Abstract
Meat is an important food source that can provide a significant amount of protein for human development. The occurrence of bacteria that are resistant to antimicrobials in meat poses a public health risk. This study evaluated the occurrence and antimicrobial resistance of E. coli (Escherichia coli) isolated from raw meats, ready-to-eat (RTE) meats and their related samples in Ghana. E. coli was isolated using the USA-FDA Bacteriological Analytical Manual and phenotypic antimicrobial susceptibility test was performed by the disk diffusion method. Of the 200 examined meats and their related samples, 38% were positive for E. coli. Notably, E. coli was highest in raw beef (80%) and lowest in RTE pork (0%). The 45 E. coli isolates were resistant ≥ 50% to amoxicillin, trimethoprim and tetracycline. They were susceptible to azithromycin (87.1%), chloramphenicol (81.3%), imipenem (74.8%), gentamicin (72.0%) and ciprofloxacin (69.5%). A relatively high intermediate resistance of 33.0% was observed for ceftriaxone. E. coli from raw meats, RTE meats, hands of meat sellers and working tools showed some differences and similarities in their phenotypic antimicrobial resistance patterns. Half (51.1%) of the E. coli isolates exhibited multidrug resistance. The E. coli isolates showed twenty-two different resistant patterns, with a multiple antibiotic resistance index of 0.0 to 0.7. The resistant pattern amoxicillin (A, n = 6 isolates) and amoxicillin-trimethoprim (A-TM, n = 6 isolates) were the most common. This study documents that raw meats, RTE meats and their related samples in Ghana are potential sources of antimicrobial-resistant E. coli and pose a risk for the transfer of resistant bacteria to the food chain, environment and humans.
1. Introduction
Escherichia coli is a Gram-negative bacteria of the enterobacteriaceae family [1]. Escherichia coli lives in the gastrointestinal tracts (GIT) of humans and animals [1,2]. They commonly end up in the environment due to the close association of animals and humans to their environment [1,3]. The interaction between animals and humans also paves the way for transfer of these bacteria between them [4]. Although most E. coli strains are noted to be commensals, pathogenic E. coli exists [2]. Pathogenic E. coli strains have been responsible for the cause of food poisoning, pneumonia and urinary tract infections [1]. E. coli infections can also lead to hospitalizations, loss of manpower and even death [5,6]. For instance, in the USA, the Center for Control and Disease Prevention (CDC) [6] reported an E. coli infection from an unknown food that caused 16 people to be ill, with eight hospitalizations resulting in one developing hemolytic uremic syndrome and one death. Outbreaks of E. coli infections in Europe have also been noted [7]. The contribution of some strains of E. coli to travelers’ diarrhea, especially among children in lower income countries, including Ghana, cannot be overemphasized, although official reports are limited [1,8].
Escherichia coli infections in humans normally result from the consumption of contaminated foods that are present in the food chain. Foods of animal origin (meat and meat products), in particular, have played a major role in the spread of E. coli infections in humans. For example, beef, chevon, pork, poultry and/or their products have been implicated in the cause of foodborne infections in the USA and Europe [5,6,9]. These meat and meat products, to a lesser extent, also serve as sources for which E. coli can cross contaminate other foods and the environment. The European Food Safety Authority (EFSA) [9] reported that meat and meat products were the food category that were tested the most for E. coli linked to human infections in a five-year period in Europe. The occurrence of E. coli in raw meats, ready-to-eat (RTE) meats, humans, working tools and/or the environment have been reported in America [5,6,10], Europe [9,11], Asia [12,13,14] and Africa [15,16,17]. Escherichia coli infections are usually self-limiting; however, when treatments are required, antimicrobials are used [18].
