The PTS Components in Klebsiella pneumoniae Affect Bacterial Capsular Polysaccharide Production and Macrophage Phagocytosis Resistance
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Plasmids, and Growth Conditions
2.2. Construction of the crr Mutant and Δcrr/ΔetcABC Double Mutant
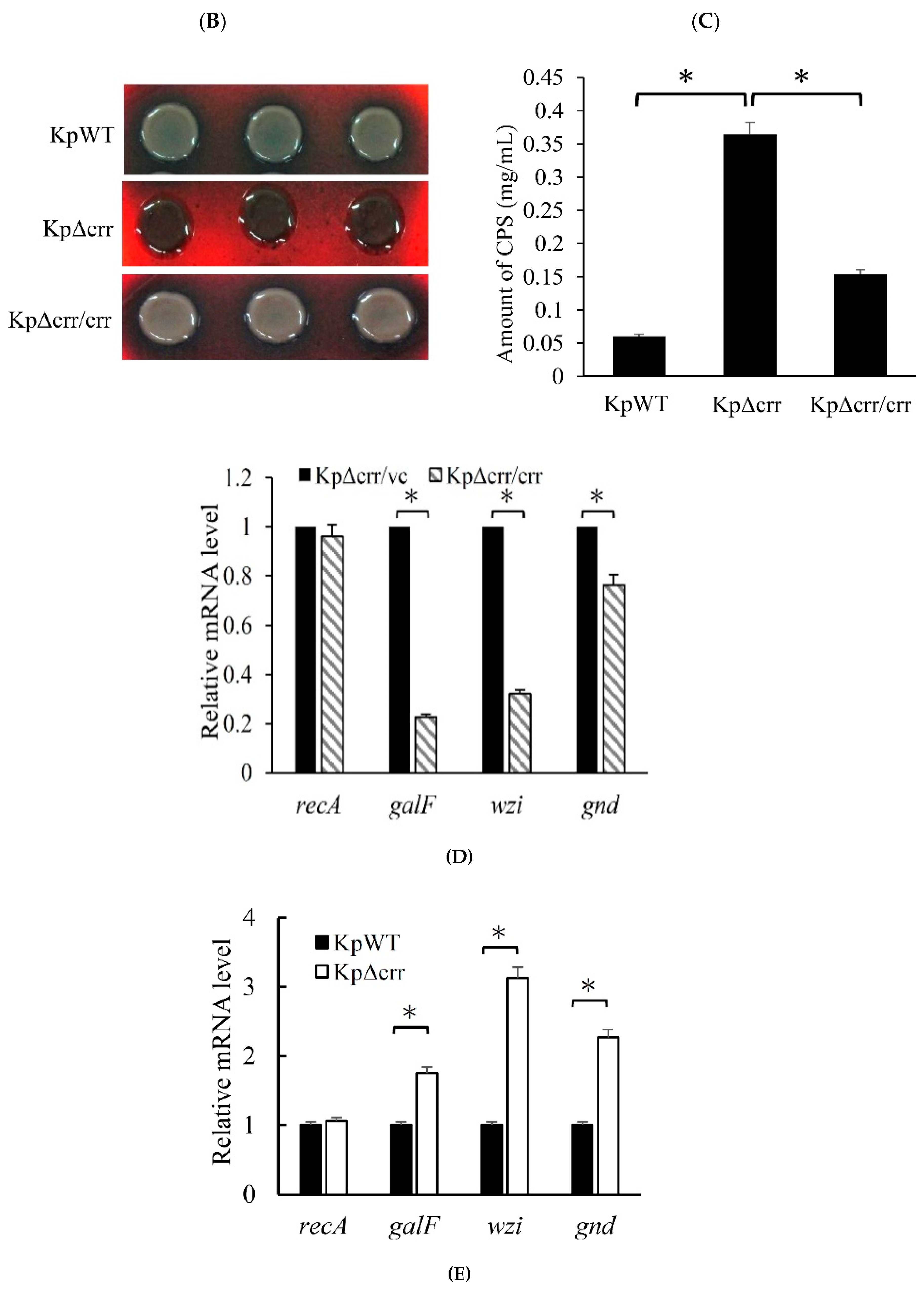
2.3. String Test
2.4. Congo Red Agar (CRA) Assay
2.5. Bacterial Mucoviscosity Assay
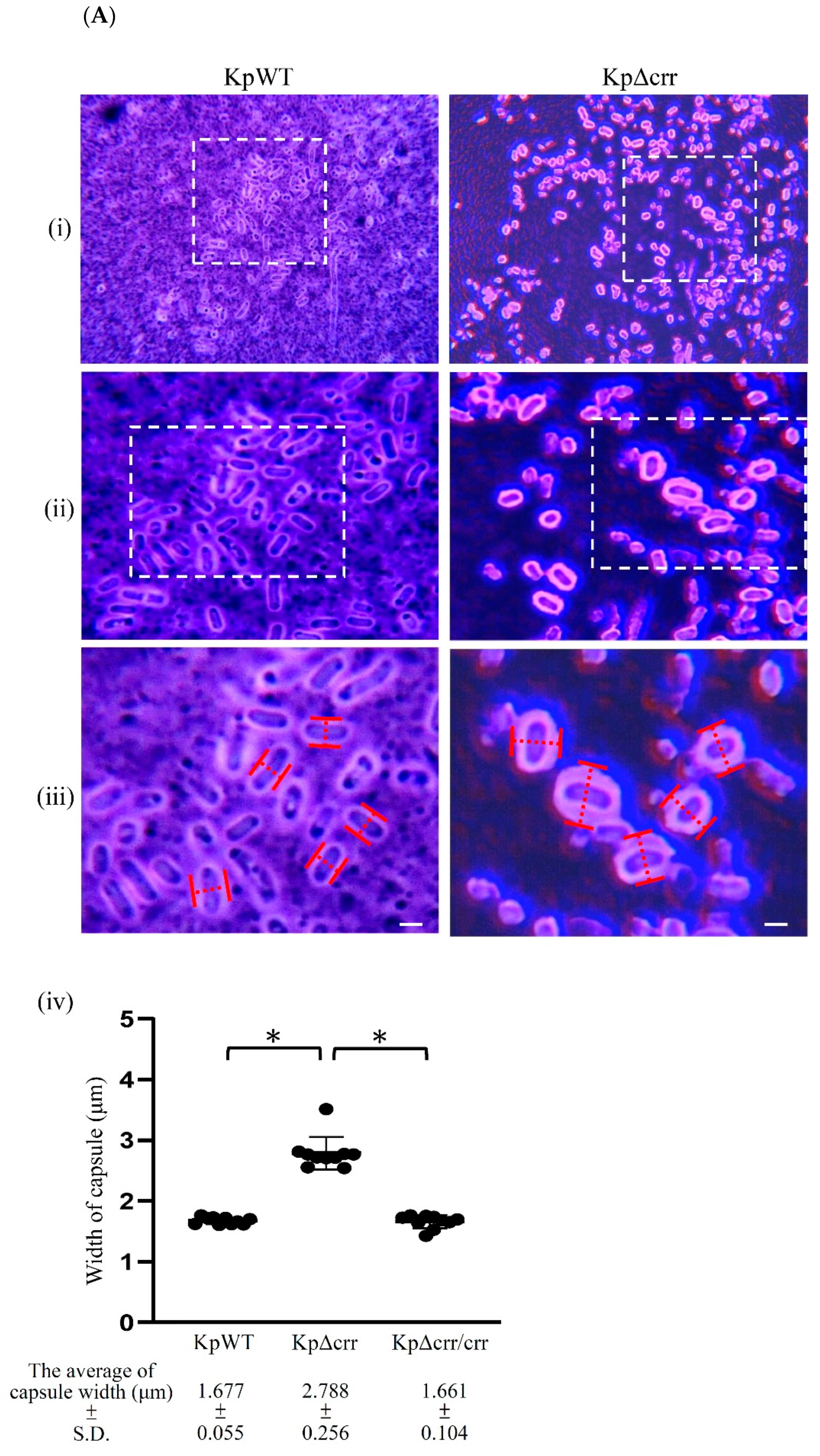
2.6. Transmission Electron Microscopy (TEM)
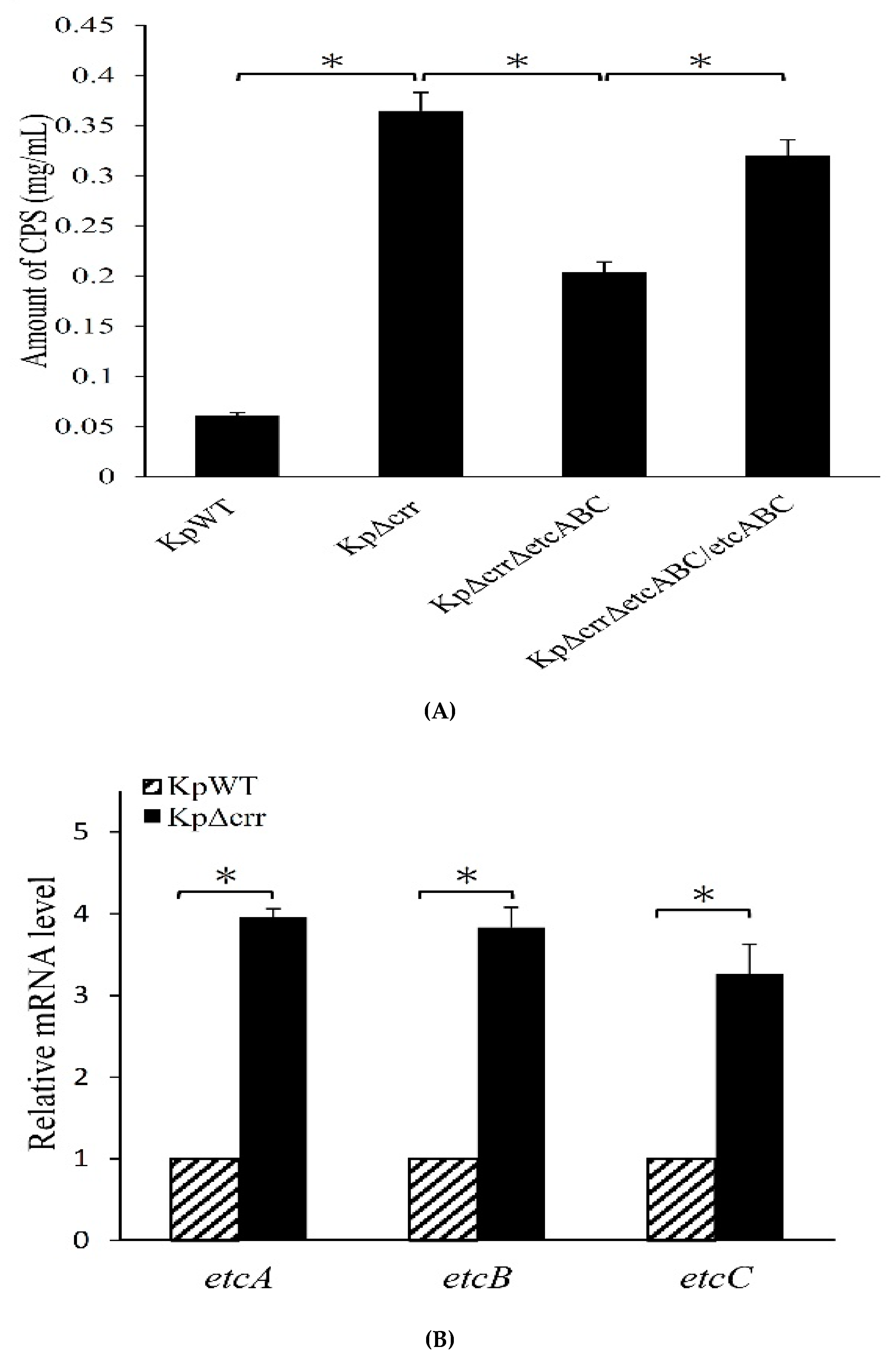
2.7. Extraction and Quantification of Capsular Polysaccharide (CPS)
2.8. Reverse Transcription Quantitative Real-Time PCR (RT-qPCR)
2.9. Hydrogen Peroxide (H2O2) Sensitivity Assay
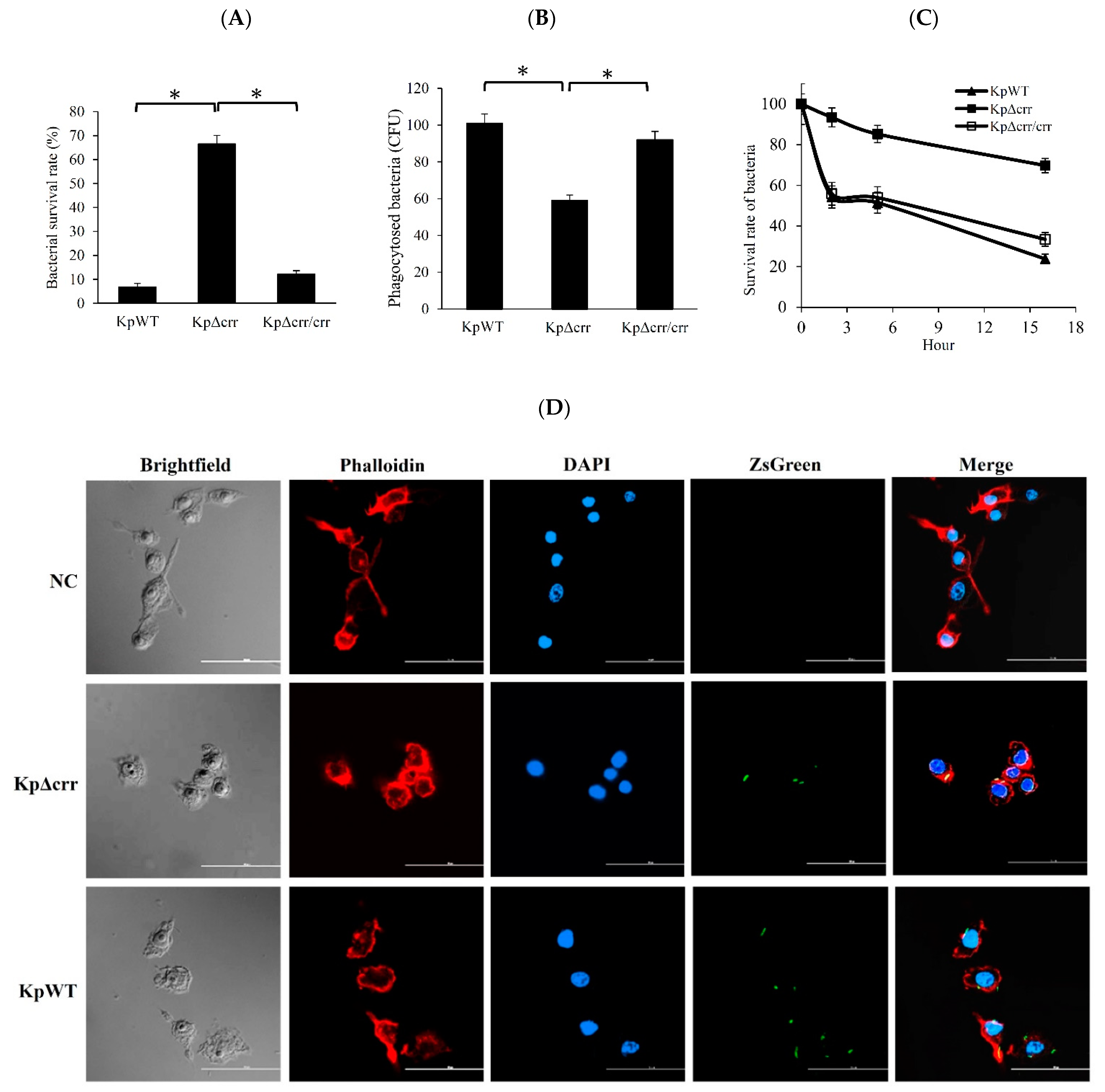
2.10. Phagocytosis Assays (Macrophage Ingestion and Bacterial Survival Ability in Macrophages)
2.11. Phagocytosis Observed by Confocal Laser Scanning Microscopy (CLSM)
2.12. Statistical Analysis
3. Results
3.1. Phenotypic Characteristics of the crr Mutant in K. pneumoniae STU1
3.2. Crr, an EIIA Component, Reduces CPS Synthesis in K. pneumoniae STU1
3.3. Deficiency of crr Rendered Bacteria Resistant to Oxidative Stress and Phagocytosis
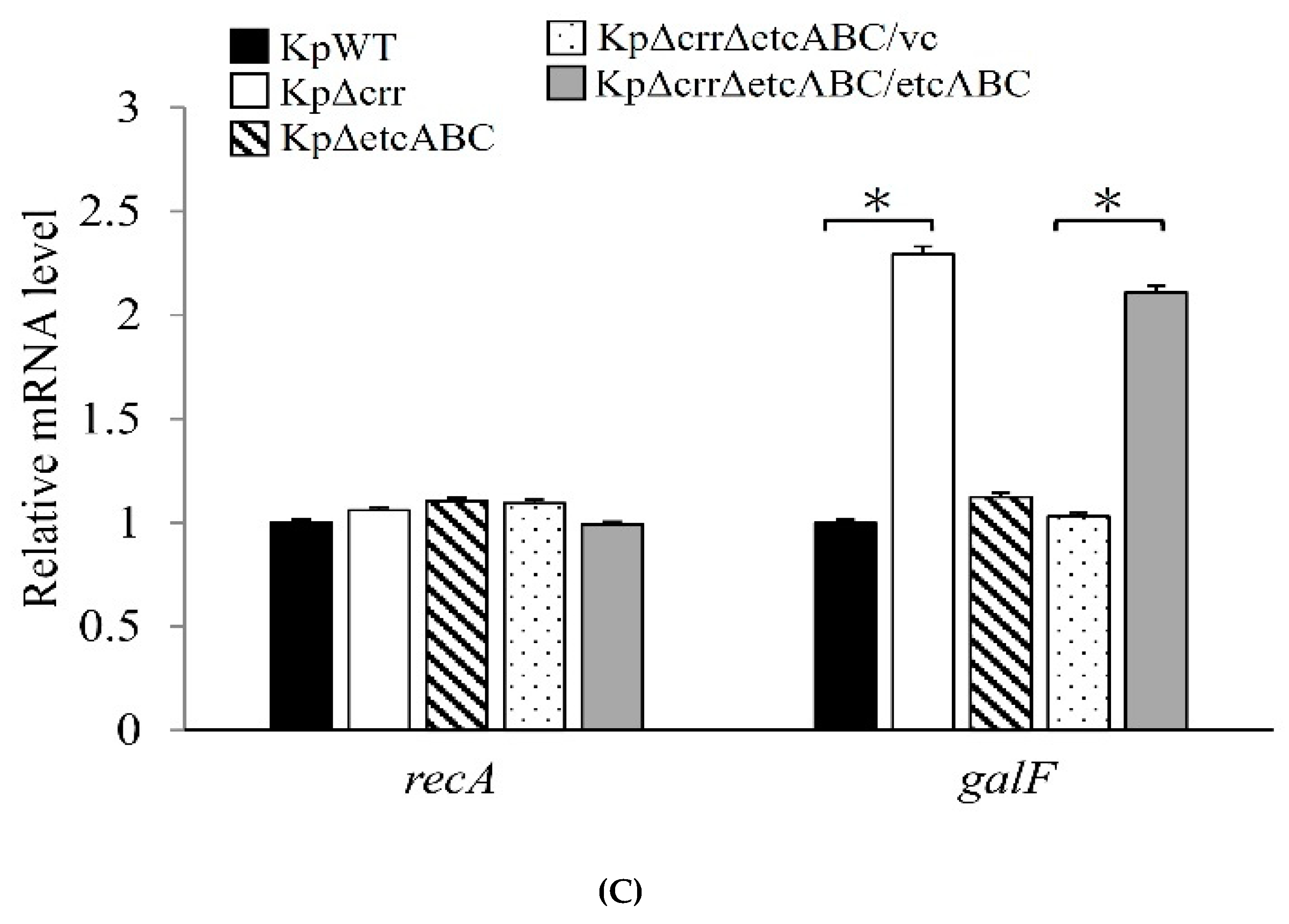
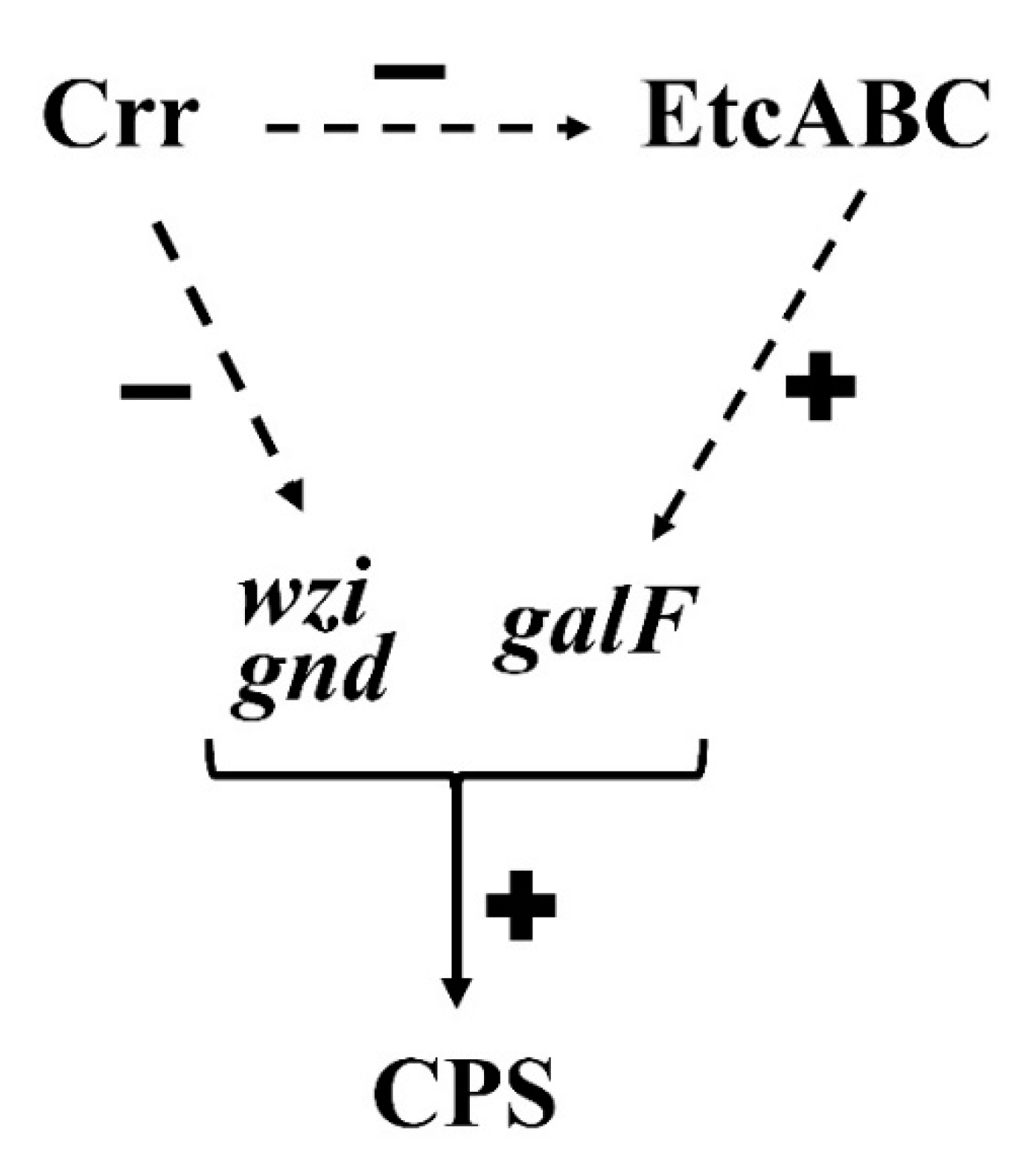
3.4. Deficiency of crr Resulted in Overexpression of Another EII Complex, EtcABC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paczosa, M.K.; Mecsas, J. Klebsiella pneumoniae: Going on the Offense with a Strong Defense. Microbiol. Mol. Biol. Rev. 2016, 80, 629–661. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, G.; Yang, Y.; Zhang, J.; Li, D.; Duan, S.; Yang, Q.; Xu, Y. Antimicrobial resistance comparison of Klebsiella pneumoniae pathogens isolated from intra-abdominal and urinary tract infections in different organs, hospital departments and regions of China between 2014 and 2017. J. Microbiol. Immunol. Infect. 2020. [Google Scholar] [CrossRef]
