Influence of Soil and Water Conservation Measures on Soil Microbial Communities in a Citrus Orchard of Southeast China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area, Sampling and Physiochemical Analysis
2.2. DNA Extraction and 16S rRNA Gene Amplicon Sequencing
2.3. Statistical Analyses
3. Results
3.1. Soil Physicochemical Properties and Microbial Community Quantity
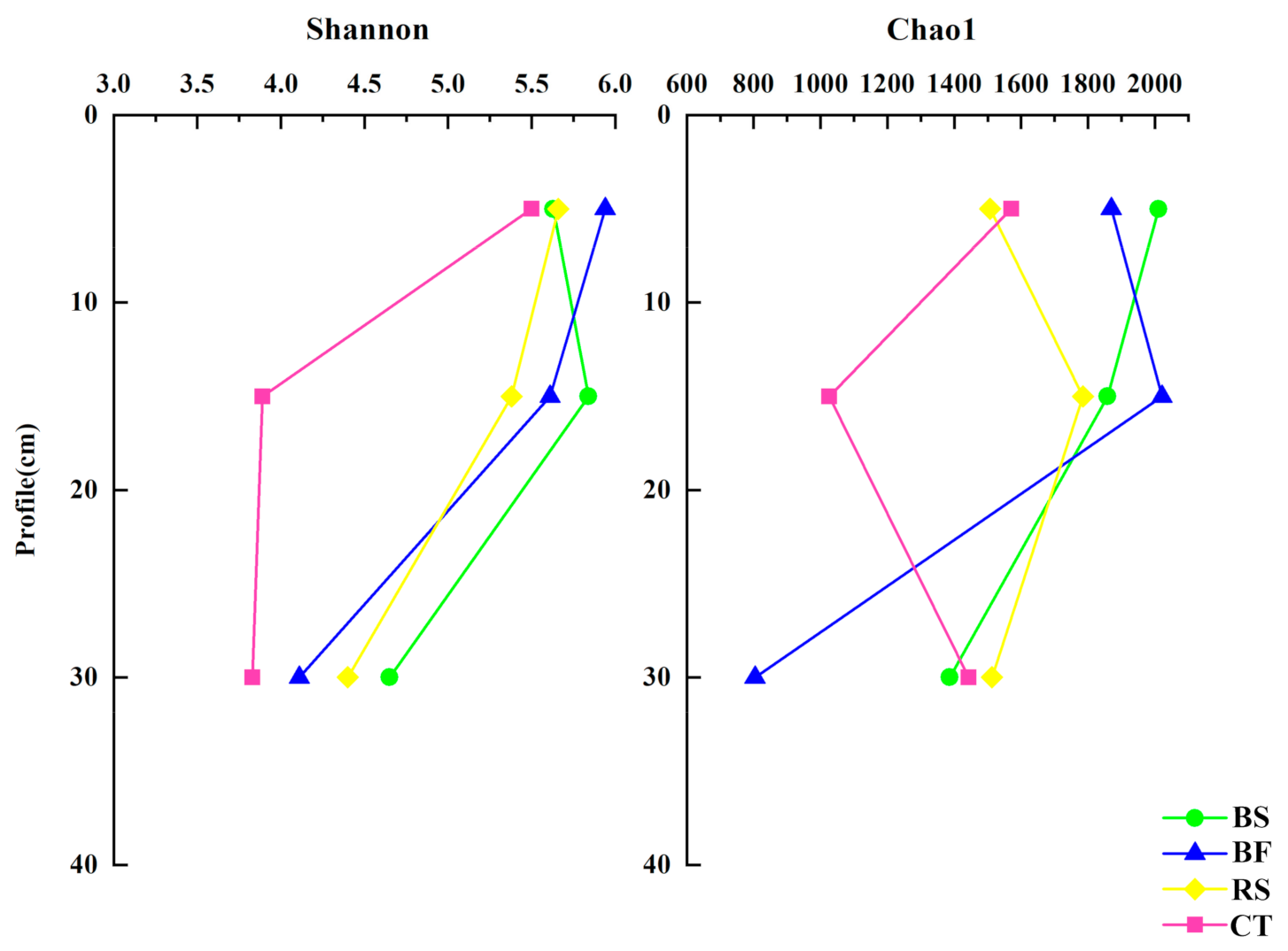
3.2. Fine Scale Spatial Distribution Patterns of Soil Microbiomes
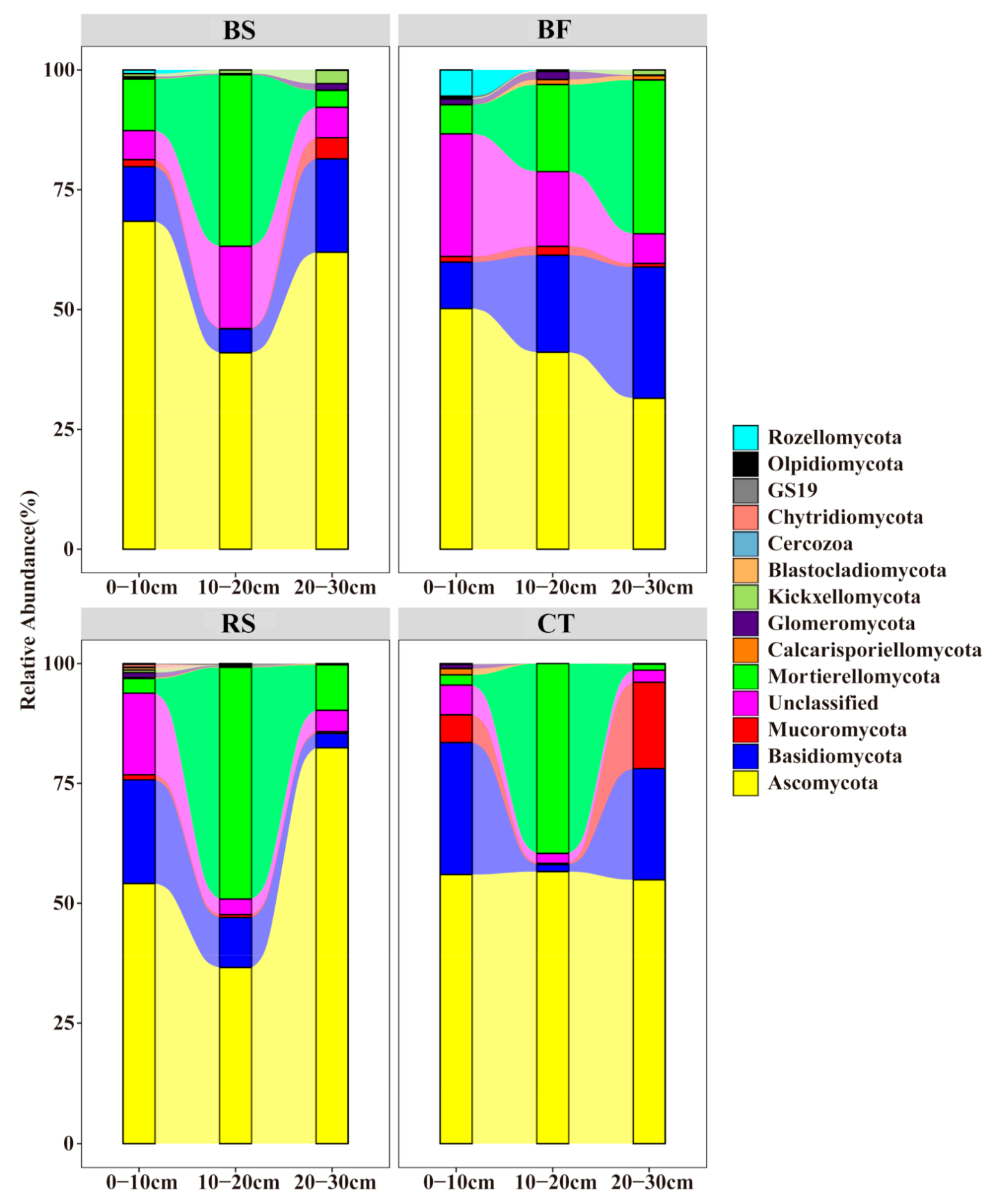
3.3. Predominant Microbial Taxa of Soil Microbial Communities
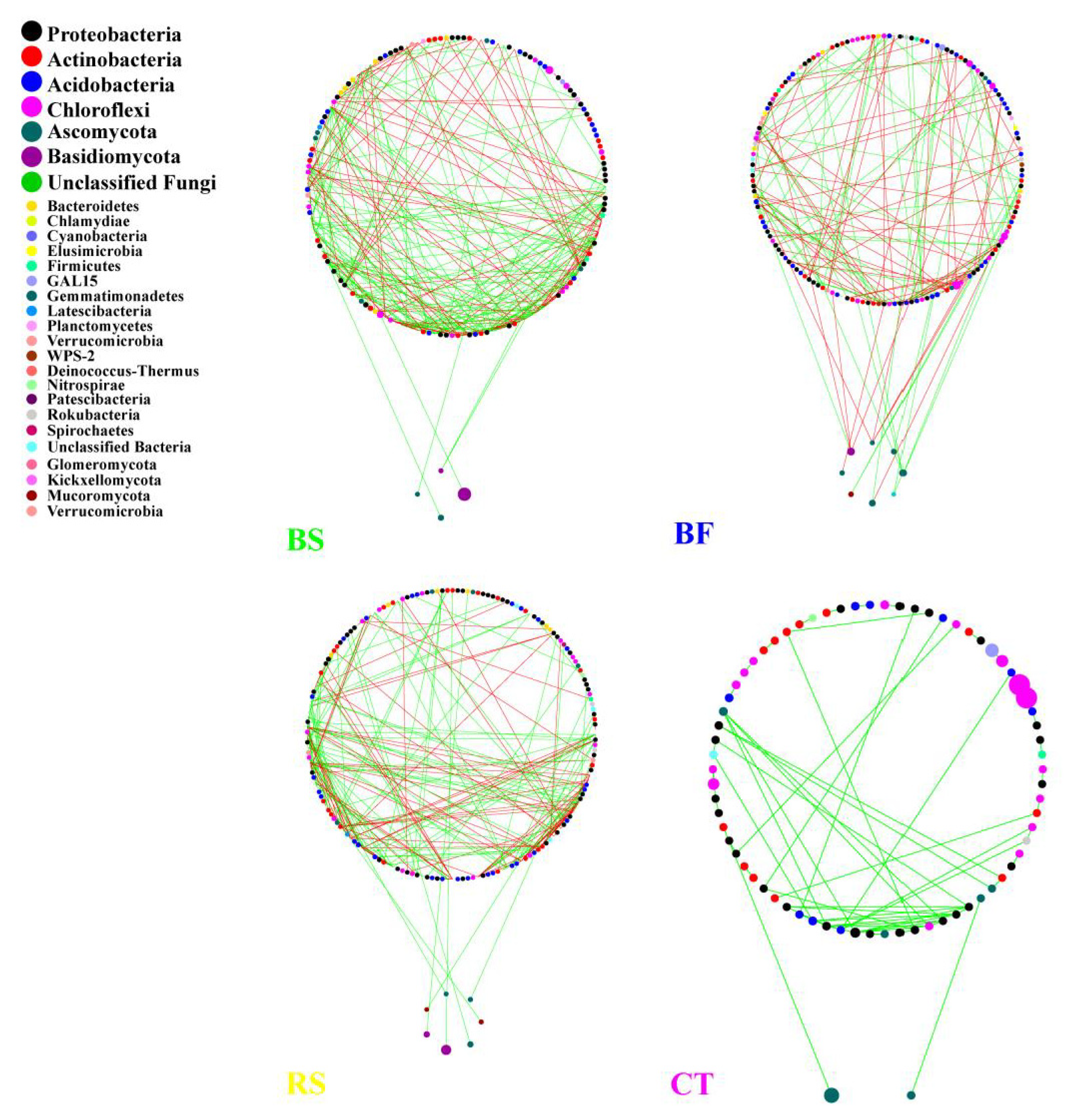
3.4. Interactions between Microbial Taxa in the Network
4. Discussion
4.1. Soil Bacterial and Fungal Abundance and α-Diversity
4.2. Soil Bacterial and Fungal Community Composition
4.3. Interactions of the Soil Microbial Community in the Networks
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiao, S.; Chen, W.; Wang, J.; Du, N.; Li, Q.; Wei, G. Soil microbiomes with distinct assemblies through vertical soil profiles drive the cycling of multiple nutrients in reforested ecosystems. Microbiome 2018, 6, 146. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; Li, X.; Su, Z.; Li, X.; Xu, M. Effects of tillage and residue incorporation on composition and abundance of microbial communities of a fluvo-aquic soil. Eur. J. Soil Biol. 2014, 65, 70–78. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, D.; Dong, L.; Shi, X.; Warner, E.; Gu, Z.; Sun, J. Regional soil erosion assessment from remote sensing data in rehabilitated high density canopy forests of southern China. Catena 2014, 123, 106–112. [Google Scholar] [CrossRef]
- Cerdà, A.; Morera, A.G.; Bodí, M.B. Soil and water losses from new citrus orchards growing on sloped soils in the western Mediterranean basin. Earth Surf. Proc. Land 2009, 34, 1822–1830. [Google Scholar] [CrossRef]
- Prosdocimi, M.; Cerdà, A.; Tarolli, P. Soil water erosion on Mediterranean vineyards: A review. Catena 2016, 141, 1–21. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, L.; Wei, A.; Gao, J.; Lu, Y.; Zhou, J. Land-use change from arable lands to orchards reduced soil erosion and increased nutrient loss in a small catchment. Sci. Total Environ. 2019, 648, 1097–1104. [Google Scholar] [CrossRef]
- López-Fando, C.; Dorado, J.; Pardo, M.T. Effects of zone-tillage in rotation with no-tillage on soil properties and crop yields in a semi-arid soil from central Spain. Soil Tillage Res. 2007, 95, 266–276. [Google Scholar] [CrossRef]
