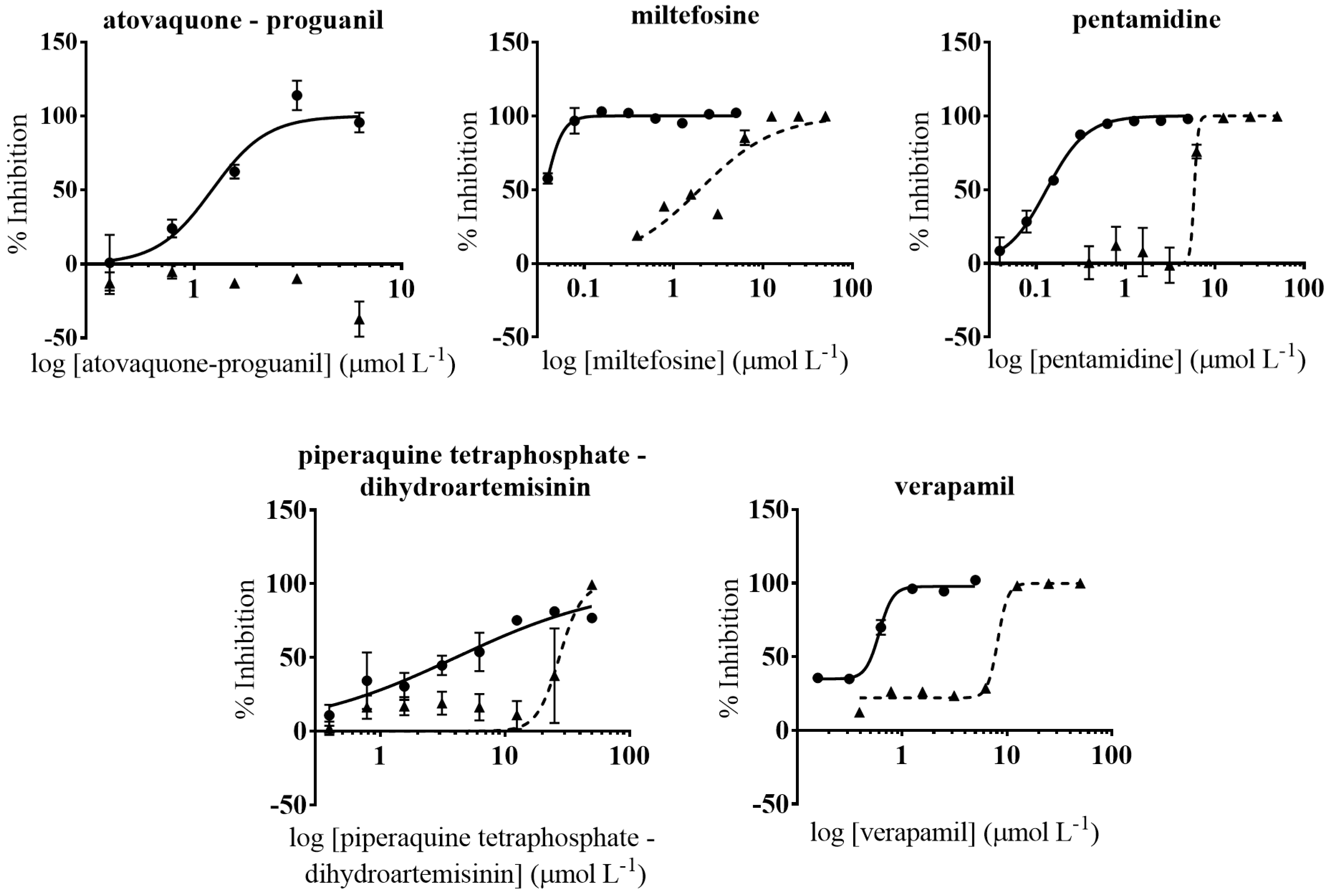
3.5. Anti-Amastigote Specific Activity of Selected Drugs
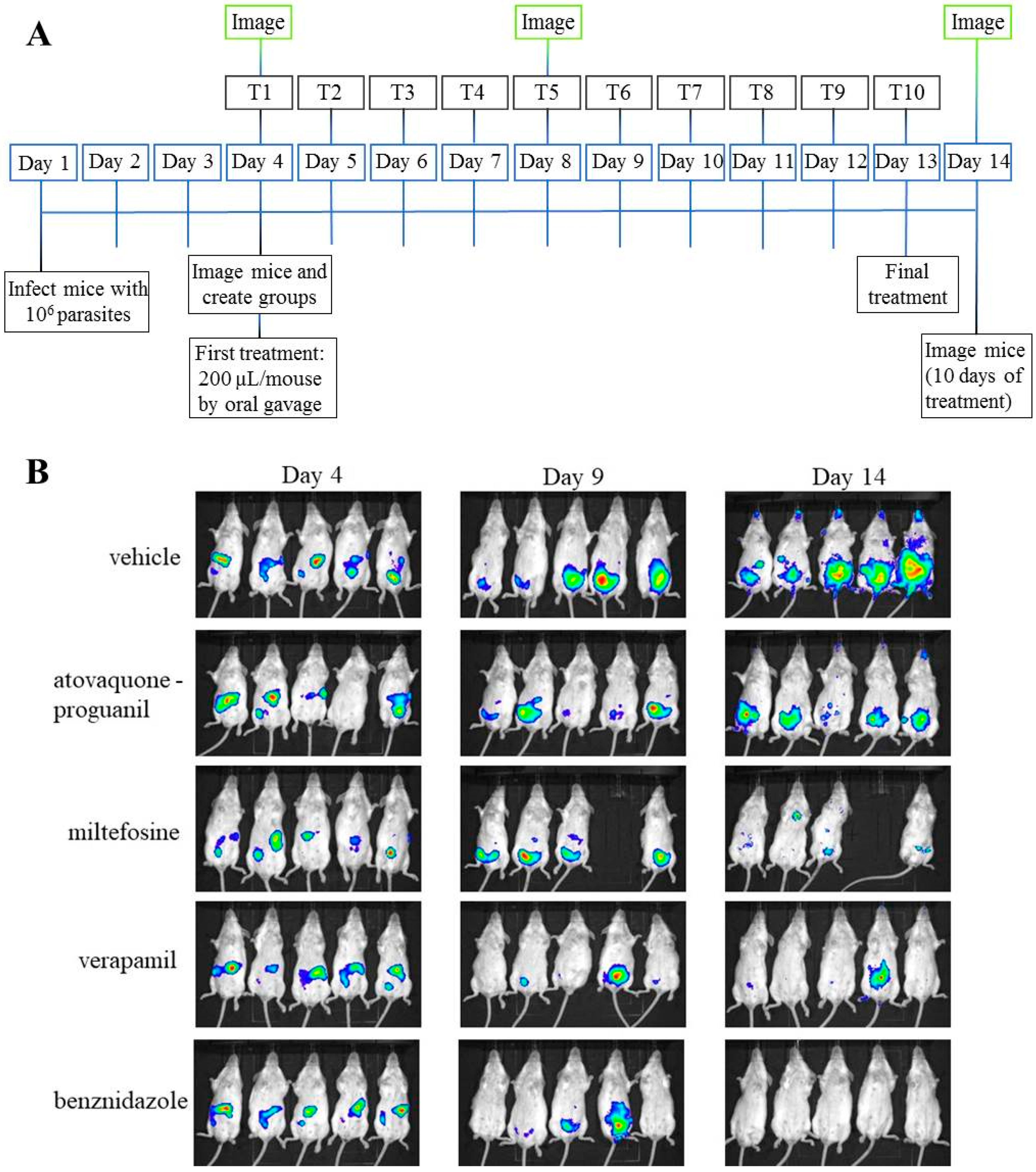
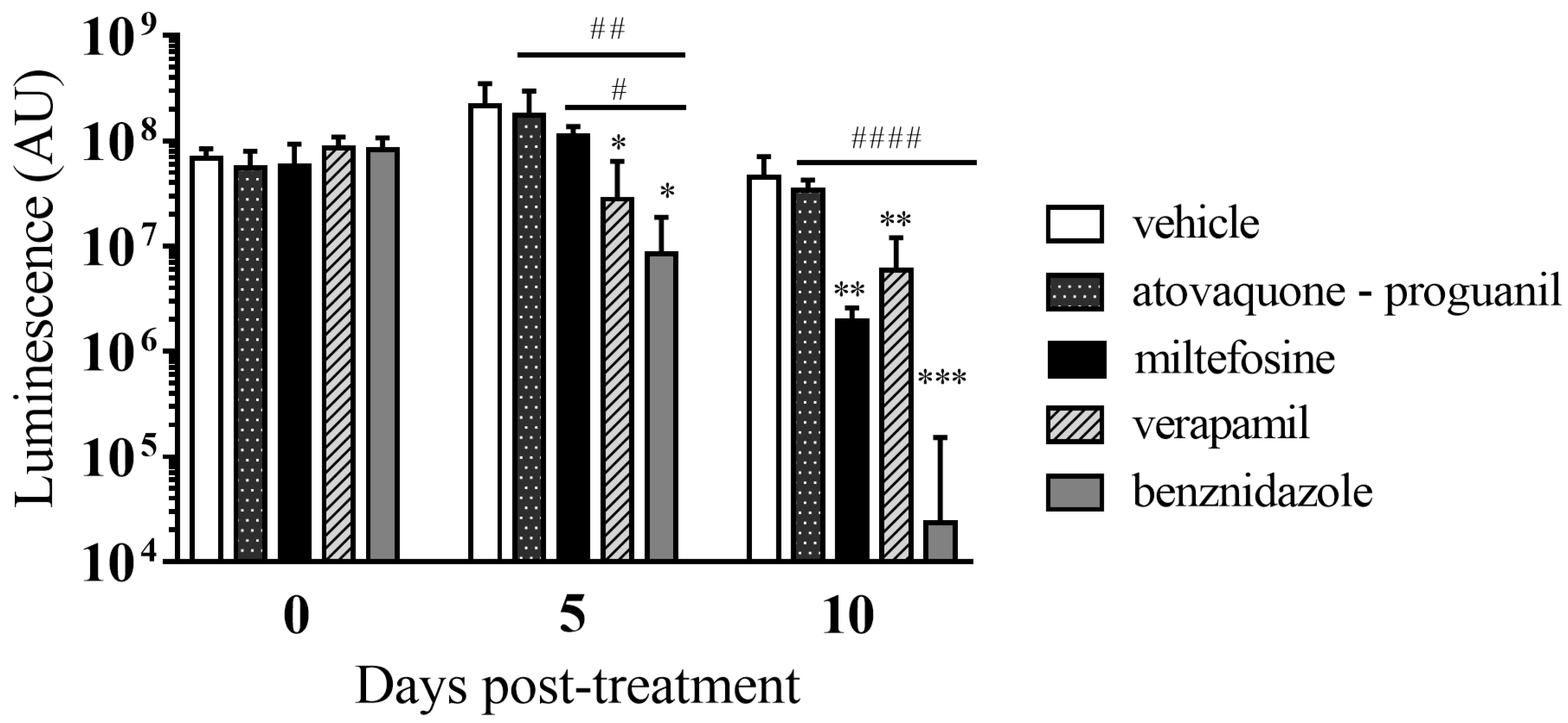
3.6. In Vivo Anti-T. cruzi Activity of the Selected Drugs in a Mouse Model of Acute Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO: Chagas Disease (American Trypanosomiasis). Available online: https://www.who.int/en/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis) (accessed on 26 January 2020).