- Klaper, K.; Wendt, S.; Lubbert, C.; Lippmann, N.; Pfeifer, Y.; Werner, G. Hypervirulent Klebsiella pneumoniae of Lineage ST66-K2 Caused Tonsillopharyngitis in a German Patient. Microorganisms 2021, 9, 133. [Google Scholar] [CrossRef]
- Bagley, S.T. Habitat association of Klebsiella species. Infect. Control. 1985, 6, 52–58. [Google Scholar] [CrossRef]
- Podschun, R.; Pietsch, S.; Holler, C.; Ullmann, U. Incidence of Klebsiella species in surface waters and their expression of virulence factors. Appl. Environ. Microbiol. 2001, 67, 3325–3327. [Google Scholar] [CrossRef] [PubMed]
- Zadoks, R.N.; Griffiths, H.M.; Munoz, M.A.; Ahlstrom, C.; Bennett, G.J.; Thomas, E.; Schukken, Y.H. Sources of Klebsiella and Raoultella species on dairy farms: Be careful where you walk. J. Dairy Sci. 2011, 94, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathi, K.; Ranganathan, L.S.; Anandi, V.; Zeyer, J. Diversity of microflora in the gut and casts of tropical composting earthworms reared on different substrates. J. Environ. Biol. 2007, 28, 87–97. [Google Scholar] [PubMed]
- Stenkat, J.; Krautwald-Junghanns, M.E.; Schmitz Ornes, A.; Eilers, A.; Schmidt, V. Aerobic cloacal and pharyngeal bacterial flora in six species of free-living birds. J. Appl. Microbiol. 2014, 117, 1564–1571. [Google Scholar] [CrossRef]
- Ranjbar, R.; Izadi, M.; Hafshejani, T.T.; Khamesipour, F. Molecular detection and antimicrobial resistance of Klebsiella pneumoniae from house flies (Musca domestica) in kitchens, farms, hospitals and slaughterhouses. J. Infect. Public Health 2016, 9, 499–505. [Google Scholar] [CrossRef]