Antimicrobials are agents used to curtail the growth of microorganisms or are used to kill them [19,20]. Antimicrobial resistance occurs when bacteria resist antimicrobials that are meant to destroy them. The unabated use of antimicrobials in farm animals for growth promotion, treatment of sick animals or as prophylactics has been the major cause of the spread of antimicrobial resistances in animals. This can spread to humans via consumption of meat and meat products from farm animals containing resistance bacteria. Humans and animals can cross contaminate other foods and the environment with resistant bacteria when they come into contact with them. There is also the possibility of resistant bacteria to spread across the food chain, humans and animals from the environment. The resistance of bacteria, including E. coli, to antimicrobials has received much attention in recent times due to the emergence of multidrug resistance and the difficulty in destroying such bacteria when they are involved in infections [18,19]. The use of antimicrobials in animal production in Ghana is not strictly regulated and monitored. As such farmers and farm attenders sometimes treat their animals with antimicrobials without prescription or adherence to instructed dosage and withdrawal periods [21,22]. In addition, farmers did not demonstrate in-depth knowledge of the antimicrobials used [21,22]. These practices pose the risk of bacteria developing resistance to antimicrobials. Furthermore, studies have shown the presence of antimicrobial resistance E. coli in meats and meats products in Ghana [23,24,25]. Dsani et al. [23] reported that E. coli isolated from meat sources were resistant to cefuroxime (17%), sulfamethoxazole-trimethoprim (21%), tetracycline (45%) and ampicillin (57%). Eibach et al. [24] found that E. coli isolates from poultry meat were resistant to ciprofloxacin (21%) and sulfamethoxazole-trimethoprim (32%), and harbored resistance genes such as BlaSHV, blaCTX-M and blaTEM. Resistance of E. coli isolated from beef to tetracycline, azithromycin and sulfamethoxazole-trimethoprim was 40%, 33% and 20%, respectively [24]. They also reported that some of the E. coli isolates were resistant to multiple antimicrobials and/or harbored BlaSHV, blaCTX-M, blaTEM, tet (A), aph (3’’)-Ib, apha (6), dfrA14 and mdf (A) resistance genes [23,24,25]. Thus, meats and meat products in Ghana are potential sources of antimicrobial-resistant bacteria.
Moreover, studies on the spread of E. coli among meats, humans and their environment in Ghana are limited. Studies have also shown that the manner in which animals are handled prior to slaughter, during slaughter and post-slaughter makes meat and their related samples potential sources of foodborne pathogens [26,27,28,29,30,31,32]. In addition, the sale of meats and meat products is sometimes conducted in open markets and by roadsides without adherence to strict hygiene [33,34,35]. RTE meats in particular are consumed without prior heating [36]. With all these handling activities, meat and meat products expose consumers to foodborne infections and antimicrobial resistance bacteria. Therefore, this study is aimed at determining whether raw meats, RTE meats and their related samples (hands of meat sellers, knives for cutting meat, tables on which meats are placed for cutting and for sale, utensils used by meat sellers) are contaminated by antimicrobial-resistant E. coli.
2. Materials and Methods
2.1. Study Area
This study was carried out in the Upper East Region of Ghana. The region shares a boundary with Burkina Faso to the north, the Republic of Togo to the east, West Mamprusi in the Northern Region to the south and Sissala in the Upper West Region to the west. The region covers an area of 8842 square kilometers and is also located between longitude 0° and 1° West, and latitudes 10°30′ North and 11° North [37].
2.2. Duration and Sampling of Raw Meats, Ready-to-Eat (RTE) Meats and Their Related Samples
Sampling was conducted between June and November, 2020. In all, 200 samples were randomly selected and examined for the presence of E. coli. The samples consisted of 10 each of raw beef, raw chevon, raw chicken, raw guinea fowl, raw mutton, raw pork, RTE beef, RTE chevon, RTE chicken, RTE guinea fowl, RTE mutton, RTE pork, hand swabs of raw meat sellers, knife swabs from raw meat sellers, table swabs from raw meat sellers, utensil swabs from raw meat sellers, hand swabs from RTE meat sellers, knife swabs from RTE meat sellers, table swabs from RTE meat sellers and utensil swabs from RTE meat sellers. For the raw and RTE meats, 10 g samples were collected into sterile bags, while for hand, knife, table and utensil samples, approximately 10 cm2 was swabbed. Raw meats, RTE meats and their related samples were transported in an ice chest containing ice and analyzed immediately on arrival at the laboratory.
2.3. Detection and Confirmation of Escherichia coli in Raw Meats, RTE Meats and Their Related Samples
The isolation of E. coli was carried out according to Feng et al. [2]. In total, 10 g of raw meats or RTE meats was pre-enriched in 90 mL of peptone buffered water (MAST Limited, Liverpool, UK) and incubated aerobically at 37 °C for 24 h. After which, the aliquots were streaked on Levine’s eosin-methylene blue (LEMB) agar (MAST Limited, Liverpool, UK) and incubated aerobically at 37 °C for 24 h. Presumptive E. coli isolates were purified on trypticase soy agar (Oxoid Limited, Basingstoke, UK) and confirmed using Gram stain (Oxoid Limited, Basingstoke, UK), growth in brilliant green bile (Oxoid Limited, Basingstoke, UK) containing Durham tubes, and E. coli latex agglutination test (Oxoid Limited, Basingstoke, UK) [2,30,36]. Prevalence results from E. coli were analyzed using binary logistic of IBM Statistical Package for the Social Sciences (SPSS) Version 20, and where significant differences existed, they were separated using Wald chi-square at 5% significance level.
