Abstract
The pathogenesis mechanisms of Campylobacter fetus subsp. venerealis (Cfv), the etiologic agent of Bovine Genital Campylobacteriosis remain elusive. This study evaluated the virulence potential and biovar characteristics of Cfv isolates (n = 13) by PCR screening of putative virulence-factor (VF) genes, Multilocus Sequence Typing (MLST) analysis, antimicrobial susceptibility to tetracycline, penicillin, enrofloxacin and streptomycin testing and whole-genome sequencing (WGS; n = 5), also comparing the latter with 26 other whole-genome sequences of Cfv strains. The putative VF genes encoding type IV secretion system of Cfv (virB2-virB11/virD4) were absent in 92% of isolates, including isolates from aborted foetuses, evidencing that these VF genes are not essential for Cfv pathogenicity. The parA gene, used as a Cfv diagnostic molecular target, was detected in only 3 of 13 isolates, invalidating its use for diagnosis purposes. Three novel sequence types were identified by MLST. Although no in vitro antimicrobial resistance was detected, WGS identified antimicrobial resistance-related genes, including those encoding the multidrug efflux pumps CmeABC and YkkCD, indicating that their presence is not enough to provide antimicrobial resistance. The SNP and accessory protein families analysis segregated the Cfv and Cfv biovar intermedius (Cfvi) strains into different clusters. In conclusion, this study evidenced virulence potential and biovar characteristics of Cfv and Cfvi, which are of relevance for the control of Bovine Genital Campylobacteriosis.
1. Introduction
Campylobacter fetus subsp. venerealis (Cfv) is the etiological agent of Bovine Genital Campylobacteriosis (BGC), a notifiable venereal disease of cattle responsible for low herd reproductive efficiency and significant economic losses worldwide [1,2]. Infected bulls asymptomatically carry Cfv in the preputial and penile mucosa and infect females during natural breeding or through semen, causing embryo loss or early fetal abortion [3]. For that reason, BGC control is based on bull preputial testing and culling of infected bulls, which requires an accurate identification of Cfv [3,4].
The Multilocus Sequencing Typing (MLST) identified a clonal structure with lower genetic diversity among C. fetus isolates than among other Campylobacter species [5]. In fact, C. fetus subsp. fetus (Cff), which colonizes the intestinal tract and occasionally the preputial cavity, causing sporadic abortion in cattle, and Cfv have more than 90% genome similarity [6]. This hampers the selection of suitable molecular targets for subspecies identification. The subspecies Cfv includes the biovar intermedius, which is differentiated by its ability to produce hydrogen sulfide from L-cysteine [7,8]. However, this is also common to Cff, hindering the subspecies differentiation by phenotypic tests. The determinants behind Cfv pathogenicity and niche restriction are still unclear. Several putative virulence factor (VF) genes were identified in both subspecies, including those encoding proteins involved in bacterial adhesion, invasion and cytotoxicity, which are common to other Campylobacter species, namely, the fibronectin binding protein, the campylobacter invasion antigen (Ciab) and the cytolethal distending toxin (CDT), among others [9]. Nevertheless, comparative genomic analyses revealed the presence of a genomic island almost exclusive of Cfv and highly prevalent in this subspecies [9,10,11], which harbors one of the most well studied genes for Cfv identification, the parA gene [12,13]. This genomic island harbors genes encoding Fic (filamentation induced by cyclic AMP)—domain proteins (fic genes) and a bacterial type IV secretion system (T4SS, virB-virD4 genes) [10]. In vitro studies demonstrated that the T4SS contributes to cytotoxicity and invasiveness of Cfv, besides being involved in interbacterial DNA transfer by conjugation [10,14]. Additionally, Fic proteins form a toxin–antitoxin network in Cfv that may favor its survival under adverse conditions [15]. These findings indicate a role of this genomic island in Cfv pathogenicity and/or adaptation to the genital tract [10,15]. A recent study revealed that C. fetus strains commonly harbor multiple T4SS encoding regions, which are phylogenetically different and were possibly acquired from different Campylobacter species [16]. Nevertheless, some T4SS encoding regions lack several virB genes and their function is still unclear.
Bulls may be treated with antibiotics, namely penicillin and streptomycin, although with limited efficacy, particularly in mature bulls [3,17,18]. These antibiotics are also routinely used in semen processing and their use is mandatory for intra-community trade of bovine semen according to the EU Directive 88/407/CEE. However, the prevalence of antimicrobial resistance among Cfv isolates has been poorly investigated. Indeed, a genomic island with two genes involved in tetracycline and streptomycin resistance was identified in Cff isolates [11,19], but its occurrence in Cfv is unknown.
This study aimed to characterize Cfv and Cfv biovar intermedius (Cfvi) isolates, assessing their genomic characteristics, genetic diversity, load of virulence-related genes and in vitro antimicrobial susceptibility.
2. Materials and Methods
2.1. Campylobacter fetus subsp. venerealis Isolates
The Cfv isolates (n = 13) were kindly provided by the Starcross Veterinary Investigation Centre from Animal and Plant Health Agency (APHA), United Kingdom, where they were phenotypically identified as Cfv (Table 1). The Cfv isolates were grown in Columbia agar plates with 5% sheep blood (COS, Biomerieux) for 48 h, under microaerobic conditions (GENbox Microaer, Biomerieux). The subspecies identification (Cfv) was further confirmed by the amplification of nahE and ISCfe1 sequences, as described by van der Graaf et al. [20].

Table 1.
Campylobacter fetus subsp. venerealis isolates used in this study.
2.2. DNA Isolation
Total DNA was extracted by a rapid boiling method. Briefly, bacterial cells were suspended in 1.5 mL PBS, centrifuged (17,000× g, 8 min), the supernatant discarded, and the cellular pellet resuspended in 500 μL of sterile water. After a second centrifugation (17,000× g, 5 min), the pellet was resuspended in 100 μL of sterile water and incubated at 95 °C for 15 min. Finally, the lysate was centrifuged (17,000× g, 8 min), and the DNA containing supernatant collected. The DNA was quantified using a Nanodrop 2000C spectrophotometer (Thermo Scientific, Waltham, USA) and diluted to 50 ng/μL.