- Gorrie, C.L.; Mirceta, M.; Wick, R.R.; Edwards, D.J.; Thomson, N.R.; Strugnell, R.A.; Pratt, N.F.; Garlick, J.S.; Watson, K.M.; Pilcher, D.V.; et al. Gastrointestinal Carriage Is a Major Reservoir of Klebsiella pneumoniae Infection in Intensive Care Patients. Clin. Infect. Dis. 2017, 65, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Brahmi, S.; Touati, A.; Dunyach-Remy, C.; Sotto, A.; Pantel, A.; Lavigne, J.P. High Prevalence of Extended-Spectrum beta-Lactamase-Producing Enterobacteriaceae in Wild Fish from the Mediterranean Sea in Algeria. Microb. Drug Resist. 2018, 24, 290–298. [Google Scholar] [CrossRef]
- Founou, L.L.; Founou, R.C.; Allam, M.; Ismail, A.; Djoko, C.F.; Essack, S.Y. Genome Sequencing of Extended-Spectrum beta-Lactamase (ESBL)-Producing Klebsiella pneumoniae Isolated from Pigs and Abattoir Workers in Cameroon. Front. Microbiol. 2018, 9, 188. [Google Scholar] [CrossRef]
- Montso, K.P.; Dlamini, S.B.; Kumar, A.; Ateba, C.N. Antimicrobial Resistance Factors of Extended-Spectrum Beta-Lactamases Producing Escherichia coli and Klebsiella pneumoniae Isolated from Cattle Farms and Raw Beef in North-West Province, South Africa. Biomed. Res. Int. 2019, 2019, 4318306. [Google Scholar] [CrossRef] [PubMed]
- Conlan, S.; Kong, H.H.; Segre, J.A. Species-level analysis of DNA sequence data from the NIH Human Microbiome Project. PLoS ONE 2012, 7, e47075. [Google Scholar] [CrossRef]
- Patro, L.P.P.; Rathinavelan, T. Targeting the Sugary Armor of Klebsiella Species. Front. Cell Infect. Microbiol 2019, 9, 367. [Google Scholar] [CrossRef]
- Shon, A.S.; Bajwa, R.P.; Russo, T.A. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: A new and dangerous breed. Virulence 2013, 4, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, M.S.; Hsu, J.; Rick, P.D.; Miller, V.L. Identification of Klebsiella pneumoniae virulence determinants using an intranasal infection model. Mol. Microbiol. 2005, 58, 1054–1073. [Google Scholar] [CrossRef]
- Doyle, C.R.; Moon, J.Y.; Daily, J.P.; Wang, T.; Pirofski, L.A. A Capsular Polysaccharide-Specific Antibody Alters Streptococcus pneumoniae Gene Expression during Nasopharyngeal Colonization of Mice. Infect. Immun 2018, 86. [Google Scholar] [CrossRef]