- Gascon, J.; Bern, C.; Pinazo, M.J. Chagas disease in Spain, the United States and other non-endemic countries. Acta Trop. 2010, 115, 22–27. [Google Scholar] [CrossRef]
- Pérez-Molina, J.A.; Molina, I. Chagas disease. Lancet 2018, 391, 82–94. [Google Scholar] [CrossRef]
- Fabbro, D.L.; Streiger, M.L.; Arias, E.D.; Bizai, M.L.; Del Barco, M.; Amicone, N.A. Trypanocide treatment among adults with chronic Chagas disease living in Santa Fe City (Argentina), over a mean follow-up of 21 years: Parasitological, serological and clinical evolution. Rev. Soc. Bras. Med. Trop. 2007, 40, 1–10. [Google Scholar] [CrossRef]
- Alonso-Padilla, J.; Cortés-Serra, N.; Pinazo, M.J.; Elena, M.; Abril, M.; Barreira, F.; Sosa-Estani, S.; Hotez, P.J.; Gascón, J. Strategies to enhance access to diagnosis and treatment for Chagas disease patients in Latin America. Expert Rev. Anti. Infect. Ther. 2019, 17, 145–157. [Google Scholar] [CrossRef]
- Crespillo, C.; Emmanuele, A.; Rullo, V.; López, R.; Begoña, V.; Maillo, M. Safety profile of benznidazole in the treatment of chronic Chagas disease: Experience of a referral centre and systematic literature review with meta-analysis. Drug Saf. 2018, 41, 1035–1048. [Google Scholar] [CrossRef]
- Forsyth, C.J.; Hernandez, S.; Olmedo, W.; Abuhamidah, A.; Traina, M.I.; Sanchez, D.R.; Soverow, J.; Meymandi, S.K. Safety profile of nifurtimox for treatment of Chagas disease in the United States. Clin. Infect. Dis. 2016, 63, 1056–1062. [Google Scholar] [CrossRef]
- Pinazo, M.J.; Muñoz, J.; Posada, E.; López-Chejade, P.; Gállego, M.; Ayala, E.; Del Cacho, E.; Soy, D.; Gascon, J. Tolerance of benznidazole in treatment of Chagas’ disease in adults. Antimicrob. Agents Chemother. 2010, 54, 4896–4899. [Google Scholar] [CrossRef]
- Jackson, Y.; Alirol, E.; Getaz, L.; Wolff, H.; Combescure, C.; Chappuis, F. Tolerance and safety of nifurtimox in patients with chronic Chagas disease. Clin. Infect. Dis. 2010, 51, e69–e75. [Google Scholar] [CrossRef]
- Urbina, J.A. New insights in Chagas’ disease treatment. Drugs Future 2010, 35, 409–419. [Google Scholar] [CrossRef]
- Khare, S.; Nagle, A.S.; Biggart, A.; Lai, Y.H.; Liang, F.; Davis, L.C.; Barnes, S.W.; Mathison, C.J.N.; Myburgh, E.; Gao, M.Y.; et al. Proteasome inhibition for treatment of leishmaniasis, Chagas disease and sleeping sickness. Nature 2016, 537, 229–233. [Google Scholar] [CrossRef]
- Peña, I.; Pilar Manzano, M.; Cantizani, J.; Kessler, A.; Alonso-Padilla, J.; Bardera, A.I.; Alvarez, E.; Colmenarejo, G.; Cotillo, I.; Roquero, I.; et al. New compound sets identified from high throughput phenotypic screening against three kinetoplastid parasites: An open resource. Sci. Rep. 2015, 5, 8771. [Google Scholar]
- Martínez-Peinado, N.; Cortes-Serra, N.; Losada-Galvan, I.; Alonso-Vega, C.; Urbina, J.A.; Rodríguez, A.; VandeBerg, J.L.; Pinazo, M.J.; Gascon, J.; Alonso-Padilla, J. Emerging agents for the treatment of Chagas disease: What is in the preclinical and clinical development pipeline? Expert Opin. Investig. Drugs 2020, 9, 947–959. [Google Scholar] [CrossRef]
- Planer, J.D.; Hulverson, M.A.; Arif, J.A.; Ranade, R.M.; Don, R.; Buckner, F.S. Synergy testing of FDA-approved drugs identifies potent drug combinations against Trypanosoma cruzi. PLoS Negl. Trop. Dis. 2014, 8, e2977. [Google Scholar] [CrossRef] [PubMed]
- Berenstein, A.J.; Magariños, M.P.; Chernomoretz, A.; Agüero, F. A multilayer network approach for guiding drug repositioning in neglected diseases. PLoS Negl. Trop. Dis. 2016, 10, 1–33. [Google Scholar] [CrossRef]
- Simões-Silva, M.R.; De Araújo, J.S.; Oliveira, G.M.; Demarque, K.C.; Peres, R.B.; D’Almeida-Melo, I.; Batista, D.G.J.; Da Silva, C.F.; Cardoso-Santos, C.; Da Silva, P.B.; et al. Drug repurposing strategy against Trypanosoma cruzi infection: In vitro and in vivo assessment of the activity of metronidazole in mono- and combined therapy. Biochem. Pharmacol. 2017, 145, 46–53. [Google Scholar]