2.3. Multilocus Sequence Typing (MLST)
The MLST analysis was performed according to a previously described scheme, based on seven housekeeping genes: aspA, glnA, gltA, glyA, pgm, tkt and uncA [5]. The sequence types (STs) were assigned using the Campylobacter MLST database (https://pubmlst.org/campylobacter/ (accessed on 4 September 2020)) sited at the University of Oxford [21]. New alleles and profiles were submitted to this database.
2.4. Surface Array Protein and L-Cysteine Transporter Typing
The isolates were classified as Cfv or Cfvi using a multiplex-PCR for detection of an L-cysteine transporter operon previously described [22]. DNA from Cfv strain NCTC 10354 and Cff strain NCTC 10842 were used as positive controls.
The surface array protein (sap) serotype (sapA and sapB) was identified as described before [23], using primers ACF/ACR and BCF/BCR. DNA from Cfv strain NCTC 10354 and Cff strain NCTC 10842 were used as positive controls in sapA and sapB PCRs, respectively.
2.5. Detection of Putative Virulence Factor Genes Using PCR
The presence of putative VF genes involved in adhesion (cadF), invasion (invA and ciaB) and cytotoxicity (cdt and pldA) of host cells [9] was assessed by PCR. For genes cadF, invA, ciaB and pldA, primers were designed with Primer-BLAST [24] using gene sequences of Cfv NCTC 10354 as template (Table S1). PCR reactions were carried out in 25 µL mixtures, containing 200 μM of each dNTP (4you4 dNTP Mix, Bioron), 400 nM of each primer, 1X reaction buffer (Complete reaction buffer, Bioron), 2 units of DFS-Taq DNA polymerase (Bioron) and 100 ng of DNA. Amplifications were performed in a Doppio thermal cycler (VWR) with the following cycling conditions: initial denaturation step at 94 °C for 2 min, followed by 30 cycles of denaturation at 94 °C for 30 s, annealing temperature for 30 s, extension at 72 °C for 1 min, with a final extension step at 72 °C for 5 min. Detection of cdtA, cdtB and cdtC genes was carried out by PCR as described previously [25].
The presence of genes encoding the most studied T4SS of Cfv [10,14], which includes virB2-virB11, virD4 and fic1 and fic2 genes were also screened by PCR. Primers were designed with primer-BLAST [24] to target virB2, virB3-virB4, virB5, virB6, virB7, virB8, virB10 and virD4 genes, using the sequences of Cfv NCTC 10354 (GenBank accession no. CP043435.1, loci CFVT_1262–1267, CFVT_1258 and CFVT_1256) as template (Table S1). Genes fic1, fic2, virB9 and virB11 were detected as recently described [26]. PCR mixtures and thermal cycling conditions were performed as described above. The amplification products were separated in a 1.5% agarose gel electrophoresis, stained with ethidium bromide and visualized using a ChemiDoc XRS + System (Bio-Rad).
2.6. Detection of parA Gene
The parA gene was detected by three PCR assays directed towards distinct nucleotide regions, comprising a conventional PCR with VenSF/VenSR primers [13], two real-time PCR assays, [12] and parA-B assay [26].
2.7. Antibiotic Susceptibility Testing
The minimum inhibitory concentrations (MICs) of streptomycin, tetracycline, enrofloxacin and penicillin G were in vitro determined using Etest gradient strips (Biomerieux). Cfv colonies grown on COS plates for 48 h were suspended in Brain Heart Infusion (BHI) broth to a turbidity of 1.0 McFarland measured with a Densimat densitometer (Biomerieux). The inoculum was spread on Mueller Hinton agar plates supplemented with 5% horse blood and 20 mg/L of β-NAD (MHF, Biomerieux) and one strip was applied on each agar plate. The concentration gradients of antibiotics in Etest strips used were 0.064–1024 μg/mL for streptomycin, 0.016–256 μg/mL for tetracycline, and 0.002–32 μg/mL for enrofloxacin and penicillin G. Plates were incubated for 48 h at 35 °C in a microaerobic atmosphere, and MICs were read at the point where the zone of inhibition intersected the MIC scale on the Etest strip. Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 25923, Enterococcus faecalis ATCC 29212 and Pseudomonas aeruginosa ATCC 27853 were used for quality control, as recommended by the manufacturer.
In the absence of specific interpretative criteria for Cfv, the MIC breakpoints of enrofloxacin and tetracycline were defined according to the ciprofloxacin and tetracycline breakpoints defined for Campylobacter jejuni and Campylobacter coli by the European Commitee on Antimicrobial Susceptibility Testing (EUCAST) [27] (Table 2). Results of penicillin G were interpreted according to the criteria defined for Gram-negative anaerobes by the EUCAST [27] (Table 2). For streptomycin MIC breakpoints, due to the absence of EUCAST breakpoints, results were interpreted according to criteria of The National Antimicrobial Resistance Monitoring System for Enteric Bacteria (NARMS) for Escherichia coli [28] (Table 2).

Table 2.
Minimum inhibitory concentration (MIC) breakpoints for antibiotic susceptibility testing.
2.8. Whole Genome Sequencing of Cfv Strains
Strains IS26-07793, IS16-01257, SA21-221439, SA21-217832 and SA21-217833 were selected for whole genome sequencing (WGS) analysis, as they have unique genomic traits, namely represent a novel ST or harbour the screened T4SS-encoding genes. Strains were grown on blood agar plates supplemented with 5% sheep blood at 37 °C for 48 h and genomic DNA isolated using the DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany), according to manufacturer’s instructions. Following preparation of DNA libraries, the genomes were sequenced with the Illumina Novaseq Platform at Stabvida (Caparica, Portugal), using 150-bp paired end reads. The reads were de novo assembled in the Pathosystems Resource Integration Center (PATRIC) version 3.6.7 web platform [29], using SPAdes version 3.12.0 [30]. Assembled genomes were submitted to the Comprehensive Genome Analysis service of PATRIC [29], which includes an annotation service using RAST tool kit (RASTtk) [31]. The whole genome shotgun projects of strains IS26-07793, IS16-01257, SA21-221439, SA21-217832 and SA21-217833 were deposited at DDBJ/ENA/GenBank under the accession numbers JAENPS000000000, JAENPT000000000, JAENPU000000000, JAENPV000000000 and JAENPW000000000, respectively.