- Zaragoza, O.; Chrisman, C.J.; Castelli, M.V.; Frases, S.; Cuenca-Estrella, M.; Rodriguez-Tudela, J.L.; Casadevall, A. Capsule enlargement in Cryptococcus neoformans confers resistance to oxidative stress suggesting a mechanism for intracellular survival. Cell Microbiol. 2008, 10, 2043–2057. [Google Scholar] [CrossRef]
- Regueiro, V.; Campos, M.A.; Pons, J.; Alberti, S.; Bengoechea, J.A. The uptake of a Klebsiella pneumoniae capsule polysaccharide mutant triggers an inflammatory response by human airway epithelial cells. Microbiology 2006, 152, 555–566. [Google Scholar] [CrossRef] [PubMed]
- March, C.; Cano, V.; Moranta, D.; Llobet, E.; Perez-Gutierrez, C.; Tomas, J.M.; Suarez, T.; Garmendia, J.; Bengoechea, J.A. Role of bacterial surface structures on the interaction of Klebsiella pneumoniae with phagocytes. PLoS ONE 2013, 8, e56847. [Google Scholar] [CrossRef]
- Evrard, B.; Balestrino, D.; Dosgilbert, A.; Bouya-Gachancard, J.L.; Charbonnel, N.; Forestier, C.; Tridon, A. Roles of capsule and lipopolysaccharide O antigen in interactions of human monocyte-derived dendritic cells and Klebsiella pneumoniae. Infect. Immun. 2010, 78, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Chang, C.C.; Liu, J.W.; Chen, R.F.; Yang, K.D. Sialic acid involved in hypermucoviscosity phenotype of Klebsiella pneumoniae and associated with resistance to neutrophil phagocytosis. Virulence 2014, 5, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Cortes, G.; Alvarez, D.; Saus, C.; Alberti, S. Role of lung epithelial cells in defense against Klebsiella pneumoniae pneumonia. Infect. Immun. 2002, 70, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.J.; Lin, T.L.; Chen, C.T.; Chen, Y.Y.; Hsieh, P.F.; Hsu, C.R.; Wu, M.C.; Wang, J.T. Genetic analysis of capsular polysaccharide synthesis gene clusters in 79 capsular types of Klebsiella spp. Sci. Rep. 2015, 5, 15573. [Google Scholar] [CrossRef]