- Nielsen, D.C.; Unger, P.W.; Miller, P.R. Efficient Water Use in Dryland Cropping Systems in the Great Plains. Agron. J. 2005, 97, 364–372. [Google Scholar] [CrossRef]
- Wang, H.; Hall, C.A.S.; Cornell, J.D.; Hall, M.H.P. Spatial dependence and the relationship of soil organic carbon and soil moisture in the Luquillo Experimental Forest, Puerto Rico. Landsc. Ecol. 2002, 17, 671–684. [Google Scholar] [CrossRef]
- Hernanz, J.L.; López, R.; Navarrete, L.; Sánchez-Girón, V. Long-term effects of tillage systems and rotations on soil structural stability and organic carbon stratification in semiarid central Spain. Soil Tillage Res. 2002, 66, 129–141. [Google Scholar] [CrossRef]
- Cui, H.; Zhou, Y.; Gu, Z.; Zhu, H.; Fu, S.; Yao, Q. The combined effects of cover crops and symbiotic microbes on phosphatase gene and organic phosphorus hydrolysis in subtropical orchard soils. Soil Biol. Biochem. 2015, 82, 119–126. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Y.; Wu, Q.S. Effects of soil tillage and planting grass on arbuscular mycorrhizal fungal propagules and soil properties in citrus orchards in southeast China. Soil Tillage Res. 2016, 155, 54–61. [Google Scholar] [CrossRef]
- Venter, Z.S.; Scott, S.L.; Strauss, J.; Jacobs, K.; Hawkins, H. Increasing crop diversity increased soil microbial activity, nitrogen-sourcing and crop nitrogen, but not soil microbial diversity. S. Afr. J. Plant Soil 2017, 34, 371–378. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, X.; Sun, Y.; Zhang, J.; Lin, X.; Liao, Y. Effect of ground mulch managements on soil bacterial community structure and diversity in the non-irrigated apple orchard in Weibei Loess Plateau. Acta Microbiol. Sin. 2015, 55, 892–904. [Google Scholar]
- Dong, W.; Liu, E.; Yan, C.; Tian, J.; Zhang, H.; Zhang, Y. Impact of no tillage vs. conventional tillage on the soil bacterial community structure in a winter wheat cropping succession in northern China. Eur. J. Soil Biol. 2017, 80, 35–42. [Google Scholar] [CrossRef]
- Sanaullah, M.; Usman, M.; Wakeel, A.; Cheema, S.A.; Ashraf, I.; Farooq, M. Terrestrial ecosystem functioning affected by agricultural management systems: A review. Soil Tillage Res. 2020, 196, 104464. [Google Scholar] [CrossRef]
- Banerjee, S.; Kirkby, C.A.; Schmutter, D.; Bissett, A.; Kirkegaard, J.A.; Richardson, A.E. Network analysis reveals functional redundancy and keystone taxa amongst bacterial and fungal communities during organic matter decomposition in an arable soil. Soil Biol. Biochem. 2016, 97, 188–198. [Google Scholar] [CrossRef]