The genomes were visualized by comparison against reference genomes (Cfv strain NCTC 10354 and Cfv strain 01/165) using the BLAST Ring Image Generator (BRIG) version 0.95 [32], with an upper identity threshold of 90% and a lower identity threshold of 70%. Genomic islands and T4SS encoding regions VG III [14], PICFV8/T4SS region 1A [9,16], T4SS region 2A and 1F [16] were included in the BRIG analysis.
The MLST allele sequences were extracted from WGS data using the MLST software version 2.0.4 [33] to confirm results of MLST analysis.
2.9. Comparative Genomic Analysis
The genomes of the five sequenced strains were compared to 26 whole genome sequences of Cfv strains retrieved from the GenBank (Table 3). Genomes were analysed by the Comprehensive Genome analysis service of PATRIC [29], which includes a k-mer-based detection method for antimicrobial resistance genes. Genes assigned to mechanisms of antibiotic inactivation and efflux pumps were considered for this analysis. Additionally, genes ant(6)-Ib and tet(44) conferring resistance to streptomycin and tetracycline [11] and putative VF encoding genes (cadF, pldA, invA, ciaB, cdtA, cdtB and cdtC) were searched by the BLAST tool in the genomes.

Table 3.
Genomes of Campylobacter fetus subsp. venerealis strains used for the comparative genomic analysis.
The PATRIC’s Family Protein Sorter service [29] was used to evaluate the distribution of protein families across the analysed genomes, and genus-specific families (PLfams) represented in more than 5% and less than 95% of the genomes (2 to 29 genomes), considered accessory protein families, were selected for further analysis. These data were used for the construction of a heat map using Next-Generation Clustered Heat Map (NG-CHM) Builder [34] with hierarchical clustering using the Euclidean distance metric with the complete agglomeration method.
Single nucleotide polymorphisms (SNP) detection and analysis were performed with CSI Phylogeny version 1.4 [35] to reconstruct a phylogenetic tree using the genome of strain NCTC 10354 as reference, with a minimum distance between SNPs set for 10 bp. The tree was illustrated using the Molecular Evolutionary Genetics Analysis (MEGA) X software version 10.1.7 [36] and bootstrap values lower than 70% were hidden.
3. Results
3.1. Multilocus Sequence Typing of Cfv Isolates
A total of 4 STs were identified among the 13 Cfv isolates (Table 4). The allelic profile of isolates IS16-01257 and SA21-221439 was not listed in the PubMLST database and after its submission, the isolates were assigned to ST-71. Two new alleles of gltA and tkt, assigned respectively to alleles 12 and 14, were deposited in the PubMLST database. The alleles 7 and 12 of gltA differ from allele 2, which is the most common, in one nucleotide position. Additionally, alleles 2 and 14 of tkt are distinguished by a single nucleotide. Overall, nine isolates were assigned to ST-4 (69.2%), two to ST-71 (15.4%), one to ST-72 (7.7%) and one to ST-73 (7.7%). Interestingly, the isolates from herds I and J were assigned to different STs (herd I—SA21-221825 to ST-4 and SA21-221439 to ST-71; herd J—SA21-217832 to ST-72 and SA21-217833 to ST-73).

Table 4.
Sequence types and corresponding allelic profiles.
3.2. Genomic Characterization of Cfv Isolates: Surface Array Protein and L-Cysteine Transporter Typing, Virulence Factor Genes and parA Gene
All the 13 Cfv isolates were classified as serotype A (sapA positive and sapB negative). Most isolates (n = 9) revealed a Cfvi pattern in L-cysteine transporter PCR (Table 5). The remaining four isolates harbour the L-cysteine transporter encoding operon partially deleted and, consequently, were classified as Cfv.

Table 5.
Genomic characteristics of the Cfv isolates.
All isolates harbour the CDT operon genes (cdtABC), which encode the cytolethal distending toxin, and genes cadF, ciaB, invA, pldA, which encode the fibronectin-binding protein, Campylobacter invasion antigen B, invasin A and phospholipase A, respectively. These genes were also found in the 26 whole genome sequences of Cfv strains, using BLAST search.
Only three isolates (23.1%) were parA positive and this result was consistent using the three different assays. Genes fic1 and fic2 were found in all Cfv isolates, whereas T4SS encoding genes (virB2-virB11 and virD4) were present only in Cfv isolate IS26-07793 (Table 5).
3.3. Antibiotic Susceptibility Testing of Cfv Isolates
Antibiotic resistances were not found among the Cfv isolates. All the 13 isolates were susceptible to tetracycline, streptomycin and enrofloxacin. Eight isolates were categorized as susceptible to penicillin G with standard dose regimen and the remaining 5 isolates (38.5%) were considered susceptible with increased exposure. The MIC values of tetracycline, streptomycin and enrofloxacin for all isolates were below the susceptibility breakpoints. The range of MIC values for each antibiotic and the MIC inhibiting 50% (MIC50) and 90% of the isolates (MIC90) are shown in Table 6.

Table 6.
Minimum inhibitory concentrations of selected antibiotics for Cfv isolates.
3.4. Whole Genome Sequencing of Five Cfv Strains
The WGS analysis confirmed the PCR result of absence of genes parA, virB2-virB11 and virD4 of T4SS encoding region 1A, in four of the five sequenced strains. Only Cfv strain IS26-07793 harbours the genomic island with the T4SS encoding region 1A [16]. However, all the five strains have other T4SS encoding regions, with gene sequences and gene composition distinct from region 1A (Table 7).

Table 7.
T4SS encoding genes found in different genomic regions.
Plotting the sequenced genomes against the genome of Cfv NCTC 10354 as reference showed that all five strains harbour the T4SS encoding region 2A (Figure 1), whereas the region 1A is present only in strain IS16-07793. An additional comparison using the genome of Cfv strain 01/165 as reference showed the presence of a T4SS encoding region 1F, in strains SA21-217832 and SA21-217833.