- Ferreira, D.D.; Mesquita, J.T.; Da Costa Silva, T.A.; Romanelli, M.M.; Da Gama Jaen Batista, D.; Da Silva, C.F.; Da Gama, A.N.S.; Neves, B.J.; Melo-Filho, C.C.; Correia Soeiro, M.D.N.; et al. Efficacy of sertraline against Trypanosoma cruzi: An in vitro and in silico study. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 30. [Google Scholar] [CrossRef]
- Kaiser, M.; Mäser, P.; Tadoori, L.P.; Ioset, J.R.; Brun, R.; Sullivan, D.J. Antiprotozoal activity profiling of approved drugs: A starting point toward drug repositioning. PLoS One 2015, 10, e0135556. [Google Scholar] [CrossRef]
- Alonso-Vega, C.; Losada-Galván, I.; Pinazo, M.J.; Sancho Mas, J.; Brustenga, J.G.; Alonso-Padilla, J. The senseless orphanage of Chagas Disease. Expert Opin. Orphan Drugs 2019, 7, 535–545. [Google Scholar] [CrossRef]
- Molina, I.; Goḿez I Prat, J.; Salvador, F.; Treviño, B.; Sulleiro, E.; Serre, N.; Pou, D.; Roure, S.; Cabezos, J.; Valerio, L.; et al. Randomized trial of posaconazole and benznidazole for chronic Chagas’ disease. N. Engl. J. Med. 2014, 370, 1899–1908. [Google Scholar] [CrossRef]
- Torrico, F.; Gascon, J.; Ortiz, L.; Alonso-Vega, C.; Pinazo, M.J.; Schijman, A.; Almeida, I.C.; Alves, F.; Strub-Wourgaft, N.; Ribeiro, I.; et al. Treatment of adult chronic indeterminate Chagas disease with benznidazole and three E1224 dosing regimens: A proof-of-concept, randomised, placebo-controlled trial. Lancet Infect. Dis. 2018, 18, 419–430. [Google Scholar] [CrossRef]
- Mosca, J.D.; Pitha, P.M. Transcriptional and posttranscriptional regulation of exogenous human beta interferon gene in simian cells defective in interferon synthesis. Mol. Cell. Biol. 1986, 6, 2279–2283. [Google Scholar] [CrossRef]
- Bettiol, E.; Samanovic, M.; Murkin, A.S.; Raper, J.; Buckner, F.; Rodriguez, A. Identification of three classes of heteroaromatic compounds with activity against intracellular Trypanosoma cruzi by chemical library screening. PLoS Negl. Trop. Dis. 2009, 3, e384. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.M.; Oh, S.J.; Lee, S.Y.; Im, J.H.; Oh, J.M.; Ryu, C.S.; Kwak, H.C.; Lee, J.Y.; Kang, K.W.; Kim, S.K. HepG2 cells as an in vitro model for evaluation of cytochrome P450 induction by xenobiotics. Arch. Pharm. Res. 2015, 38, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Buckner, F.S.; Verlinde, C.L.M.J.; La Flamme, A.C.; Van Voorhis, W.C. Efficient technique for screening drugs for activity against Trypanosoma cruzi using parasites expressing β-Galactosidase. Antimicrob. Agents Chemother. 1996, 40, 2592–2597. [Google Scholar] [CrossRef] [PubMed]
- Reimão, J.Q.; Scotti, M.T.; Tempone, A.G. Anti-leishmanial and anti-trypanosomal activities of 1,4-dihydropyridines: In vitro evaluation and structure-activity relationship study. Bioorganic Med. Chem. 2010, 18, 8044–8053. [Google Scholar] [CrossRef]
- Borges Migliavaca, C.; Stein, C.; Colpani, V.; René Pinto de Sousa Miguel, S.; Nascimento Cruz, L.; Oliveira Dantas, R.; Falavigna, M. Isosorbide and nifedipine for Chagas’ megaesophagus: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2018, 12, 1–13. [Google Scholar] [CrossRef]
- De Rycker, M.; Thomas, J.; Riley, J.; Brough, S.J.; Miles, T.J.; Gray, D.W. Identification of trypanocidal activity for known clinical compounds using a new Trypanosoma cruzi hit-discovery screening cascade. PLoS Negl. Trop. Dis. 2016, 10, e0004584. [Google Scholar] [CrossRef]
- Paolini, E.; Stronati, G.; Guerra, F.; Capucci, A. Flecainide: Electrophysiological properties, clinical indications, and practical aspects. Pharmacol. Res. 2019, 148, 10443. [Google Scholar]
- Quiros, F.R.; Morillo, C.A.; Casas, J.P.; Cubillos, L.A.; Silva, F.A. Charity: Chagas cardiomyopathy bisoprolol intervention study: A randomized double-blind placebo force-titration controlled study with bisoprolol in patients with chronic heart failure secondary to Chagas cardiomyopathy [NCT00323973]. Trials 2006, 7, 21. [Google Scholar] [CrossRef]
- Stein, C.; Castanotto, D.; Krishnan, A.; Nikolaenko, L. Defibrotide (Defitelio): A new addition to the stockpile of food and drug administration-approved oligonucleotide drugs. Molecular Therapy - Nucleic Acids. 2016, 5, e346. [Google Scholar] [CrossRef]