- Barberán, A.; Bates, S.T.; Casamayor, E.O.; Fierer, N. Using network analysis to explore co-occurrence patterns in soil microbial communities. ISME J. 2012, 6, 343–351. [Google Scholar] [CrossRef]
- Mo, M.; Liu, Z.; Yang, J.; Song, Y.; Tu, A.; Liao, K.; Zhang, J. Water and sediment runoff and soil moisture response to grass cover in sloping citrus land, Southern China. Soil Water Res. 2019, 14, 10–21. [Google Scholar] [CrossRef]
- Eshel, G.; Levy, G.J.; Mingelgrin, U.; Singer, M.J. Critical Evaluation of the Use of Laser Diffraction for Particle-Size Distribution Analysis. Soil Sci. Soc. Am. J. 2004, 68, 736–743. [Google Scholar] [CrossRef]
- Faust, K.; Raes, J. CoNet app: Inference of biological association networks using Cytoscape. F1000Research 2016, 5, 1519. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wendy, L.; Zou, Q.; Ma, Z. Network analysis of the hot spring microbiome sketches out possible niche differentiations among ecological guilds. Ecol. Model. 2020, 431, 109147. [Google Scholar] [CrossRef]
- Vick-Majors, T.J.; Priscu, J.C.; Zettler, L.A. Modular community structure suggests metabolic plasticity during the transition to polar night in ice-covered Antarctic lakes. ISME J. 2014, 8, 778–789. [Google Scholar] [CrossRef]
- Reckling, M.; Hecker, J.; Bergkvist, G.; Watson, C.A.; Zander, P.; Schläfke, N.; Stoddard, F.L.; Eory, V.; Topp, C.F.E.; Maire, J.; et al. A cropping system assessment framework—Evaluating effects of introducing legumes into crop rotations. Eur. J. Agron. 2016, 76, 186–197. [Google Scholar] [CrossRef]
- Gu, C.; Liu, Y.; Mohamed, I.; Zhang, R.; Wang, X.; Nie, X.; Jiang, M.; Brooks, M.; Chen, F.; Li, Z. Dynamic Changes of Soil Surface Organic Carbon under Different Mulching Practices in Citrus Orchards on Sloping Land. PLoS ONE 2016, 11, e168384. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Cheng, X.; Zhou, W.; Han, H. Microbial regulation of soil carbon properties under nitrogen addition and plant inputs removal. PeerJ 2019, 7, e7343. [Google Scholar] [CrossRef]
- Kušlienė, G.; Rasmussen, J.; Kuzyakov, Y.; Eriksen, J. Medium-term response of microbial community to rhizodeposits of white clover and ryegrass and tracing of active processes induced by 13C and 15N labelled exudates. Soil Biol. Biochem. 2014, 76, 22–33. [Google Scholar] [CrossRef]
- Cederlund, H.; Wessén, E.; Enwall, K.; Jones, C.M.; Juhanson, J.; Pell, M.; Philippot, L.; Hallin, S. Soil carbon quality and nitrogen fertilization structure bacterial communities with predictable responses of major bacterial phyla. Appl. Soil Ecol. 2014, 84, 62–68. [Google Scholar] [CrossRef]