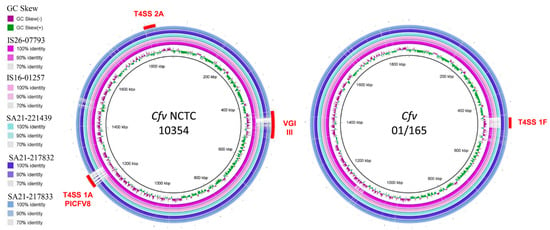
Figure 1.
Comparative genomic analysis of Cfv strains with reference strains NCTC 10354 and 01/165. Image created using Blast Ring Image Generator version 0.95. The inner ring represents the GC Skew and the remaining rings represent a BLASTN comparison of genomes of IS26-07793, IS16-01257, SA21-221439, SA21-217832 and SA21-217833 with the reference strains NCTC 10354 (left) and 01/165 (right). Red curved bars indicate chromosomal genomic islands previously identified by other authors (T4SS encoding regions 1A, 1F, 2A) [16].
All except strain IS26-07793 harbour a T4SS encoding region 1B, and strains SA21-217832 and SA21-217833 also present T4SS tra/trb encoding regions.
The comparison with the genome of Cfv NCTC 10354 revealed the absence of a prophage in VGI III [6] in strains IS16-01257, SA21-221439, SA21-217832 and SA21-217833 within the sap locus.
3.5. Comparative Genomic Analysis of Cfv Strains
The SNP analysis using the strain NCTC 10354 as reference was based on 1,630,344 nucleotide positions that were common to all genomes. As shown in Figure 2, the strain IS26-07793 is phylogenetically related to strains CCUG 33900, B6 and NCTC 10354. The remaining four sequenced strains are phylogenetically distant from IS26-07793. Strains SA21-217832 and SA21-217833, isolated from the same herd, are highly related despite having different STs. These strains typed as ST-72 and ST-73 are phylogenetically close to IS16-01257 and SA21-221439 typed as ST-71, and the ST-4 WBT011/09.
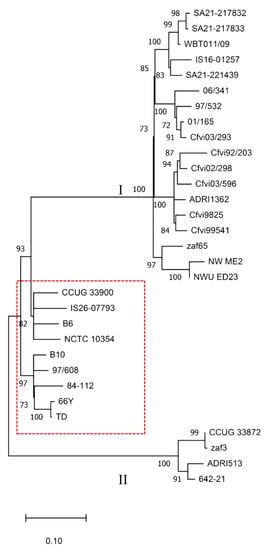
Figure 2.
Phylogenetic tree based on single nucleotide polymorphisms (SNP) of 31 C. fetus subsp. venerealis strains. Numbers at the nodes represent bootstrap values and values lower than 70% were hidden. The red border rectangle separates Cfv strains (inside) from strains biotyped as Cfv biovar intermedius (outside).
The phylogenetic tree also shows a clear distinction between strains typed as Cfvi or Cfv in previous studies [5,22,37]. Although NWU_ED23 and NW_ME2 strains were not typed in these studies, the BLAST search identified the complete L-cysteine transporter encoding gene in NWU_ED23 (contig 72) and the sequence divided into two contigs (contigs 26 and 417) in NW_ME2, which is compatible with a Cfvi classification. Overall, nine strains are classified as Cfv and 22 as Cfvi. Cfvi strains are divided into two distant groups (Clusters I and II), with strains CCUG 33872, zaf3, ADRI513 and 642-21 segregated from the remaining Cfvi strains. This comparative genomic analysis of Cfv strains evidences the presence of similar SNP patterns in isolates from the same geographic region. For instance, strains zaf65, NW_ME2 and NWU_ED23 were isolated from different regions of South Africa. Moreover, Cfvi strains sequenced in this study cluster with the strain WBT011/09 from the UK. The results also showed that Cfvi strains from Argentina are included in two related clusters grouped with a bootstrap value of 100%.
Regarding the antimicrobial resistance genes, those encoding the multidrug efflux system CmeABC, the broad-specificity multidrug efflux pump YkkCD, the Macrolide-specific efflux protein MacA, the Macrolide export ATP-binding/permease protein MacB and the nitroimidazole resistance protein were found in the genome of the 31 strains under study, whereas the genes tet(44) and ant(6)-Ib searched using BLAST were not identified in the genomes under analysis.
The analysis of protein families (PLfams) identified 2425 genus-specific protein families, from which 1641 were represented in all the 31 genomes. A total of 1693 proteins are encoded in the genome of 30 or more isolates (≥96.8%), which represent the core gene families considering the commonly accepted cut-off value of 95% and are listed in Supplementary Table S2. The accessory protein families, found in less than 95% of the strains, are represented in a heatmap with hierarchical clustering (Figure 3). A total of 540 accessory protein families were found encoded in the 31 analysed genomes (Table S3), of which 461 have an unknown function (hypothetical proteins). The groups formed based on the accessory protein families almost match those formed based on the SNP phylogenetic tree, with exception of strain Cfvi 06/341, 97/532 and UK Cfvi strains that were split into two groups. The Cfvi strains 642-21, ADRI 513, zaf3 and CCUG33872 are closely related and segregated from the remaining Cfvi strains, which is in accordance with SNP analysis. There were no exclusive Cfv protein families common to all strains. As expected, two protein families (PLF_194_00014554 and PLF_194_00049089) related to L-cysteine transporters were exclusively found in Cfvi strains, with the exception of strain cfvi9925. Excluding this strain, 180 protein families were unique to Cfvi and 10 protein families were exclusively represented in the nine Cfv genomes.
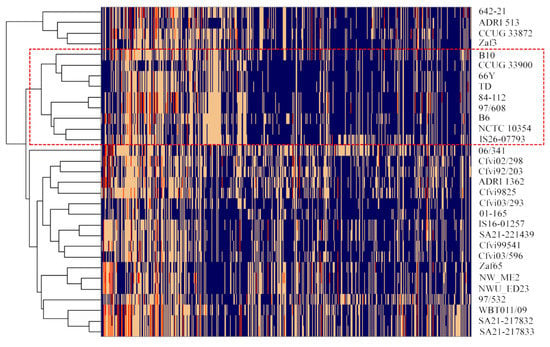
Figure 3.