2.4. Phenotypic Antimicrobial Resistance Test
The phenotypic antimicrobial susceptibility test was conducted according to the disk diffusion method [38]. In total, 45 E. coli isolates were subjected to this test using the following antimicrobials: amoxicillin 30 μg (A), azithromycin 15 µg (ATH), ceftriaxone 30 µg (CRO), chloramphenicol 30 µg (C), ciprofloxacin 5 µg (CIP), gentamicin 10 µg (GM), trimethoprim 2.5 µg (TM), tetracycline 30 ug (T) and imipenem 10 µg (IMI) purchased from MAST Limited, Liverpool, UK. Pure cultures of E. coli were grown in 10 mL trypticase soy broth (Oxoid Limited, Basingstoke, UK) overnight at 37 °C and the concentration adjusted to 0.5 McFarland solution. The adjusted solution was spread on Muller Hinton agar (Oxoid Limited, Basingstoke, UK), antimicrobial discs were placed on it and incubated overnight at 37 °C. After incubation, the inhibition zones were measured, and the results interpreted using the Clinical and Laboratory Standards Institute Guidelines [39]. The procedure of Krumperman [40] was used to determine the multiple antibiotic resistance index (MAR index) of the E. coli isolates. MAR index was defined as a/b where “a” is the number of antibiotics to which a particular isolate was resistant, and “b” is the total number of antibiotics examined. Multidrug resistance was defined as resistant to ≥3 different classes of antimicrobials [41].
3. Results
3.1. Distribution of Escherichia coli in Raw Meats, Ready-to-Eat (RTE) Meats and Their Related Samples
The distribution of the E. coli isolates in the raw meats, RTE meats and their related samples is shown in Table 1. Raw beef (80%) was the most contaminated source, followed by raw mutton (70%), and raw chevon (60%), raw pork (60%) and knife of raw meat sellers (60%). In addition, the prevalence of E. coli in raw beef was significantly higher (p < 0.05) than the rest of the samples examined except for raw mutton, raw chevon, raw pork and knife of raw meat sellers. Hands of raw meat sellers and their utensils had the least contamination rate of 10% each. E. coli was not detected in RTE pork.

Table 1.
Distribution of Escherichia coli in raw meats, ready-to-eat (RTE) meats and their related samples.
3.2. Phenotypic Antimicrobial Susceptibility of Escherichia coli Isolated from Raw Meats, RTE Meats and Their Related Samples
The overall phenotypic antimicrobial resistance of the meats and their related samples is presented in Figure 1. The E. coli isolates were highly resistant to amoxicillin (70.9%), tetracycline (57.0%) and trimethoprim (55.0%). Considerable rate of intermediate resistance occurred for ceftriaxone (33.3%), amoxicillin (29.1%), ciprofloxacin (21.1%) and gentamicin (19.6%). The E. coli isolates were, however, susceptible to azithromycin (87.1%), chloramphenicol (81.3%), imipenem (74.8%), gentamicin (72.0%) and ciprofloxacin (69.5%).
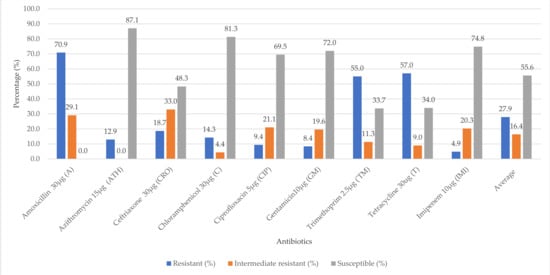
Figure 1.
Overall antibiotic susceptibility of E. coli (n = 45) isolated from raw meats, RTE meats and their related samples.
The phenotypic antimicrobial resistance of E. coli isolated from raw meats, RTE meats, hands and working tools of meat sellers can be found in Table 2. Escherichia coli isolates from these sources were all resistant ≥55% to amoxicillin. E. coli isolates isolated from the hands of meat sellers were resistant to tetracycline (60.0%) and trimethoprim (60.0%), while those isolated from working tools showed 76.9% resistance each to tetracycline and trimethoprim. Resistance to trimethoprim and tetracycline were 52.9% and 41.2%, respectively, for E. coli isolates obtained from raw meats. It was 30.0% (trimethoprim) and 50.0% (tetracycline), respectively, for E. coli of RTE meat origin.

Table 2.