- Landman, G.W.D.; De Bock, G.H.; Van Hateren, K.J.J.; Van Dijk, P.R.; Groenier, K.H.; Gans, R.O.B.; Houweling, S.T.; Bilo, H.J.G.; Kleefstra, N. Safety and efficacy of gliclazide as treatment for type 2 diabetes: A systematic review and meta-analysis of randomized Trials. PLoS One 2014, 9, e82880. [Google Scholar] [CrossRef]
- Penitente, A.R.; Leite, A.L.J.; Costa, G.D.P.; Shrestha, D.; Horta, A.L.; Natali, A.J.; Neves, C.A.; Talvani, A. Enalapril in combination with benznidazole reduces cardiac inflammation and creatine kinases in mice chronically infected with Trypanosoma cruzi. Am. J. Trop. Med. Hyg. 2015, 93, 976–982. [Google Scholar] [CrossRef]
- Loo, C.S.N.; Lam, N.S.K.; Yu, D.; Su, X.Z.; Lu, F. Artemisinin and its derivatives in treating protozoan infections beyond malaria. Pharmacol. Res. 2017, 117, 192–217. [Google Scholar] [CrossRef]
- Moreira, V.R.; De Jesus, L.C.L.; Soares, R.E.P.; Silva, L.D.M.; Pinto, B.A.S.; Melo, M.N.; De AndradePaes, A.M.; Pereira, S.R.F. Meglumine antimoniate (Glucantime) causes oxidative stress-derived DNA damage in Balb/c mice infected by Leishmania (Leishmania) infantum. Antimicrob. Agents Chemother. 2017, 61, e02360-16. [Google Scholar] [CrossRef]
- Andrade, S.G.; Magalhães, L.D.A.; Pessina, D.H. Importance of TNF-α in the course of acute infection with Trypanosoma cruzi: Influence of its inhibition by pentoxifylline treatment. Mem. Inst. Oswaldo Cruz 2008, 103, 21–26. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pereira, I.R.; Vilar-Pereira, G.; Moreira, O.C.; Ramos, I.P.; Gibaldi, D.; Britto, C.; Moraes, M.O.; Lannes-Vieira, J. Pentoxifylline reverses chronic experimental chagasic cardiomyopathy in association with repositioning of abnormal CD8+ T-cell response. PLoS Negl. Trop. Dis. 2015, 9, e0003659. [Google Scholar]
- Hobbie, S.N.; Kaiser, M.; Schmidt, S.; Shcherbakov, D.; Janusic, T.; Brun, R.; Böttger, E.C. Genetic reconstruction of protozoan RRNA decoding sites provides a rationale for paromomycin activity against Leishmania and Trypanosoma. PLoS Negl. Trop. Dis. 2011, 5, e1161. [Google Scholar] [CrossRef] [PubMed]
- Croft, S.L.; Snowdon, D.; Yardley, V. The activities of four anticancer alkyllysophospholipids against Leishmania donovani, Trypanosoma cruzi and Trypanosoma brucei. J. Antimicrob. Chemother. 1996, 38, 1041–1047. [Google Scholar] [CrossRef]
- Luna, K.P.; Hernandez, I.P.; Rueda, C.M.; Zorro, M.M.; Croft, S.L.; Escobar, P. In vitro susceptibility of Trypanosoma cruzi strains from Santander, Colombia, to hexadecylphosphocholine (Miltefosine), nifurtimox and benznidazole. Biomedica 2009, 29, 448–455. [Google Scholar]
- Santa-Rita, R.M.; Santos Barbosa, H.; Meirelles, M.D.N.S.L.; De Castro, S.L. Effect of the alkyl-lysophospholipids on the proliferation and differentiation of Trypanosoma cruzi. Acta Trop. 2000, 75, 219–228. [Google Scholar]
- Saraiva, V.B.; Gibaldi, D.; Previato, J.O.; Mendonça-Previato, L.; Bozza, M.T.; Freire-de-Lima, C.G.; Heise, N. Proinflammatory and cytotoxic effects of hexadecylphosphocholine (miltefosine) against drug-resistant strains of Trypanosoma cruzi. Antimicrob. Agents Chemother. 2002, 46, 3472–3477. [Google Scholar] [CrossRef]
- Dias, J.C.P.; Schofield, C.J.; Machado, E.M.M.; Fernandes, A.J. Ticks, ivermectin, and experimental Chagas disease. Mem. Inst. Oswaldo Cruz 2005, 100, 829–832. [Google Scholar] [CrossRef][Green Version]
- Achan, J.; Talisuna, A.O.; Erhart, A.; Yeka, A.; Tibenderana, J.K.; Baliraine, F.N.; Rosenthal, P.J.; D’Alessandro, U. Quinine, an old anti-malarial drug in a modern world: Role in the treatment of malaria. Malar. J. 2011, 10, 144. [Google Scholar] [CrossRef]
- Flannery, E.L.; Foquet, L.; Chuenchob, V.; Fishbaugher, M.; Billman, Z.; Navarro, M.J.; Betz, W.; Olsen, T.M.; Lee, J.; Camargo, N.; et al. Assessing drug efficacy against Plasmodium falciparum liver stages in vivo. JCI Insight 2018, 3, e92587. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.A.; Weiss, L.M.; Factor, S.; Bilezikian, J.P.; Tanowitz, H.; Wittner, M. Verapamil ameliorates clinical, pathologic and biochemical manifestations of experimental chagasic cardiomyopathy in mice. J. Am. Coll. Cardiol. 1989, 14, 782–789. [Google Scholar] [CrossRef]