Heat map representing the distribution of accessory protein families (n = 540) in the genomes of 31 Cfv strains. The absence of the protein family is represented in blue and the number of proteins per family is represented in yellow (n = 1), orange (n = 2) and red (n = 3). Cfv strains are grouped by hierarchical clustering, using the Euclidean distance and the complete agglomeration method. The rectangle with red border separates Cfv strains (inside) from Cfv biovar inter-medius strains (outside).
4. Discussion
This study evaluated the virulence potential of Cfv strains through genomic and phenotypic approaches and uncovered characteristics of this subspecies that are relevant for BGC control. MLST genotyping showed that most Cfv isolates were clustered in ST-4, which is reported to be the most prevalent ST among Cfv [5]. Thus, this genotyping method could be considered an effective tool for typing C. fetus at the subspecies level [20]. Interestingly, this study revealed a considerable ST diversity and identified three novel STs. These STs differ from ST-4 in one to two nucleotide positions, which denotes the genetic stability of Cfv. Nevertheless, the ST variability was higher than expected and the use of MLST for subspecies identification should be further evaluated. In fact, the suitability of MLST for subspecies typing was questionable since the description of one Cff strain belongs to ST-4 [38].
The pathogenicity mechanisms of Cfv are still unclear. All Cfv isolates and 26 genomes from different geographic regions harbour genes encoding the fibronectin-binding protein (cadF), Campylobacter invasion antigen B (ciaB), invasin A (invA), phospholipase A (pldA) and cytolethal distending toxin (cdtABC), which contribute to the virulence potential of other Campylobacter species, playing roles in adhesion, invasion and/or cytotoxicity of host cells [8,39]. The presence of these genes in all genomes of Cfv suggests their relevance for host colonization and/or pathogenicity. Further research is needed to understand the contribution of these genes to Cfv virulence.
A T4SS encoded by virB2-virB11 and virD4 genes, within a genomic island formerly considered unique to Cfv [9,10], was suggested as being involved in Cfv virulence, namely in cell invasion, cytotoxicity and conjugative DNA transfer [10,14]. Genes encoding this T4SS, corresponding to region 1A [16], were detected in 91% of 67 Cfv strains [10]. However, in this study, only 1 out of 13 isolates harbour genes encoding this T4SS, and 7 of the 12 negative strains were isolated from aborted foetuses, which still evidences their pathogenicity even in the absence of this genomic island. The WGS of the five sequenced isolates identified other loci with T4SS encoding genes, with distinct gene sequences from encoding region 1A, whose putative role in Cfv pathogenicity or niche specialization have so far not been addressed. These T4SS encoding regions 1B, 1F and 2A lack some virB/virD4 genes, which require further studies to evaluate their functionality. Other genes that were not analysed in this study may also contribute to the pathogenicity of these isolates and should be evaluated in a larger sample.
Tested by three different PCR assays, only 3 out of 13 isolates harbour the parA gene, and this negative status was confirmed in the WGS of four strains. A previous study reported that most Cfv isolates from the UK were negative for parA gene [40]. The doubt remained whether this resulted from sequence variations in primer-binding sites or from absence of the gene. The present study confirms the absence of the gene in a large proportion of UK strains and clarifies the reason of sensitivity failures of parA detection methods for Cfv identification [4,20]. In accordance, a recent study [41] reported the absence of this gene in 45% of C. fetus genomes proposed as belonging to subspecies venerealis, including some of the strains analysed in the present study. The parA gene is located in a genomic island, which was already described in Cff [11,16] and other Campylobacter species [42]. Therefore, the sole use of this molecular marker for Cfv identification should be avoided due to its lack of specificity and sensitivity.
To the authors’ best knowledge, this is the first report on antibiotic minimum inhibitory concentrations in Cfv field isolates. No antimicrobial resistance to streptomycin, penicillin, tetracycline and enrofloxacin was found in the 13 Cfv isolates, and streptomycin and tetracycline resistance genes were not detected in the 31 Cfv analysed genomes. These latter genes were identified in Cff strains harbouring the Cfv-associated genomic island with T4SS encoding genes [11,19]. The antimicrobial susceptibility results of this study are in accordance with a previous study, in which all isolates were susceptible to penicillin, streptomycin and tetracycline and only 5% of the isolates were susceptible to enrofloxacin [43]. Similarly, in another study with Cfv isolates from Germany, only 4% of the isolates revealed increased susceptibility to streptomycin and 2% for ciprofloxacin and tetracycline [44]. Antimicrobial resistance data from this study must be regarded with caution, as they refer to a very limited number of isolates from a narrow world geographical region. Nevertheless, they provide proof of concept for the simultaneous presence of in vitro susceptibility to antimicrobials and genes encoding for its resistance. A wide geographical survey with a large sampling is needed to ascertain the presence of antimicrobial resistance in different scenarios.
In contrast, genes encoding two multidrug efflux pumps were detected in all 31 analysed genomes. The CmeABC efflux pump, well-studied in C. jejuni, provides resistance to bile salts, heavy metals and antibiotics [45,46]. Mutational analysis of the cmeB gene in several Campylobacter species, including C. fetus, revealed its involvement in antimicrobial resistance [47]. However, results showed that all five sequenced isolates harbour genes encoding this efflux pump and those encoding the ykkCD efflux pump without exhibiting phenotypic resistance to antimicrobials. The role of these efflux pumps in C. fetus antimicrobial resistance deserve further research with other antimicrobials, as these systems may act synergistically with other genes conferring antimicrobial resistance.