- Deutscher, J.; Francke, C.; Postma, P.W. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol. Mol. Biol. Rev. 2006, 70, 939–1031. [Google Scholar] [CrossRef] [PubMed]
- Deutscher, J.; Ake, F.M.; Derkaoui, M.; Zebre, A.C.; Cao, T.N.; Bouraoui, H.; Kentache, T.; Mokhtari, A.; Milohanic, E.; Joyet, P. The bacterial phosphoenolpyruvate:carbohydrate phosphotransferase system: Regulation by protein phosphorylation and phosphorylation-dependent protein-protein interactions. Microbiol. Mol. Biol. Rev. 2014, 78, 231–256. [Google Scholar] [CrossRef]
- Park, S.; Park, Y.H.; Lee, C.R.; Kim, Y.R.; Seok, Y.J. Glucose induces delocalization of a flagellar biosynthesis protein from the flagellated pole. Mol. Microbiol. 2016, 101, 795–808. [Google Scholar] [CrossRef]
- Oh, B.R.; Hong, W.K.; Heo, S.Y.; Luo, L.H.; Kondo, A.; Seo, J.W.; Kim, C.H. The production of 1,3-propanediol from mixtures of glycerol and glucose by a Klebsiella pneumoniae mutant deficient in carbon catabolite repression. Bioresour. Technol. 2013, 130, 719–724. [Google Scholar] [CrossRef]
- Jeng, W.Y.; Panjaitan, N.S.D.; Horng, Y.T.; Chung, W.T.; Chien, C.C.; Soo, P.C. The Negative Effects of KPN00353 on Glycerol Kinase and Microaerobic 1,3-Propanediol Production in Klebsiella pneumoniae. Front. Microbiol. 2017, 8, 2441. [Google Scholar] [CrossRef]
- Horng, Y.T.; Wang, C.J.; Chung, W.T.; Chao, H.J.; Chen, Y.Y.; Soo, P.C. Phosphoenolpyruvate phosphotransferase system components positively regulate Klebsiella biofilm formation. J. Microbiol. Immunol. Infect. 2018, 51, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Panjaitan, N.S.D.; Horng, Y.T.; Cheng, S.W.; Chung, W.T.; Soo, P.C. EtcABC, a Putative EII Complex, Regulates Type 3 Fimbriae via CRP-cAMP Signaling in Klebsiella pneumoniae. Front. Microbiol. 2019, 10, 1558. [Google Scholar] [CrossRef]