Phenotypic antibiotic resistance of E. coli (n = 45) isolated raw meats, RTE meats, hands and working tools of meat sellers.
3.3. Multidrug Resistant of Individual E. coli Isolates
Table 3 shows the multiple antibiotic resistance (MAR) index and antimicrobial resistance pattern of individual E. coli species isolated from raw meats, RTE meats and their related samples. The MAR index of the E. coli isolates was within the range of 0.0 to 0.7 and displayed 22 resistant patterns. Resistance to A (amoxicillin) and A-T-TM (amoxicillin-tetracycline-trimethoprim) was found in six E. coli isolates each and was the most common pattern observed. Resistance to as high as six different classes of antibiotics—that is, A-ATH-T-C-CRO-T and A-T-C-CRO-TM-IMI—was observed for FP9 (E. coli isolated from raw pork) and FP6 (E. coli isolated from raw pork), respectively. RH7 (E. coli isolated from hand swab of raw meat seller), RB4 (E. coli isolated from raw beef) and RK7 (E. coli isolated from knife swab of raw meat seller) were resistant to five (A-ATH-T-CRO-TM, CIP-A-T-C-CRO and CIP-A-T-C-TM) different classes of antibiotics, respectively. Resistance to zero, one, two, three, four, five and six antimicrobials was 11.1%, 28.9%, 8.9%, 17.8%, 22.2%, 6.7% and 4.4%, respectively. Multidrug resistance (that is, resistant to ≥3 different antimicrobials) was 51.1%.

Table 3.
Multiple antibiotic resistance index and antimicrobial resistance profile of individual E. coli species of raw meats, ready-to-eat (RTE) meats, and their related samples.
4. Discussions
Escherichia coli harbor the GIT of animals including humans and are normally distributed in the environment by these organisms [1,3]. Their distribution among humans, animals and the environment is associated with the re-circulation of antimicrobial resistant and pathogenic strains. Raw meats, ready-to-eat (RTE) meats (except RTE pork), hands of meat sellers, knives used by meat sellers for cutting meat, tables on which meats are sold or cut into pieces and utensils for processing meat were all contaminated with E. coli species. This reveals lapses in the processing of the meat and meat products. It also suggests possible cross contamination among the samples examined. It must be noted that knives, tables and utensils are not primary sources of E. coli and thus were cross contaminated. E. coli has been reported as an indicator organism for revealing unhygienic conditions in the food processing chain [1]. The rate of contamination of raw meats by E. coli was higher than the RTE meats, which was expected. The heat treatment RTE meats were subjected to during cooking accounted for this occurrence [36]. The occurrence of E. coli in raw meats, RTE meats and their related samples has been reported in other countries. In the USA, Zhao et al. [10] reported that retail chicken (83.5%), beef (68.9%) and pork (44.0%) were contaminated by E. coli. Fresh bovine meat (1.0%), fresh ovine (5.3%) and fresh pigs (3.0%) of Europe origin were contaminated with E. coli [9]. Again, the EFSA [9] reported that meat preparations and meat products from mixed sources were contaminated by shiga-toxin producing E. coli non O157 (2.7%) and shiga-toxin producing E. coli (2.2%). Zhao et al. [10] and the EFSA [9] reported lower incidences of E. coli as compared to this study. Their works were conducted in countries where adherence to hygienic handling and processing of animals into meat and meat products are given much priority and standards are followed and monitored. The same cannot be said of Ghana. Escherichia coli was observed in 32% of RTE meat products collected from Latvia [11], which was within the range (0–50%) found in this study. In Egypt, E. coli was detected in raw beef (54%), chicken (16%) and hand swabs from sellers (24%) [16]. According to Parvin et al. [14], 76% of frozen chicken meats collected from Bangladesh were positive for E. coli. The results of Gwida et al. [16] in Egypt and that of Parvin et al. [14] in Bangladesh were quite similar to the findings of the current work. These developing countries might have similar situations in the handling of animals and processing of meat and meat products to that of Ghana. However, it is emphasized that factors, such as the accuracy, type of the isolation or detection method employed, number of samples examined, type of samples examined, period or season of sampling, level of hygiene observed or practiced and location of sampling, are responsible for wide differences in the occurrences of E. coli and other bacteria in humans, foods, animals and the environment [2,4,9,10]. The presence of E. coli in this current study also signifies that meat and meat products are important sources of E. coli and possible risk sources for E. coli infection.