The resolution provided by MLST to differentiate Cfv strains was weak, compared with SNP or accessory protein family analysis. The housekeeping genes used in MLST are very stable among Cfv strains, which makes this method very limited for genetic diversity analysis. Although a Cfv clonal nature was reported [5], this study identified genomic features that consistently grouped most strains by their SNPs or accessory protein families. Both methods segregated Cfvis in two distant clusters, which is indicative of genetic diversity within this biovar. Cfvi were also segregated from Cfv strains, indicating a higher variability between biovars than the described by the L-cysteine transporter encoding operon. Analysis of protein families revealed 2 proteins (PLF_194_00014554 and PLF_194_00049089) present in Cfvi that are absent in Cfv. Genes encoding these proteins in Cfvi were described as responsible for the phenotypic differences found between biovars [37]. Strain 9825 was the exception, as, while not exhibiting the above two protein families, it was still clustered by SNP and MLST as Cfvi. Strain 9825 was initially classified as Cfvi in a first study [5], although the isolate failed to produce H2S in two other reports [22,37]. This study indicates that this strain is closer to Cfvi than to Cfv, even lacking genes encoding the L-cysteine transporter.
5. Conclusions
This study combined MLST genotyping, VF genes PCR testing, antimicrobial resistance phenotyping, WGS and comparative genomic analysis to evaluate the virulence potential of Cfv isolates and strains. Three novel STs were identified by MLST (ST-71, ST-72 and ST-73). Most VF genes common to Campylobacter genus were detected, but genes encoding the T4SS, previously regarded as involved in Cfv virulence or niche adaptation, were absent in most Cfv strains. This indicates that T4SS is not essential for Cfv pathogenicity, as strains were isolated from aborted foetuses. The parA gene, still used for Cfv identification was absent in most Cfv strains, which precludes the sole use of this marker for BGC molecular diagnosis. As genes encoding the CmeABC and YkkCD efflux pumps were detected and in vitro antimicrobial resistance towards streptomycin, penicillin, tetracycline and enrofloxacin was not detected, it is demonstrated that the sole presence of those genes is not enough to provide antimicrobial resistance to tested antimicrobials. Genetic diversity was found in isolates from different geographic regions, and WGS and comparative genomic analysis of SNPs and accessory protein families allowed to differentiate biovars Cfv from Cfvi. Results of this study provided novel knowledge related to Cfv virulence potential evaluation and BGC control.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2607/9/2/340/s1, Table S1: Primers designed in this study for detection of putative virulence genes and T4SS-encoding genes, Table S2: Genus-specific protein families (PLFams) represented in more than 95% of the Cfv genomes; Table S3: Genus-specific protein families (PLFams) represented in more than 5% and less than 95% of the Cfv genomes.
Author Contributions
Conceptualization, M.F.S., L.L.-d.-C. and E.S.; methodology, M.F.S., M.J.F., L.L.-d.-C. and E.S.; validation, M.F.S., L.L.-d.-C. and E.S.; formal analysis, M.F.S., G.P., L.L.-d.-C. and E.S.; investigation, M.F.S. and A.L.P.; writing—original draft preparation, M.F.S.; writing—review and editing, A.L.P., M.J.F., G.P., L.M., L.L.-d.-C. and E.S.; visualization, M.F.S., L.M., L.L.-d.-C. and E.S.; supervision, L.L.-d.-C. and E.S.; project administration, L.L.-d.-C. and E.S.; funding acquisition, L.L.-d.-C. and E.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Fundação para a Ciência e a Tecnologia (FCT) and Fundo Europeu de Desenvolvimento Regional (FEDER), under the project PTDC/CVT-CVT/30145/2017. This study was also supported by Centro de Investigação Interdisciplinar em Sanidade Animal—CIISA (Project UIDP/CVT/00276/2020, funded by FCT). Marta Silva and Gonçalo Pereira are PhD students supported by grants from FCT, SFRH/BD/125657/2016 and SFRH/BD/130923/2017, respectively. Elisabete Silva is funded by FCT (DL 57/2016/CP1438/CT0001). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The whole genome shotgun projects of strains IS26-07973, IS16-01257, SA21-221439, SA21-217832 and SA21-217833 were deposited at DDBJ/ENA/GenBank under the accession numbers JAENPS000000000, JAENPT000000000, JAENPU000000000, JAENPV000000000 and JAENPW000000000, respectively. Additional data presented in this study are available in Supplementary Tables S1–S3.
Acknowledgments
The authors would like to acknowledge Elena Velo-Rego and the Animal and Plant Health Agency (APHA) for providing the C. fetus subsp. venerealis isolates. Additionally, the authors thank Manuela Oliveira from the Microbiology and Immunology Lab of CIISA for sharing the strains used for quality control of in vitro antimicrobial susceptibility testing.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Mshelia, G.D.; Amin, J.D.; Woldehiwet, Z.; Murray, R.D.; Egwu, G.O. Epidemiology of bovine venereal campylobacteriosis: Geographic distribution and recent advances in molecular diagnostic techniques. Reprod. Domest. Anim. 2010, 45. [Google Scholar] [CrossRef] [PubMed]
- OIE. Bovine Genital Campylobacteriosis. In OIE Terrestrial Manual 2018; OIE: Paris, France, 2018; pp. 1031–1044. [Google Scholar]
- Michi, A.N.; Favetto, P.H.; Kastelic, J.; Cobo, E.R. A review of sexually transmitted bovine trichomoniasis and campylobacteriosis affecting cattle reproductive health. Theriogenology 2016, 85, 781–791. [Google Scholar] [CrossRef]
- McGoldrick, A.; Chanter, J.; Gale, S.; Parr, J.; Toszeghy, M.; Line, K. Real Time PCR to detect and differentiate Campylobacter fetus subspecies fetus and Campylobacter fetus subspecies venerealis. J. Microbiol. Methods 2013, 94, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Van Bergen, M.A.P.; Dingle, K.E.; Maiden, M.C.J.; Newell, D.G.; Van Der Graaf-Van Bloois, L.; Van Putten, J.P.M.; Wagenaar, J.A. Clonal nature of Campylobacter fetus as defined by multilocus sequence typing. J. Clin. Microbiol. 2005, 43, 5888–5898. [Google Scholar] [CrossRef]