- Fang, C.T.; Chuang, Y.P.; Shun, C.T.; Chang, S.C.; Wang, J.T. A novel virulence gene in Klebsiella pneumoniae strains causing primary liver abscess and septic metastatic complications. J. Exp. Med. 2004, 199, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Rampelotto, R.F.; Lorenzoni, V.V.; Silva, D.D.C.; Coelho, S.S.; Wust, V.; Garzon, L.R.; Nunes, M.S.; Meneghetti, B.; Brites, P.C.; Horner, M.; et al. Assessment of different methods for the detection of biofilm production in coagulase-negative staphylococci isolated from blood cultures of newborns. Rev. Soc. Bras. Med. Trop. 2018, 51, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.C.; Lin, T.L.; Hsieh, P.F.; Yang, H.C.; Wang, J.T. Isolation of genes involved in biofilm formation of a Klebsiella pneumoniae strain causing pyogenic liver abscess. PLoS ONE 2011, 6, e23500. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Gomes, A.E.I.; Stuchi, L.P.; Siqueira, N.M.G.; Henrique, J.B.; Vicentini, R.; Ribeiro, M.L.; Darrieux, M.; Ferraz, L.F.C. Selection and validation of reference genes for gene expression studies in Klebsiella pneumoniae using Reverse Transcription Quantitative real-time PCR. Sci. Rep. 2018, 8, 9001. [Google Scholar] [CrossRef]
- Pericone, C.D.; Park, S.; Imlay, J.A.; Weiser, J.N. Factors contributing to hydrogen peroxide resistance in Streptococcus pneumoniae include pyruvate oxidase (SpxB) and avoidance of the toxic effects of the fenton reaction. J. Bacteriol. 2003, 185, 6815–6825. [Google Scholar] [CrossRef]
- Liu, P.; Xue, J.; Tong, S.; Dong, W.; Wu, P. Structure Characterization and Hypoglycaemic Activities of Two Polysaccharides from Inonotus obliquus. Molecules 2018, 23, 1948. [Google Scholar] [CrossRef]
- Freeman, D.J.; Falkiner, F.R.; Keane, C.T. New method for detecting slime production by coagulase negative staphylococci. J. Clin. Pathol. 1989, 42, 872–874. [Google Scholar] [CrossRef]