Thus, the resistance of E. coli to antimicrobials in this study should be worrying. This study found high phenotypic resistance of E. coli to penicillins (amoxicillin), trimethoprims (trimethoprim), and tetracyclines (tetracycline). The EFSA [42] reported that ampicillin, sulfamethoxazole, tetracycline and trimethoprim were the antimicrobials most often represented in the pattern of multidrug resistance E. coli isolates from bovines, porcine and poultry species. Other studies have also reported higher resistances to tetracycline [10,13,14] and ampicillin [12,14]. For example, Zhao et al. [10] reported resistance to tetracycline (50.3%) and gentamicin (18.6%) in E. coli isolated from different retail meats in the USA. This study found higher resistance to tetracycline, but lower for gentamicin. In the current study, average phenotypic resistance was higher in the human hands (hands of meat sellers) and working tools (tables, knives and utensils used by meat sellers) E. coli isolates than the raw and RTE meats E. coli isolates. There were some variations and similarities in the phenotypic resistance patterns among the raw meats, RTE meats, hands of sellers and the working tools used by meat sellers. Phenotypic antimicrobial resistance patterns allow for the identification of emerging or specific patterns of resistance. For instance, the resistance pattern, A-T-TM (amoxicillin-tetracycline-trimethoprim) was shared by E. coli isolated from knife swabs from raw meat seller (FK4), table swabs from raw meat seller (FT1), RTE chevon (Rch1) and table swabs from RTE meat seller (RT3) (Table 3). In addition, FT1 and FT8 or Rch1 and Rch5, which are E. coli species isolated from table swabs of raw meat sellers or RTE chevon, respectively, exhibited the same resistant pattern of A-T-TM (amoxicillin-tetracycline-trimethoprim). This means that E. coli species from similar sources or locations do not necessarily exhibit the same behavior in their resistant capabilities. Although the meat and their related samples were collected at random to avoid biasness and the assignment of a particular result to a vendor, RH4 and RH4-2 were E. coli isolated from the hands of an RTE meat seller, but they exhibited different resistant patterns (amoxicillin-gentamicin-trimethoprim versus amoxicillin, respectively). Bacteria in close association are capable of exchanging genes that confer resistance to each other [43]. This can bring about similarities and differences in their resistance patterns. Therefore, it is essential to understand the resistance mechanisms, distribution of resistant determinants in bacteria and to determine humans, animal, food and environmental factors that facilitate their dissemination. Processes such as antibiotic degradation/modification, antibiotic sequestration, antibiotic target modifications and antibiotic target bypass are among the processes proposed as means for transfer and exchange of antimicrobial resistances in bacteria [42,43].
The multiple antibiotic resistance (MAR) index of the E. coli isolates ranged from resistant to zero antibiotics to resistant to six antibiotics. It has been shown that bacteria with MAR index of >0.2 originate from high-risk sources, that is, sources where growth promoters or several antibiotics are used, whilst MAR index of <0.2 are isolates originating from sources where less antibiotics are used [44]. In the present study, 50.1% had MAR index between 0.3 to 0.7, meaning that most E. coli isolates were isolated from sources exposed to antibiotics and their usage. A much higher percentage of the E. coli exhibited multidrug resistance. Two, three, ten and eight E. coli isolates were resistant to six, five, four and three different classes of antimicrobials, respectively. Multidrug resistance E. coli isolates from raw meats, RTE meats and/or their environment have also been reported by Zhao et al. [10], Parvin et al. [14], EFSA [9] and Altalhi et al. [12] in different countries. Zhao et al. [10] found that E. coli isolates from chicken (38.9%), pork (17.3%) and beef (9.3%) were multidrug resistant. Parvin et al. [14] reported that all E. coli isolated from frozen chicken meats were multidrug resistant. In addition, Altalhi et al. [12] indicated that 15 strains of E. coli isolated from retail raw chicken meat exhibited multidrug resistance phenotype and harbored at least three resistance genes. Resistance genes, such as blaCTX-M and blaTEM (beta-lactamase), tet (A) and tet (B) (tetracycline), aph (3’’)-Ib and apha (6) (aminoglycoside), dfrA17 and dfrA14 (trimethoprim) and mdf(A) macrolide, have been found in E. coli isolated from Ghana [23,24]. In addition, virulence factors, such as lpfA, gad, mchF, iha, mchB, mchC eilA, iss, cftB and mcmA, have been associated with E. coli isolates from various meat samples in Ghana [45], revealing the potential and possibility of E. coli isolates from this study to be pathogenic, although this study did not seek to establish that. The developments of resistances are elicited by factors including the inappropriate use of antimicrobials and poor hygiene practices in healthcare and food chain setups [19,20], facilitating the transfer of resistant E. coli. E. coli resistant to antimicrobials present in meats and meat products can spread to humans via consumption. They can also infest humans via contamination from their own hands and working tools. Multiple antimicrobial-resistant E. coli will make antimicrobials less effective against them and result in treatment failures [9,19]. E. coli is a commensal organism and can also serve as a reservoir for antimicrobial-resistant genes among other commensals and pathogenic bacteria [9,20]. Monitoring antimicrobial resistance in E. coli and other bacteria in meats, meat products and foods is required to elucidate the reason for which they develop and spread resistance among humans, animals, the environment and food chain.