- Kienesberger, S.; Sprenger, H.; Wolfgruber, S.; Halwachs, B.; Thallinger, G.G.; Perez-perez, G.I.; Blaser, M.J.; Zechner, E.L.; Gorkiewicz, G. Comparative genome analysis of Campylobacter fetus subspecies revealed horizontally acquired genetic elements important for virulence and niche specificity. PLoS ONE 2014, 9, e85491. [Google Scholar] [CrossRef]
- Veron, M.; Chatelain, R. Taxonomic Study of the Genus Campylobacter Sebald and Veron and Designation of the Neotype Strain for the Type Species, Campylobacter fetus (Smith and Taylor) Sebald and Veron. Int. J. Syst. Bacteriol. 1973, 23, 122–134. [Google Scholar] [CrossRef]
- Sprenger, H.; Zechner, E.L.; Gorkiewicz, G. So close and yet so far—Molecular microbiology of Campylobacter fetus subspecies. Eur. J. Microbiol. Immunol. 2012, 2, 66–75. [Google Scholar] [CrossRef]
- Ali, A.; Soares, S.C.; Santos, A.R.; Guimarães, L.C.; Barbosa, E.; Almeida, S.S.; Abreu, V.A.C.; Carneiro, A.R.; Ramos, R.T.J.; Bakhtiar, S.M.; et al. Campylobacter fetus subspecies: Comparative genomics and prediction of potential virulence targets. Gene 2012, 508, 145–156. [Google Scholar] [CrossRef]
- Gorkiewicz, G.; Kienesberger, S.; Schober, C.; Scheicher, S.R.; Gülly, C.; Zechner, R.; Zechner, E.L. A genomic island defines subspecies-specific virulence features of the host-adapted pathogen Campylobacter fetus subsp. venerealis. J. Bacteriol. 2010, 192, 502–517. [Google Scholar] [CrossRef] [PubMed]
- Abril, C.; Brodard, I.; Perreten, V. Two novel antibiotic resistance genes, tet(44) and ant(6)-Ib, are located within a transferable pathogenicity island in Campylobacter fetus subsp. fetus. Antimicrob. Agents Chemother. 2010, 54, 3052–3055. [Google Scholar] [CrossRef]
- McMillen, L.; Fordyce, G.; Doogan, V.J.; Lew, A.E. Comparison of culture and a novel 5′ Taq nuclease assay for direct detection of Campylobacter fetus subsp. venerealis in clinical specimens from cattle. J. Clin. Microbiol. 2006, 44, 938–945. [Google Scholar] [CrossRef]
- Hum, S.; Quinn, K.; Brunner, J.; On, S.L.W. Evaluation of a PCR assay for identification and differentiation of Campylobacter fetus subspecies. Aust. Vet. J. 1997, 75, 827–831. [Google Scholar] [CrossRef]
- Kienesberger, S.; Trummler, C.S.; Fauster, A.; Lang, S.; Sprenger, H.; Gorkiewicz, G.; Zechner, E.L. Interbacterial macromolecular transfer by the Campylobacter fetus subsp. venerealis type IV secretion system. J. Bacteriol. 2011, 193, 744–758. [Google Scholar] [CrossRef]
- Sprenger, H.; Kienesberger, S.; Pertschy, B.; Pöltl, L.; Konrad, B.; Bhutada, P.; Vorkapic, D.; Atzmüller, D.; Feist, F.; Högenauer, C.; et al. Fic proteins of Campylobacter fetus subsp. venerealis form a network of functional toxin-antitoxin systems. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Graaf-van Bloois, L.; Miller, W.G.; Yee, E.; Gorkiewicz, G.; Forbes, K.J.; Zomer, A.L.; Wagennar, J.A.; Duim, B. Campylobacter fetus Subspecies Contain Conserved Type IV Secretion Systems on Multiple Genomic Islands and Plasmids. PLoS ONE 2016, 11, e0152832. [Google Scholar] [CrossRef] [PubMed]
- Truyers, I.; Luke, T.; Wilson, D.; Sargison, N. Diagnosis and management of venereal campylobacteriosis in beef cattle. BMC Vet. Res. 2014, 10, 1–7. [Google Scholar] [CrossRef]
- Hum, S.; Brunner, J.; Gardiner, B. Failure of therapeutic vaccination of a bull infected with Campylobacter fetus. Aust. Vet. J. 1993, 70, 386–387. [Google Scholar] [CrossRef]
- Escher, R.; Brunner, C.; von Steiger, N.; Brodard, I.; Droz, S.; Abril, C.; Kuhnert, P. Clinical and epidemiological analysis of Campylobacter fetus subsp. fetus infections in humans and comparative genetic analysis with strains isolated from cattle. BMC Infect. Dis. 2016, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- van der Graaf-van Bloois, L.; van Bergen, M.A.P.; van der Wal, F.J.; de Boer, A.G.; Duim, B.; Schmidt, T.; Wagenaar, J.A. Evaluation of molecular assays for identification Campylobacter fetus species and subspecies and development of a C. fetus specific real-time PCR assay. J. Microbiol. Methods 2013, 95, 93–97. [Google Scholar] [CrossRef]
- Jolley, K.A.; Bray, J.E.; Maiden, M.C.J. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 2018, 3, 1–20. [Google Scholar] [CrossRef]
- Farace, P.D.; Morsella, C.G.; Cravero, S.L.; Sioya, B.A.; Amadio, A.F.; Paolicchi, F.A.; Gioffré, A.K. L-cysteine transporter-PCR to detect hydrogen sulfide-producing Campylobacter fetus. PeerJ 2019, 2019, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, J.; Tummuru, M.K.R.; Blaser, M.J. Segmental conservation of sapA sequences in type B Campylobacter fetus cells. J. Biol. Chem. 1995, 270, 15093–15101. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef]
- Asakura, M.; Samosornsuk, W.; Hinenoya, A.; Misawa, N.; Nishimura, K.; Matsuhisa, A.; Yamasaki, S. Development of a cytolethal distending toxin (cdt) gene-based species-specific multiplex PCR assay for the detection and identification of Campylobacter jejuni, Campylobacter coli and Campylobacter fetus. FEMS Immunol. Med. Microbiol. 2008, 52, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.F.; Duarte, A.; Pereira, G.; Mateus, L.; Lopes-da-Costa, L.; Silva, E. Assessment of Campylobacter fetus subsp. venerealis molecular diagnosis using clinical samples of bulls. BMC Vet. Res. 2020, 16. [Google Scholar] [CrossRef] [PubMed]
- The European Commitee on Antimicrobial Susceptibility Testing (Ed.) Breakpoint Tables for Interpretation of MICs and Zone Diameters; The European Commitee on Antimicrobial Susceptibility Testing: Central Hospital Växjö, Sweden, 2020; Volume 10. [Google Scholar]
- NARMS Interpretive Criteria for Susceptibility Testing. Available online: https://www.fda.gov/media/108180/download (accessed on 4 September 2020).