- Tanowitz, H.B.; Morris, S.A.; Weiss, L.M.; Bilezikian, J.P.; Factor, S.M.; Wittner, M. Effect of verapamil on the development of chronic experimental Chagas’ disease. Am. J. Trop. Med. Hyg. 1989, 41, 643–649. [Google Scholar] [CrossRef]
- Tanowitz, H.B.; Wittner, M.; Chen, B.; Huang, H.; Weiss, L.M.; Christ, G.J.; Braunstein, V.; Bilezikian, J.P.; Morris, S.A. Effects of verapamil on acute murine Chagas’ disease. J. Parasitol. 1996, 82, 814. [Google Scholar] [CrossRef] [PubMed]
- De Souza, A.P.; Tanowitz, H.B.; Chandra, M.; Shtutin, V.; Weiss, L.M.; Morris, S.A.; Factor, S.M.; Huang, H.; Wittner, M.; Shirani, J.; et al. Effects of early and late verapamil administration on the development of cardiomyopathy in experimental chronic Trypanosoma cruzi (Brazil Strain) infection. Parasitol. Res. 2004, 92, 496–501. [Google Scholar]
- Díaz, M.V.; Miranda, M.R.; Campos-Estrada, C.; Reigada, C.; Maya, J.D.; Pereira, C.A.; López-Muñoz, R. Pentamidine exerts in vitro and in vivo anti Trypanosoma cruzi activity and inhibits the polyamine transport in Trypanosoma cruzi. Acta Trop. 2014, 134, 1–9. [Google Scholar] [CrossRef]
- Seguel, V.; Castro, L.; Reigada, C.; Cortes, L.; Díaz, M.V.; Miranda, M.R.; Pereira, C.A.; Lapier, M.; Campos-Estrada, C.; Morello, A.; et al. Pentamidine antagonizes the benznidazole’s effect in vitro, and lacks of synergy in vivo: Implications about the polyamine transport as an anti-Trypanosoma cruzi target. Exp. Parasitol. 2016, 171, 23–32. [Google Scholar] [CrossRef] [PubMed]
- McCabe, R. Primaquine is lethal for intracellular but not extracellular Trypanosoma cruzi. J. Parasitol. 1988, 74, 748–753. [Google Scholar] [CrossRef]
- van der Pluijm, R.W.; Tripura, R.; Hoglund, R.M.; Pyae Phyo, A.; Lek, D.; ul Islam, A.; Anvikar, A.R.; Satpathi, P.; Satpathi, S.; Behera, P.K.; et al. Triple artemisinin-based combination therapies versus artemisinin-based combination therapies for uncomplicated Plasmodium falciparum malaria: A multicentre, open-label, randomised clinical trial. Lancet 2020, 395, 1345–1360. [Google Scholar] [CrossRef]
- Leite, L.R.; Fenelon, G.; Simoes, A.; Silva, G.G.; Friedman, P.A.; De Paola, A.A.V. Clinical usefulness of electrophysiologic testing in patients with ventricular tachycardia and chronic chagasic cardiomyopathy treated with amiodarone or sotalol. J. Cardiovasc. Electrophysiol. 2003, 14, 567–573. [Google Scholar]
- Andriani, G.; Chessler, A.-D.C.; Courtemanche, G.; Burleigh, B.A.; Rodriguez, A. Activity in vivo of anti-Trypanosoma cruzi compounds selected from a high throughput screening. PLoS Negl. Trop. Dis. 2011, 5, e1298. [Google Scholar] [CrossRef]
- Martinez-Peinado, N.; Cortes-Serra, N.; Torras-Claveria, L.; Pinazo, M.-J.; Gascon, J.; Bastida, J.; Alonso-Padilla, J. Amaryllidaceae alkaloids with anti-Trypanosoma cruzi activity. Parasit. Vectors 2020, 13, 299. [Google Scholar] [PubMed]
- Martinez-Peinado, N.; Martori, C.; Cortes-Serra, N.; Sherman, J.; Rodriguez, A.; Gascon, J.; Alberola, J.; Pinazo, M.-J.; Rodriguez-Cortes, A.; Alonso-Padilla, J. Anti-Trypanosoma cruzi activity of metabolism modifier compounds. Int. J. Mol. Sci. 2021, 22, 688. [Google Scholar] [CrossRef]
- Alonso-Padilla, J.; Cotillo, I.; Presa, J.L.; Cantizani, J.; Peña, I.; Bardera, A.I.; Martín, J.J.; Rodriguez, A. Automated high-content assay for compounds selectively toxic to Trypanosoma cruzi in a myoblastic cell line. PLoS Negl. Trop. Dis. 2015, 9, e0003493. [Google Scholar] [CrossRef]
- Franco, C.H.; Alcântara, L.M.; Chatelain, E.; Freitas-Junior, L.; Moraes, C.B. Drug discovery for Chagas disease: Impact of different host cell lines on assay performance and hit compound selection. Trop. Med. Infect. Dis. 2019, 4, 82. [Google Scholar]
- Mitchel, F.L. The implementation of quality control and factors affecting its success. Ann. Clin. Biochem. 1969, 6, 119–122. [Google Scholar] [CrossRef]
- Anti-Infectives Screening Core Services | NYU Langone Health. Available online: https://med.nyu.edu/research/scientific-cores-shared-resources/anti-infectives-screening-core/services (accessed on 28 January 2021).