5. Conclusions
The raw meats, RTE meats, and hands, tables, knives and utensils of meat sellers were contaminated by E. coli, revealing possible unhygienic practices. This poses the risk of E. coli infection. Raw beef and mutton were the most contaminated source. E. coli was not detected in RTE pork. Generally, raw meats were more contaminated with E. coli than the RTE meats, warranting appropriate cooking or heating prior to consumption. Most (50.1%) of the E. coli exhibited resistance to multiple antimicrobials. This is a sign of emerging resistances and reduction in the effectiveness of antimicrobials. Resistance was high for amoxicillin, tetracycline and trimethoprim. Resistance to only amoxicillin or amoxicillin-tetracycline-trimethoprim was most common. Hygienic practices among fresh and RTE meat sellers is recommended to prevent the spread of antimicrobial-resistant E. coli in the food chain and among humans, animals and their environment. Molecular characterization and detection of ESBL genes of the E. coli isolates is recommended.
Author Contributions
Conceptualization, F.A., N.H. and A.H.M.S.; methodology, F.A.; validation F.A., N.H. and A.H.M.S.; formal Analysis, F.A., N.H. and A.H.M.S.; investigation, F.A.; resources, F.A., N.H. and A.H.M.S.; data curation, F.A., N.H. and A.H.M.S.; writing–original draft preparation, F.A.; writing, review and editing, F.A., N.H. and A.H.M.S.; funding acquisition, F.A., N.H. and A.H.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
University Malaysia Sabah, Jalan UM, 88400, Kota Kinabula.
Institutional Review Board Statement
Not applicable. This study did not subject animals or humans to any treatment.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
University for Development Studies, Rejoice Ekli and Martin Aduah for their role in this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Center for Control and Disease Prevention (CDC). E. coli (Escherichia coli). 2014. Available online: https://www.cdc.gov/ecoli/general/index.html (accessed on 23 August 2020).
- Feng, P.; Weagant, S.D.; Jinneman, K. Bacteriological Analytical Manuel Chapter 4A: Diarrheagenic Escherichia coli. Available online: https://www.fda.gov/food/laboratory-methods-food/bam-chapter-4a-diarrheagenic-escherichia-coli (accessed on 23 August 2019).
- Founou, L.L.; Amoako, G.G.; Raspail Carrel Founou, R.C.; Essack, S.Y. Antibiotic resistance in food animals in Africa: A systematic review and meta-analysis. Microb. Drug Resist. 2018, 24, 648–665. [Google Scholar] [CrossRef]
- Jang, J.; Hur, H.G.; Sadowsky, M.J.; Byappanahalli, M.N.; Yan, T.; Ishi, S. Environmental Escherichia coli: Ecology and public health implications—A review. J. Appl. Microbiol. 2017, 123, 570–581. [Google Scholar] [CrossRef]
- Center for Control and Disease Prevention (CDC). Multistate Outbreak of E. coli O157:H7 Infections Associated with Beef from National Steak and Poultry (final update). 2010. Available online: https://www.cdc.gov/ecoli/2010/national-steak-poultry-1-6-10.html (accessed on 23 August 2020).
- Center for Control and Disease Prevention (CDC). Outbreak of E. coli infections—Unknown Source 1. 2020. Available online: https://www.cdc.gov/ecoli/2020/o157h7-10-20a/index.html (accessed on 23 August 2020).
- World Health Organization. Outbreaks of E. coli O104:H4 Infection. 2020. Available online: https://www.euro.who.int/en/health-topics/disease-prevention/food-safety/outbreaks-of-e.-coli-o104h4-infection (accessed on 23 August 2020).