- Liu, C.; Jin, Y.; Hu, Y.; Tang, J.; Xiong, Q.; Xu, M.; Bibi, F.; Beng, K.C. Drivers of soil bacterial community structure and diversity in tropical agroforestry systems. Agric. Ecosyst. Environ. 2019, 278, 24–34. [Google Scholar] [CrossRef]
- Kerfahi, D.; Tripathi, B.M.; Dong, K.; Go, R.; Adams, J.M. Rainforest Conversion to Rubber Plantation May Not Result in Lower Soil Diversity of Bacteria, Fungi, and Nematodes. Microb. Ecol. 2016, 72, 359–371. [Google Scholar] [CrossRef]
- de Carvalho, T.S.; Jesus, E.D.; Barlow, J.; Gardner, T.A.; Soares, I.C.; Tiedje, J.M.; Moreira, F.M. Land use intensification in the humid tropics increased both alpha and beta diversity of soil bacteria. Ecology 2016, 97, 2760–2771. [Google Scholar] [CrossRef]
- He, H.; Li, W.; Zhang, Y.; Cheng, J.; Jia, X.; Li, S.; Yang, H.; Chen, B.; Xin, G. Effects of Italian ryegrass residues as green manure on soil properties and bacterial communities under an Italian ryegrass (Lolium multiflorum L.)-rice (Oryza sativa L.) rotation. Soil Tillage Res. 2020, 196, 104487. [Google Scholar] [CrossRef]
- Goldfarb, K.C.; Karaoz, U.; Hanson, C.A.; Santee, C.A.; Bradford, M.A.; Treseder, K.K.; Wallenstein, M.D.; Brodie, E.L. Differential Growth Responses of Soil Bacterial Taxa to Carbon Substrates of Varying Chemical Recalcitrance. Front Microbiol. 2011, 2, 94. [Google Scholar] [CrossRef]
- Yan, S.; Song, J.; Fan, J.; Yan, C.; Dong, S.; Ma, C.; Gong, Z. Changes in soil organic carbon fractions and microbial community under rice straw return in Northeast China. Glob. Ecol. Conserv. 2020, 22, e962. [Google Scholar] [CrossRef]
- Fierer, N.; Lauber, C.L.; Ramirez, K.S.; Zaneveld, J.; Bradford, M.A.; Knight, R. Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J. 2012, 6, 1007–1017. [Google Scholar] [CrossRef]
- Gschwend, F.; Aregger, K.; Gramlich, A.; Walter, T.; Widmer, F. Periodic waterlogging consistently shapes agricultural soil microbiomes by promoting specific taxa. Appl. Soil Ecol. 2020, 155, 103623. [Google Scholar] [CrossRef]
- Magill, A.H.; Aber, J.D. Variation in soil net mineralization rates with dissolved organic carbon additions. Soil Biol. Biochem. 2000, 32, 597–601. [Google Scholar] [CrossRef]