- Zeng, G.Q.; De Reuse, H.; Danchin, A. Mutational analysis of the enzyme IIIGlc of the phosphoenolpyruvate phosphotransferase system in Escherichia coli. Res. Microbiol. 1992, 143, 251–261. [Google Scholar] [CrossRef]
- Pelton, J.G.; Torchia, D.A.; Remington, S.J.; Murphy, K.P.; Meadow, N.D.; Roseman, S. Structures of active site histidine mutants of IIIGlc, a major signal-transducing protein in Escherichia coli. Effects on the mechanisms of regulation and phosphoryl transfer. J. Biol. Chem. 1996, 271, 33446–33456. [Google Scholar] [CrossRef] [PubMed]
- Ares, M.A.; Sansabas, A.; Rodriguez-Valverde, D.; Siqueiros-Cendon, T.; Rascon-Cruz, Q.; Rosales-Reyes, R.; Jarillo-Quijada, M.D.; Alcantar-Curiel, M.D.; Cedillo, M.L.; Torres, J.; et al. The Interaction of Klebsiella pneumoniae With Lipid Rafts-Associated Cholesterol Increases Macrophage-Mediated Phagocytosis Due to Down Regulation of the Capsule Polysaccharide. Front. Cell Infect. Microbiol. 2019, 9, 255. [Google Scholar] [CrossRef]
- Cano, V.; March, C.; Insua, J.L.; Aguilo, N.; Llobet, E.; Moranta, D.; Regueiro, V.; Brennan, G.P.; Millan-Lou, M.I.; Martin, C.; et al. Klebsiella pneumoniae survives within macrophages by avoiding delivery to lysosomes. Cell Microbiol. 2015, 17, 1537–1560. [Google Scholar] [CrossRef] [PubMed]
- Morabbi Heravi, K.; Altenbuchner, J. Cross Talk among Transporters of the Phosphoenolpyruvate-Dependent Phosphotransferase System in Bacillus subtilis. J. Bacteriol. 2018, 200. [Google Scholar] [CrossRef] [PubMed]
- Chaillou, S.; Postma, P.W.; Pouwels, P.H. Contribution of the phosphoenolpyruvate:mannose phosphotransferase system to carbon catabolite repression in Lactobacillus pentosus. Microbiology 2001, 147, 671–679. [Google Scholar] [CrossRef][Green Version]
- Lin, C.T.; Chen, Y.C.; Jinn, T.R.; Wu, C.C.; Hong, Y.M.; Wu, W.H. Role of the cAMP-dependent carbon catabolite repression in capsular polysaccharide biosynthesis in Klebsiella pneumoniae. PLoS ONE 2013, 8, e54430. [Google Scholar] [CrossRef]