- Malm, K.L.; Nyarko, K.M.; Yawson, A.E.; Gogo, B.; Lawson, A.; Afari, E. Foodborne illness among school children in Ga East, Accra. Ghana Med. J. 2015, 49, 72–76. [Google Scholar] [CrossRef]
- European Food Safety Authority. The European union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018, 16, 1–262. [Google Scholar]
- Zhao, S.; Blickenstaff, K.; Bodeis-Jones, S.; Gaines, S.A.; Tong, E.; McDermott, P.F. Comparison of the prevalences and antimicrobial resistances of Escherichia coli isolates from different retail meats in the United States, 2002 to 2008. Appl. Environ. Microbiol. 2012, 78, 1701–1707. [Google Scholar] [CrossRef] [PubMed]
- Ciekure, E.; Siksna, I.; Valcina, O.; Viksna, L.; Krumina, A. Microbiological quality of ready-to-eat products and potential risks for consumers in Latvia. Nat. Exact Appl. Sci. 2016, 70, 245–251. [Google Scholar]
- Altalhi, A.D.; Gherbawy, Y.A.; Hassan, S.A. Antibiotic resistance in Escherichia coli isolated from retail raw chicken meat in Taif, Saudi Arabia. Foodborne Path. Dis. 2010, 7, 281–285. [Google Scholar] [CrossRef]
- Yulistiani, R.; Praseptiangga, D.; Supyani, S.; Raharjo, D.; Shirakawa, T. Prevalence of antibiotic-resistance enterobacteriaceae strains isolated from chicken meat at traditional markets in Surabaya, Indonesia. IOP Conf. Ser. Mat. Sci. Eng. 2017, 193, 012007. [Google Scholar] [CrossRef]
- Parvin, M.S.; Talukder, S.; Ali, M.Y.; Chowdhury, E.H.; Rahman, M.T.; Islam, M.T. Antimicrobial resistance pattern of Escherichia coli isolated from frozen chicken meat in Bangladesh. Pathogens 2020, 9, 420. [Google Scholar] [CrossRef]
- Bekele, T.; Zewde, G.; Tefera, G.; Feleke, F.; Zerom, K. Escherichia coli O157:H7 in raw meat in Addis Ababa, Ethiopia: Prevalence at an abattoir and retailers and antimicrobial susceptibility. Int. J. Food Cont. 2014, 1, 1–8. [Google Scholar] [CrossRef]
- Gwida, M.; Hotze, H.; Geue, L.; Tomaso, H. Occurrence of enterobacteriaceae in raw meat and in human samples from Egyptian retail sellers. Int. Scholarly Res. Notices 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Adzitey, F.; Teye, G.A.; Anachinaba, I.A. Microbial quality of fresh and smoked guinea fowl meat sold in the Bolgatanga Municipality, Ghana. Asian J. Poult. Sci. 2015, 9, 165–171. [Google Scholar] [CrossRef][Green Version]
- Gill, C.J.; Hamer, D.H. Foodborne illnesses. Curr. Treat. Options. Gastroenterol. 2001, 4, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Fair, R.J.; Tor, Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Med. Chem. 2014, 6, 25–64. [Google Scholar] [CrossRef]
- Purssell, E. Antimicrobials. Underst. Pharm Nurs. Pract. 2019, 2019, 147–165. [Google Scholar]
- Akansale, R.; Adzitey, F.; Teye, G.A. Knowledge of farmers in antibiotic usage and investigation of antibiotic residues in meats in Sunyani Municipality, Ghana. J. Food Saf. Hyg. 2019, 5, 155–164. [Google Scholar]
- Ekli, R.; Adzitey, F.; Agbolosu, A.A. Farmers’ knowledge in antibiotic usage, antibiotic residues, and susceptibility of Salmonella enterica in beef samples from the Wa Municipality, Ghana. Bull. Anim. Health Prod. Africa 2020, 68, 89–101. [Google Scholar]
- Dsani, E.; Afari, E.A.; Danso-Appiah, A.; Kenu, E.; Kaburi, B.B.; Egyir, B. Antimicrobial resistance and molecular detection of extended spectrum β-lactamase producing Escherichia coli isolates from raw meat in Greater Accra region, Ghana. BMC Microbiol. 2020, 20, 1–8. [Google Scholar] [CrossRef]
- Eibach, D.; Dekker, D.; Gyau Boahen, K.; Wiafe Akenten, C.; Sarpong, N.; Belmar Campos, C.; Berneking, L.; Aepfelbacher, M.; Krumkamp, R.; Owusu-Dabo, E.; et al. Extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in local and imported poultry meat in Ghana. Vet. Microbiol. 2018, 217, 7–12. [Google Scholar] [CrossRef]
- Adzitey, F. Incidence and antimicrobial susceptibility of Escherichia coli isolated from beef (meat muscle, liver and kidney) samples in Wa Abattoir, Ghana. Cog. Food Agric. 2020, 6, 2–10. [Google Scholar] [CrossRef]
- Agbodaze, D.; Nmai, P.N.; Robertson, F.; Yeboah-Manu, D.; Owusu-Darko, K.; Addo, K. Microbiological quality of khebab consumed in the Accra metropolis. Ghana Med. J. 2015, 39, 46–49. [Google Scholar] [CrossRef]
- Twum, E. Microbial Quality of Fresh Beef Sold in the Birim North District of the Eastern Region of Ghana. Master’s Thesis, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana, April 2016. Available online: http://ir.knust.edu.gh/xmlui/handle/123456789/8792?show=full (accessed on 23 August 2020).