- Jones, R.T.; Robeson, M.S.; Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. A comprehensive survey of soil acidobacterial diversity using pyrosequencing and clone library analyses. ISME J. 2009, 3, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, Y.; Zhang, P.; Trivedi, P.; Riera, N.; Wang, Y.; Liu, X.; Fan, G.; Tang, J.; Coletta-Filho, H.D.; et al. The structure and function of the global citrus rhizosphere microbiome. Nat. Commun. 2018, 9, 4894. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.; Chang, S.X.; Liang, X.; Qin, H.; Chen, J.; Xu, Q. Linking soil fungal community structure and function to soil organic carbon chemical composition in intensively managed subtropical bamboo forests. Soil Biol. Biochem. 2017, 107, 19–31. [Google Scholar] [CrossRef]
- Hussain, Q.; Liu, Y.; Zhang, A.; Pan, G.; Li, L.; Zhang, X.; Song, X.; Cui, L.; Jin, Z. Variation of bacterial and fungal community structures in the rhizosphere of hybrid and standard rice cultivars and linkage to CO2 flux. FEMS Microbiol. Ecol. 2011, 78, 116–128. [Google Scholar] [CrossRef]
- Caesar-TonThat, T.; Lenssen, A.W.; Caesar, A.J.; Sainju, U.M.; Gaskin, J.F. Effects of tillage on microbial populations associated to soil aggregation in dryland spring wheat system. Eur. J. Soil Biol. 2010, 46, 119–127. [Google Scholar] [CrossRef]
- Sui, X.; Zhang, R.; Frey, B.; Yang, L.; Li, M.; Ni, H. Land use change effects on diversity of soil bacterial, Acidobacterial and fungal communities in wetlands of the Sanjiang Plain, northeastern China. Sci. Rep. UK 2019, 9, 18535. [Google Scholar] [CrossRef]
- Hill, P.W.; Marsden, K.A.; Jones, D.L. How significant to plant N nutrition is the direct consumption of soil microbes by roots? New Phytol. 2013, 199, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Howe, A.; Hofmockel, K.S. Demonstrating microbial co-occurrence pattern analyses within and between ecosystems. Front Microbiol. 2014, 5, 358. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, K.; Zhou, W.; Qiu, S.; Huang, S.; He, P. Changes in soil microbial community, enzyme activities and organic matter fractions under long-term straw return in north-central China. Agric. Ecosyst. Environ. 2016, 216, 82–88. [Google Scholar] [CrossRef]
- Mau, R.L.; Liu, C.M.; Aziz, M.; Schwartz, E.; Dijkstra, P.; Marks, J.C.; Price, L.B.; Keim, P.; Hungate, B.A. Linking soil bacterial biodiversity and soil carbon stability. ISME J. 2015, 9, 1477–1480. [Google Scholar] [CrossRef] [PubMed]
- Dundore-Arias, J.P.; Felice, L.; Dill-Macky, R.; Kinkel, L.L. Carbon Amendments Induce Shifts in Nutrient Use, Inhibitory, and Resistance Phenotypes Among Soilborne Streptomyces. Front. Microbiol. 2019, 10, 498. [Google Scholar] [CrossRef]