- Crouch, S.P.M.; Kozlowski, R.; Slater, K.J.; Fletcher, J. The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J. Immunol. Methods 1993, 160, 81–88. [Google Scholar]
- Francisco, A.F.; Jayawardhana, S.; Lewis, M.D.; Taylor, M.C.; Kelly, J.M. Biological factors that impinge on Chagas disease drug development. Parasitology 2017, 144, 1871–1880. [Google Scholar] [CrossRef]
- Lewis, M.D.; Francisco, A.F.; Taylor, M.C.; Kelly, J.M. A new experimental model for assessing drug efficacy against Trypanosoma cruzi infection based on highly sensitive in vivo imaging. J. Biomol. Screen. 2015, 20, 36–43. [Google Scholar]
- Timm, B.L.; Da Silva, P.B.; Batista, M.M.; Farahat, A.A.; Kumar, A.; Boykin, D.W.; Soeiro, M.N.C. In vitro investigation of the efficacy of novel diamidines against Trypanosoma cruzi. Parasitology 2014, 141, 1272–1276. [Google Scholar] [CrossRef]
- Wispelwey, B.; Pearson, R. Pentamidine: A risk-benefit analysis. Drug Saf. 1990, 5, 212–219. [Google Scholar] [CrossRef]
- Lewis, M.D.; Francisco, A.F.; Taylor, M.C.; Jayawardhana, S.; Kelly, J.M. Host and parasite genetics shape a link between Trypanosoma cruzi infection dynamics and chronic cardiomyopathy. Cell Microbiol. 2016, 18, 1429–1443. [Google Scholar] [CrossRef]
- Sunyoto, T.; Potet, J.; Boelaert, M. Why miltefosine—A life-saving drug for leishmaniasis—Is unavailable to people who need it the most. BMJ Glob. Health 2018, 3, e000709. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Martinez, A.K.; Rodriguez-Durán, J.; Serrano-Martin, X.; Hernandez-Rodriguez, V.; Benaim, G. Mechanism of action of miltefosine on Leishmania donovani involves the impairment of acidocalcisome function and the activation of the sphingosine-dependent plasma membrane Ca2+ channel. Antimicrob. Agents Chemother. 2018, 62, e01614-17. [Google Scholar] [CrossRef]
- Rohloff, P.; Montalvetti, A.; Docampo, R. Acidocalcisomes and the contractile vacuole complex are involved in osmoregulation in Trypanosoma cruzi. J. Biol. Chem. 2004, 279, 52270–52281. [Google Scholar]
- Benaim, G.; Paniz-Mondolfi, A.E.; Sordillo, E.M.; Martinez-Sotillo, N. Disruption of intracellular calcium homeostasis as a therapeutic target against Trypanosoma cruzi. Front. Cell. Infect. Microbiol. 2020, 10, 46. [Google Scholar] [CrossRef]
- Braga, S.S. Multi-target drugs active against leishmaniasis: A paradigm of drug repurposing. Eur. J. Med. Chem. 2019, 183, 111660. [Google Scholar] [CrossRef]
- Sundar, S.; Olliaro, P.L. Miltefosine in the treatment of leishmaniasis: Clinical evidence for informed clinical risk management. Ther. Clin. Risk Manag. 2007, 3, 733–740. [Google Scholar]




| Trade Name | Active Ingredient | Class | Route of Administration | Molecular Weight (g/mol) | References |
|---|---|---|---|---|---|
| Adalat | nifedipine | calcium channel blocker | oral | 346.34 | [26,27] |
| Amlodipine Normon | amlodipine | calcium channel blocker | oral | 408.88 | [14,26,28] |
| Apocard | flecainide | antiarrhythmic | oral | 414.34 | [29] |
| Atenolol Normon | atenolol | beta blocker | oral | 266.34 | [14] |
| Biocoryl | procainamide | antiarrhythmic | oral | 235.33 | [14] |
| Bisoprolol Normon | bisoprolol | beta blocker | oral | 325.44 | [30] |
| Daraprim | pyrimethamine | antiprotozoal | oral | 248.71 | [14] |
| Defitelio | defibrotide | antithrombotic | intravenous | 444.40 | [31] |
| Diamicron | gliclazide | antidiabetic | oral | 323.41 | [32] |
| Enalapril Normon | enalapril | ACE inhibitor | oral | 376.45 | [14,33] |
| Eskazole | albendazole | antihelminthic and antiprotozoal | oral | 256.33 | [14] |
| Eurartesim | piperaquine tetraphosphate-dihydroartemisinin | antimalarial | oral | 927.49–284.35 | [34] |
| Glucantime | meglumine antimoniate | antileishmanial | intramuscular | 365.98 | [26,35] |
| Glucophage | metformin hydrochloride | antidiabetic | oral | 129.16 | [14] |
| Hemovas | pentoxifylline | hemorrheologic agent | oral | 278.31 | [14,36,37] |