- Davis, J.J.; Wattam, A.R.; Aziz, R.K.; Brettin, T.; Butler, R.; Butler, R.M.; Chlenski, P.; Conrad, N.; Dickerman, A.; Dietrich, E.M.; et al. The PATRIC Bioinformatics Resource Center: Expanding data and analysis capabilities. Nucleic Acids Res. 2020, 48, 606–612. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.O.N.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Brettin, T.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Olsen, G.J.; Olson, R.; Overbeek, R.; Parrello, B.; Pusch, G.D.; et al. RASTtk: A modular and extensible implementation of the RAST algorithm for building custom annotation pipelines and annotating batches of genomes. Sci. Rep. 2015, 5, 8365. [Google Scholar] [CrossRef]
- Alikhan, N.; Petty, N.K.; Zakour, N.L.B.; Beatson, S.A. BLAST Ring Image Generator (BRIG): Simple prokaryote genome comparisons. BMC Genom. 2011, 402. [Google Scholar] [CrossRef]
- Larsen, M.V.; Cosentino, S.; Rasmussen, S.; Friis, C.; Hasman, H.; Marvig, L.; Jelsbak, L.; Sicheritz-pontén, T.; Ussery, D.W.; Aarestrup, F.M.; et al. Multilocus sequence typing of total-genome-sequenced bacteria. J. Clin. Microbiol. 2012, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.C.; Stucky, M.; Wakefield, C.; Melott, J.M.; Akbani, R.; Weinstein, J.N.; Broom, B.M. Interactive Clustered Heat Map Builder: An easy web-based tool for creating sophisticated clustered heat maps. F1000Research 2020, 8, 1750. [Google Scholar] [CrossRef] [PubMed]
- Kaas, R.S.; Leekitcharoenphon, P.; Aarestrup, F.M.; Lund, O. Solving the problem of comparing whole bacterial genomes across different sequencing platforms. PLoS ONE 2014, 9, e104984. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- van der Graaf-van Bloois, L.; Duim, B.; Miller, W.G.; Forbes, K.J.; Wagenaar, J.A.; Zomer, A. Whole genome sequence analysis indicates recent diversification of mammal-associated Campylobacter fetus and implicates a genetic factor associated with H2S production. BMC Genom. 2016, 17, 1–10. [Google Scholar] [CrossRef]
- Iraola, G.; Betancor, L.; Calleros, L.; Gadea, P.; Algorta, G.; Galeano, S.; Muxi, P.; Greif, G.; Pérez, R. A rural worker infected with a bovine-prevalent genotype of Campylobacter fetus subsp. fetus supports zoonotic transmission and inconsistency of MLST and whole-genome typing. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1593–1596. [Google Scholar] [CrossRef]
- Bolton, D.J. Campylobacter virulence and survival factors. Food Microbiol. 2015, 48, 99–108. [Google Scholar] [CrossRef]
- Willoughby, K.; Nettleton, P.F.; Quirie, M.; Maley, M.A.; Foster, G.; Toszeghy, M. A multiplex polymerase chain reaction to detect and differentiate Campylobacter fetus subspecies fetus and Campylobacter fetus-species venerealis: Use on UK isolates of C. fetus and other Campylobacter spp. J. Appl. Microbiol. 2005, 99, 758–766. [Google Scholar] [CrossRef]
- Abdel-glil, M.Y.; Hotzel, H.; Tomaso, H.; Linde, J. Phylogenomic analysis of Campylobacter fetus reveals a clonal structure of insertion element ISCfe1 positive genomes. Front. Microbiol. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Silva, M.F.; Gonçalo, P.; Carneiro, C.; Hemphill, A.; Mateus, L.; Lopes-da-Costa, L.; Silva, E. Campylobacter portucalensis sp. nov, a new species of Campylobacter isolated from the preputial mucosa of bulls. PLoS ONE 2020, 15, e0227500. [Google Scholar] [CrossRef]
- Vargas, A.C.; Costa, M.M.; Groff, A.C.M.; Viana, L.R.; Krewer, C.C.; Spricigo, D.A.; Kirinus, J.K. Susceptibilidade antimicrobiana de Campylobacter fetus subsp. venerealis isolado de bovinos. Pesqui. Veterinária Bras. 2005, 25, 1–3. [Google Scholar] [CrossRef]
- Hänel, I.; Hotzel, H.; Müller, W.; Tomaso, H. Antimicrobial susceptibility testing of German Campylobacter fetus subsp. venerealis isolates by agar disk diffusion method. Berl. Munch. Tierarztl. Wochenschr. 2011, 124, 198–202. [Google Scholar] [CrossRef]
- Lin, J.; Michel, L.O.; Zhang, Q. CmeABC functions as a multidrug efflux system in Campylobacter jejuni. Antimicrob. Agents Chemother. 2002, 46, 2124–2131. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Sahin, O.; Michel, L.O.; Zhang, Q. Critical role of multidrug efflux pump CmeABC in bile resistance and in vivo colonization of Campylobacter jejuni. Infect. Immun. 2003, 71, 4250–4259. [Google Scholar] [CrossRef]
- Guo, B.; Lin, J.; Reynolds, D.L.; Zhang, Q. Contribution of the Multidrug Efflux Transporter CmeABC to Antibiotic Resistance in Different Campylobacter Species. Foodborne Pathog. Dis. 2010, 7, 77–83. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).