- Coyte, K.Z.; Schluter, J.; Foster, K.R. The ecology of the microbiome: Networks, competition, and stability. Science 2015, 350, 663. [Google Scholar] [CrossRef]
- Constancias, F.; Chemidlin Prevost-Boure, N.; Terrat, S.; Aussems, S.; Nowak, V.; Guillemin, J.; Bonnotte, A.; Biju-Duval, L.; Navel, A.; Martins, J.; et al. Microscale evidence for a high decrease of soil bacterial density and diversity by cropping. Agron. Sustain. Dev. 2014, 34, 831–840. [Google Scholar] [CrossRef]
- Banerjee, S.; Baah-Acheamfour, M.; Carlyle, C.N.; Bissett, A.; Richardson, A.E.; Siddique, T.; Bork, E.W.; Chang, S.X. Determinants of bacterial communities in Canadian agroforestry systems. Environ. Microbiol. 2016, 18, 1805–1816. [Google Scholar] [CrossRef]
- Deng, Y.; Jiang, Y.; Yang, Y.; He, Z.; Luo, F.; Zhou, J. Molecular ecological network analyses. BMC Bioinform. 2012, 13, 113. [Google Scholar] [CrossRef]
- Mougi, A.; Kondoh, M. Diversity of Interaction Types and Ecological Community Stability. Science 2012, 337, 349–351. [Google Scholar] [CrossRef]
- Ratzke, C.; Barrere, J.; Gore, J. Strength of species interactions determines biodiversity and stability in microbial communities. Nat. Ecol. Evol. 2020, 4, 376–383. [Google Scholar] [CrossRef]
- Jiao, S.; Wang, J.; Wei, G.; Chen, W.; Lu, Y. Dominant role of abundant rather than rare bacterial taxa in maintaining agro-soil microbiomes under environmental disturbances. Chemosphere 2019, 235, 248–259. [Google Scholar] [CrossRef]






| Depths (cm) | Sample Name | TP (g kg−1) | TN (g kg−1) | pH | SOC (g kg−1) | GC (%) | DOC (mg kg−1) | MBC (mg kg−1) | Bacteria 16S rRNA | Fungal ITS rRNA |
|---|---|---|---|---|---|---|---|---|---|---|
| 0–10 | BS1 | 0.33 (0.02) bd | 1.30 (0.02)c | 7.28 (0.04) b | 10.74 (1.17) e | 0.0093 (0.0025) ab | 28.70 (3.25) ad | 147.23 (44.46) a | 8.49 (0.02) a | 6.67 (0.02) e |
| BF1 | 0.32 (0.07) b | 1.44 (0.06)c | 6.36 (1.22) b | 12.48 (0.60) c | 0.0071 (0.0024) ab | 29.53 (1.14) abc | 150.66 (76.16)a | 8.80 (0.02) d | 6.58 (0.06) b | |
| RS1 | 0.50 (0.04) c | 1.15 (0.12)d | 6.93 (0.91) b | 9.79 (0.37) e | 0.0116 (0.0036) ab | 28.44 (5.05) ac | 132.29 (54.61) a | 8.45 (0.03) a | 6.36 (0.05) c | |
| CT1 | 0.33 (0.03) b | 0.82 (0.03) e | 7.08 (0.13) bd | 7.69 (0.47) a | 0.0103 (0.0039) ab | 29.60 (0.58) a | 79.17 (43.42) ab | 8.40 (0.03) c | 6.27 (0.01) d | |
| 10–20 | BS2 | 0.38 (0.06) ad | 0.96 (0.08) b | 7.23 (0.09) b | 7.26 (0.66) ab | 0.0092 (0.0024) b | 30.42 (5.91) ad | 143.29 (69.01) a | 8.48 (0.02) a | 6.52 (0.03) f |