| Humatin | paramomycin | antimicrobial | oral | 615.63 | [38] |
| Impavido | miltefosine | antiprotozoal | oral | 407.57 | [39,40,41,42] |
| Iver P (ELEA) | ivermectin | antihelminthic | oral | 875.10 | [43] |
| Quinine sulfate (Hospital Clinic) | quinine sulfate | antimalarial | oral | 782.96 | [44] |
| Lidocaine Braun | lidocaine | local anesthetic | intravenous | 234.34 | [14] |
| Lomper | mebendazole | antihelminthic | oral | 295.29 | [14] |
| Malarone | atovaquone-proguanil | antimalarial | oral | 366.84 | [45] |
| Manidon | verapamil | calcium channel blocker | oral/intravenous | 454.60 | [46,47,48,49] |
| Masdil | diltiazem hydrochloride | calcium channel blocker | oral | 414.52 | [14] |
| Menaderm Otológico | beclometasone dipropionate-clioquinol | fungal/antibacterial | otic | 521.04–304.91 | - |
| Nerdipina | nicardipine | calcium channel blocker | oral | 479.53 | [14,26] |
| Pentacarinat | pentamidine | antiprotozoal | intravenous/intramuscular/inhalation | 340.42 | [14,26,50,51] |
| Primaquine (Hospital Clinic) | primaquine | antimalarial | oral | 259.35 | [14,52] |
| Riamet | artemether-lumefrantine | antimalarial | oral | 298.37–528.94 | [53] |
| Solgol | nadolol | beta blocker | oral | 309.40 | [14] |
| Sotapor | sotalol | beta blocker | oral | 272.36 | [54] |
| Tricolam | tinidazole | antiprotozoal | oral | 247.27 | [14] |
| Drug | Vero Cells Assays | NIH-3T3 Cells Assays | HepG2 Assay | Anti-Amastigote Assay | |||||
|---|---|---|---|---|---|---|---|---|---|
| [IC50 (SD)] µmol L−1 | [TC50 (SD)] µmol L−1 | SI | [IC50 (SD)] µmol L−1 | [TC50 (SD)] µmol L−1 | SI | [TC50 (SD)] µmol L−1 | [IC50 (SD)] µmol L−1 | SI | |
| Benznidazole | 1.93 (0.82) | 242.2 (13.93) | 125.5 | - | - | - | 229.8 (18.54) | 2.66 (0.14) | 91.1 |
| Atovaquone-proguanil | 1.26 (0.14) | 27.13 (5.05) | 21.5 | 1.32 (0.07) | >50 | >50 | 34.36 (5.88) | 1.85 (0.06) | 14.7 |
| Miltefosine | 0.018 (0.0015) | 78.99 (10.55) | 4388.3 | 0.037 (0.001) | 1.95 (0.57) | 52.7 | 51.28 (7.51) | 1.25 (0.05) | 63.2 |
| Lidocaine # | 0.016 (0.0015) | 0.23 (0.027) | 14.4 | - | - | - | - | - | - |
| Nifedipine | 0.19 (0.018) | 1.97 (0.267) | 10.4 | - | - | - | - | - | - |
| Pentamidine | 1.01 (0.55) | 78.96 (15.55) | 78.2 | 0.13 (0.005) | 5.9 (0.15) | 45.4 | 39.4 (5.20) | - | - |
| Piperaquine tetraphosphate-dihydroartemisinin | 3.95 (0.51) | 75.27 (16.56) | 19.1 | 4.05 (0.72) | 27.33 (3.68) | 6.8 | - | - | - |
| Verapamil | 3.44 (0.44) | 197.4 (25.54) | 57.4 | 0.60 (0.04) | 8.16 (1.63) | 13.6 | 170.5 (13.14) | 122.5 (7.04) | 1.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Peinado, N.; Cortes-Serra, N.; Sherman, J.; Rodriguez, A.; Bustamante, J.M.; Gascon, J.; Pinazo, M.-J.; Alonso-Padilla, J. Identification of Trypanosoma cruzi Growth Inhibitors with Activity In Vivo within a Collection of Licensed Drugs. Microorganisms 2021, 9, 406. https://doi.org/10.3390/microorganisms9020406
Martinez-Peinado N, Cortes-Serra N, Sherman J, Rodriguez A, Bustamante JM, Gascon J, Pinazo M-J, Alonso-Padilla J. Identification of Trypanosoma cruzi Growth Inhibitors with Activity In Vivo within a Collection of Licensed Drugs. Microorganisms. 2021; 9(2):406. https://doi.org/10.3390/microorganisms9020406
Chicago/Turabian StyleMartinez-Peinado, Nieves, Nuria Cortes-Serra, Julian Sherman, Ana Rodriguez, Juan M. Bustamante, Joaquim Gascon, Maria-Jesus Pinazo, and Julio Alonso-Padilla. 2021. "Identification of Trypanosoma cruzi Growth Inhibitors with Activity In Vivo within a Collection of Licensed Drugs" Microorganisms 9, no. 2: 406. https://doi.org/10.3390/microorganisms9020406
APA StyleMartinez-Peinado, N., Cortes-Serra, N., Sherman, J., Rodriguez, A., Bustamante, J. M., Gascon, J., Pinazo, M.-J., & Alonso-Padilla, J. (2021). Identification of Trypanosoma cruzi Growth Inhibitors with Activity In Vivo within a Collection of Licensed Drugs. Microorganisms, 9(2), 406. https://doi.org/10.3390/microorganisms9020406