- Galinier, A.; Deutscher, J. Sophisticated Regulation of Transcriptional Factors by the Bacterial Phosphoenolpyruvate: Sugar Phosphotransferase System. J. Mol. Biol. 2017, 429, 773–789. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Chen, I.L.; Chuah, S.K.; Tai, W.C.; Chang, C.C.; Chen, F.J.; Chen, J.F. Impact of glycemic control on capsular polysaccharide biosynthesis and opsonophagocytosis of Klebsiella pneumoniae: Implications for invasive syndrome in patients with diabetes mellitus. Virulence 2016, 7, 770–778. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panjaitan, N.S.D.; Horng, Y.-T.; Chien, C.-C.; Yang, H.-C.; You, R.-I.; Soo, P.-C. The PTS Components in Klebsiella pneumoniae Affect Bacterial Capsular Polysaccharide Production and Macrophage Phagocytosis Resistance. Microorganisms 2021, 9, 335. https://doi.org/10.3390/microorganisms9020335
Panjaitan NSD, Horng Y-T, Chien C-C, Yang H-C, You R-I, Soo P-C. The PTS Components in Klebsiella pneumoniae Affect Bacterial Capsular Polysaccharide Production and Macrophage Phagocytosis Resistance. Microorganisms. 2021; 9(2):335. https://doi.org/10.3390/microorganisms9020335
Chicago/Turabian StylePanjaitan, Novaria Sari Dewi, Yu-Tze Horng, Chih-Ching Chien, Hung-Chi Yang, Ren-In You, and Po-Chi Soo. 2021. "The PTS Components in Klebsiella pneumoniae Affect Bacterial Capsular Polysaccharide Production and Macrophage Phagocytosis Resistance" Microorganisms 9, no. 2: 335. https://doi.org/10.3390/microorganisms9020335
APA StylePanjaitan, N. S. D., Horng, Y.-T., Chien, C.-C., Yang, H.-C., You, R.-I., & Soo, P.-C. (2021). The PTS Components in Klebsiella pneumoniae Affect Bacterial Capsular Polysaccharide Production and Macrophage Phagocytosis Resistance. Microorganisms, 9(2), 335. https://doi.org/10.3390/microorganisms9020335