| BF2 | 0.29 (0.02) b | 0.94 (0.00) b | 7.06 (0.17) bd | 7.94 (0.04) b | 0.0086 (0.0007) b | 32.04 (3.89) a | 181.52 (33.36) a | 8.53 (0.05) ae | 6.81 (0.05) c | |
| RS2 | 0.34 (0.00) ab | 0.85 (0.06)b | 7.38 (0.19) b | 7.75 (0.06) b | 0.0132 (0.0030) b | 32.63 (2.89) ac | 114.53 (29.44) a | 8.73 (0.02) b | 6.67 (0.02) d | |
| CT2 | 0.32 (0.05) ab | 0.71 (0.03) ae | 6.74 (0.33) d | 6.52 (0.51) a | 0.0052 (0.0026) a | 26.35 (6.70) a | 54.02 (11.38) b | 8.51 (0.04) a | 6.09 (0.00) b | |
| 20–40 | BS3 | 0.44 (0.21) abdef | 0.85 (0.07) b | 6.24 (0.65) a | 5.78 (0.72) d | 0.0094 (0.0016) ab | 33.66 (6.60) d | 150.40 (25.17) a | 8.44 (0.04) a | 6.30 (0.04) ab |
| BF3 | 0.38 (0.14) abef | 0.86 (0.11) b | 6.38 (0.65) ab | 6.06 (1.78) bd | 0.0095 (0.0018) ab | 25.37 (2.03) b | 186.92 (29.92) a | 8.59 (0.01) be | 6.37 (0.05) a | |
| RS3 | 0.28 (0.02) e | 0.63 (0.02) a | 6.06 (0.78) ab | 4.90 (0.54) d | 0.0117 (0.0039) b | 32.64 (1.20) cd | 146.92 (17.18) a | 8.29 (0.03) c | 6.26 (0.04) b | |
| CT3 | 0.85 (0.40) f | 0.63 (0.11) a | 5.39 (0.14) a | 4.66 (0.62) d | 0.0068 (0.0025) a | 22.18 (0.27) ab | 97.92 (19.16) b | 8.62 (0.01) b | 6.67 (0.04) c |
| TP | TN | pH | SOC | GC | DOC | MBC | ||
|---|---|---|---|---|---|---|---|---|
| measures | BS | 0.44 | 0.46 | 0.36 | 0.47 | −0.06 | 0.15 | 0.20 |
| BF | −0.03 | 0.88 ** | −0.34 | 0.74 * | −0.55 | −0.06 | −0.54 | |
| RS | 0.17 | 0.33 | 0.70 * | 0.52 | 0.27 | 0.11 | −0.40 | |
| CT | 0.68 * | −0.81 ** | −0.86 ** | −0.93 ** | −0.47 | −0.62 | 0.45 | |
| layers | Surface | −0.39 | 0.72 ** | −0.41 | 0.79 ** | −0.51 | 0.14 | 0.33 |
| Middle | −0.001 | −0.13 | 0.46 | 0.34 | 0.49 | 0.24 | 0.09 | |
| Bottom | 0.60 * | 0.17 | −0.19 | 0.03 | −0.5 | −0.70 * | −0.19 |
| TP | TN | pH | SOC | GC | DOC | MBC | ||
|---|---|---|---|---|---|---|---|---|
| measures | BS | −0.42 | 0.92 ** | 0.81 ** | 0.92 ** | −0.08 | −0.46 | −0.13 |
| BF | −0.30 | 0.11 | 0.28 | 0.29 | −0.03 | 0.79 * | 0.11 | |
| RS | 0.04 | 0.22 | 0.66 | 0.41 | 0.27 | 0.05 | −0.30 | |
| CT | 0.84 ** | −0.50 | −0.86 ** | −0.73 * | 0.17 | −0.46 | 0.62 | |
| layers | Surface | −0.38 | 0.82 ** | −0.05 | 0.77 ** | −0.22 | −0.13 | 0.57 |
| Middle | −0.14 | 0.76 ** | 0.58 | 0.84 ** | 0.65 * | 0.51 | 0.77 ** | |
| Bottom | 0.72 ** | −0.33 | −0.50 | −0.29 | −0.56 | −0.82 ** | −0.62 * |
| Soil Conservation Measures | Interaction Networks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nodes | Links | Negative Links | Positive Links | Negative/ Positive | Average Geodesic Distance | Average Clustering Coefficient | Network Diameter | Average Connectivity | Modularity | |