- Adzitey, F.; Ekli, R.; Abu, A. Prevalence and antibiotic susceptibility of Staphylococcus aureus isolated from raw and grilled beef in Nyankpala community in the Northern Region of Ghana. Cogent Food Agric. 2019, 5, 1671115. [Google Scholar] [CrossRef]
- Yafetto, L.; Adator, E.H.; Ebuako, A.A.; Ekloh, E.; Afeti, F.Y. Microbial quality of raw beef and chevon from selected markets in Cape Coast, Ghana. J. Biol. Life Sci. 2019, 10, 78–97. [Google Scholar] [CrossRef]
- Adzitey, F.; Assoah-Peprah, P.; Teye, G.A.; Somboro, A.M.; Kumalo, H.M.; Amoako, D.G. Prevalence and antimicrobial resistance of Escherichia coli isolated from various meat types in the Tamale, Metropolis of Ghana. Int. J. Food Sci. 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- Adzitey, F.; Teye, G.A.; Amoako, D.G. Prevalence, phylogenetic insights, and phenotypic characterization of Salmonella enterica isolated from meats in the Tamale Metropolis of Ghana. Food Sci. Nutr. 2020, 8, 1–9. [Google Scholar]
- Adzitey, F. Prevalence of Escherichia coli and Salmonella spp. in beef samples sold at Tamale Metropolis, Ghana. Int. J. Meat Sci. 2015, 5, 8–13. [Google Scholar] [CrossRef][Green Version]
- Adzitey, F.; Sulleyman, K.W.; Mensah, S.S. Knowledge and practices of meat safety by meat sellers in the Kumasi Metropolis of Ghana. Res. Rev. J. Food Sci. Technol. 2018, 7, 34–41. [Google Scholar]
- Sulleyman, K.W.; Adzitey, F.; Boateng, E.F. Knowledge and practices of meat safety by meat sellers in the Accra Metropolis of Ghana. Int. J. Vet. Sci. 2018, 7, 167–171. [Google Scholar]
- Adzitey, F.; Sulleyman, K.W.; Kum, P.K. Knowledge and practices of meat safety by meat sellers in the Tamale Metropolis of Ghana. Food Prot. Trends 2020, 40, 40–47. [Google Scholar]
- Abass, A.; Adzitey, F.; Huda, N. Escherichia coli of ready-to-eat (RTE) meats origin showed resistance to antibiotics used by farmers. Antibiotics 2020, 9, 869. [Google Scholar] [CrossRef]
- Anonymous. Upper East Region. 2020. Available online: https://www.modernghana.com/GhanaHome/regions/uppereast.asp?menu_id=6&sub_menu_id=14&gender= (accessed on 23 August 2020).
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turk, M. Antibiotic susceptibility testing by a standardized single disc method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standard Institute. Document M100. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 27th ed.; Approved Standard; Clinical and Laboratory Standard Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Krumperman, P.H. Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of fecal contamination of food. Appl. Environ. Microbiol. 1983, 46, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2017/2018. EFSA J. 2020, 18, 1–166. [Google Scholar]
- Peterson, E.; Kaur, P. Antibiotic resistance mechanisms in bacteria: Relationships between resistance determinants of antibiotic producers, environmental bacteria, and clinical pathogens. Front. Microbiol. 2018, 9, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Kathleen, M.M.; Samuel, L.; Felecia, C.; Reagan, E.L.; Kasing, A.; Lesley, M.; Toh, S.C. Antibiotic resistance of diverse bacteria from aquaculture in Borneo. Int. J. Microbiol. 2016, 2016, 2164761. [Google Scholar] [CrossRef]
- Adzitey, F.; Asante, J.; Kumalo, H.M.; Khan, R.B.; Somboro, A.M.; Amoako, D.G. Genomic investigation into the virulome, pathogenicity, stress response factors, clonal lineages, and phylogenetic relationship of Escherichia coli strains isolated from meat sources in Ghana. Genes 2020, 11, 1504. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).