| BS | 165 | 294 | 110 | 184 | 0.600 | 2.40 | 0.040 | 7 | 3.56 | 6 |
| BF | 166 | 192 | 95 | 97 | 0.979 | 2.56 | 0.286 | 7 | 2.31 | 16 |
| RS | 184 | 264 | 103 | 161 | 0.640 | 2.34 | 0.158 | 7 | 2.87 | 8 |
| CT | 72 | 61 | 0 | 61 | 0 | 1.70 | 0.153 | 4 | 1.69 | 3 |
| Soil Conservation Measures | Interaction Networks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nodes | Links | Negative Links | Positive Links | Negative/ Positive | Average Geodesic Distance | Average Clustering Coefficient | Network Diameter | Average Connectivity | Modularity | |
| BS | 163 | 245 | 93 | 152 | 0.612 | 2.63 | 0.121 | 7 | 3.01 | 6 |
| BF | 186 | 283 | 100 | 183 | 0.546 | 2.95 | 0.348 | 11 | 3.04 | 15 |
| RS | 183 | 266 | 95 | 171 | 0.556 | 2.08 | 0.230 | 6 | 2.91 | 8 |
| CT | 126 | 304 | 103 | 201 | 0.512 | 2.23 | 0.291 | 6 | 4.83 | 8 |
| Soil Conservation Measures | Interaction Networks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nodes | Links | Negative Links | Positive Links | Negative/ Positive | Average Geodesic Distance | Average Clustering Coefficient | Network Diameter | Average Connectivity | Modularity | |
| BS | 23 | 131 | 54 | 77 | 0.701 | 1.648 | 0.769 | 5 | 11.391 | 3 |
| BF | 11 | 23 | 11 | 12 | 0.917 | 1.378 | 0.718 | 2 | 4.182 | 2 |
| RS | 25 | 99 | 31 | 68 | 0.456 | 1.957 | 0.644 | 4 | 7.92 | 2 |
| CT | 7 | 5 | 1 | 4 | 0.250 | 1.909 | 0.000 | 4 | 1.429 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, B.; Wang, P.; Devlin, A.T.; Xiao, S.; Shu, W.; Zhang, H.; Ding, M. Influence of Soil and Water Conservation Measures on Soil Microbial Communities in a Citrus Orchard of Southeast China. Microorganisms 2021, 9, 319. https://doi.org/10.3390/microorganisms9020319
Wu B, Wang P, Devlin AT, Xiao S, Shu W, Zhang H, Ding M. Influence of Soil and Water Conservation Measures on Soil Microbial Communities in a Citrus Orchard of Southeast China. Microorganisms. 2021; 9(2):319. https://doi.org/10.3390/microorganisms9020319
Chicago/Turabian StyleWu, Bobo, Peng Wang, Adam T. Devlin, Shengsheng Xiao, Wang Shu, Hua Zhang, and Mingjun Ding. 2021. "Influence of Soil and Water Conservation Measures on Soil Microbial Communities in a Citrus Orchard of Southeast China" Microorganisms 9, no. 2: 319. https://doi.org/10.3390/microorganisms9020319
APA StyleWu, B., Wang, P., Devlin, A. T., Xiao, S., Shu, W., Zhang, H., & Ding, M. (2021). Influence of Soil and Water Conservation Measures on Soil Microbial Communities in a Citrus Orchard of Southeast China. Microorganisms, 9(2), 319. https://doi.org/10.3390/microorganisms9020319






