Influence of Extremophiles on the Generation of Acid Mine Drainage at the Abandoned Pan de Azúcar Mine (Argentina)
Abstract
:1. Introduction
2. Materials and Methods
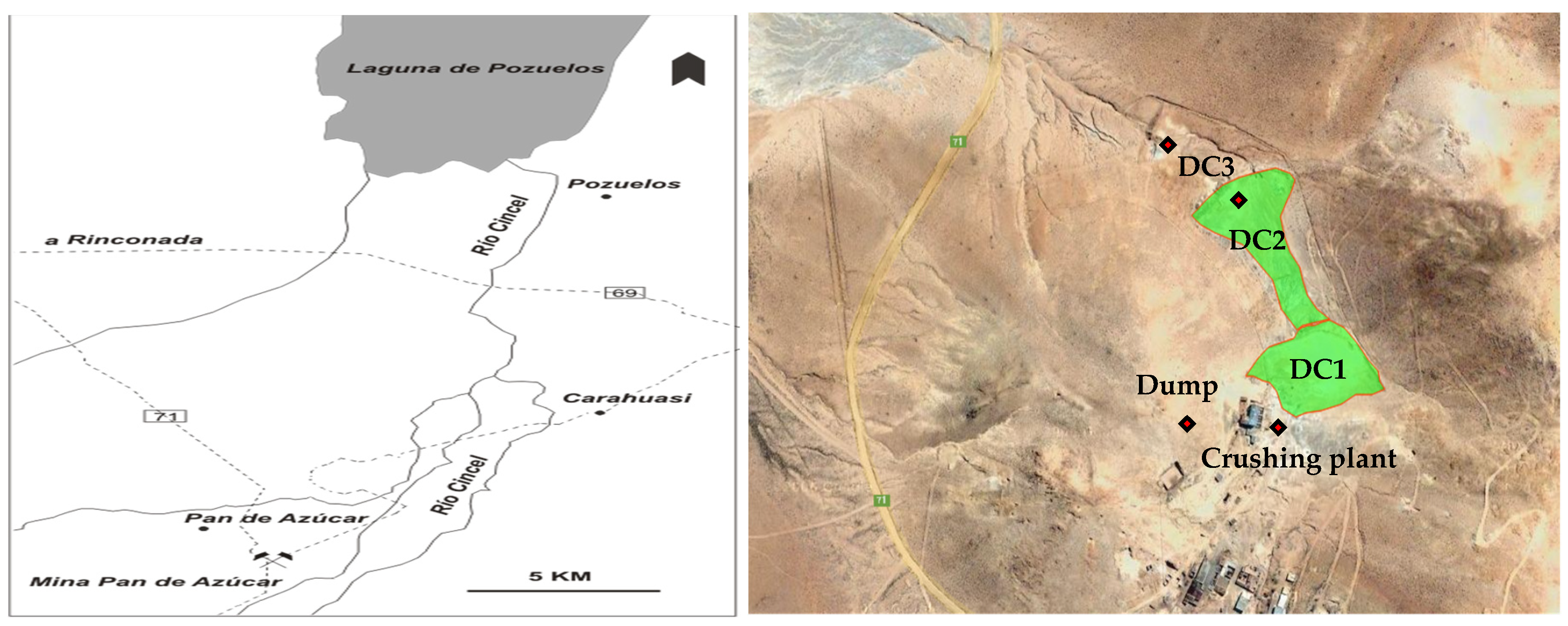
2.1. Description of the Site
2.2. Acid Drainage Samples
2.2.1. Physicochemical Analysis
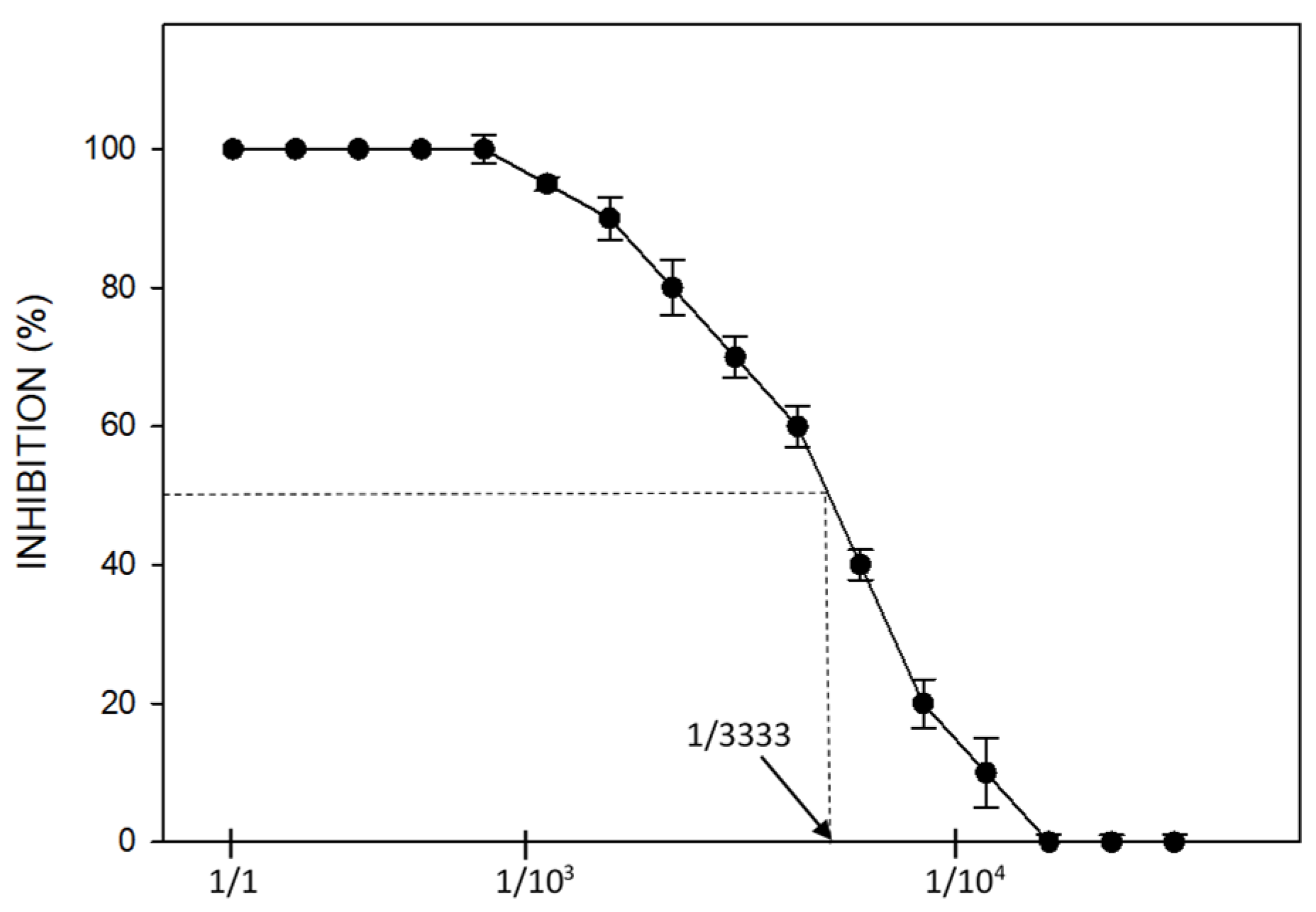
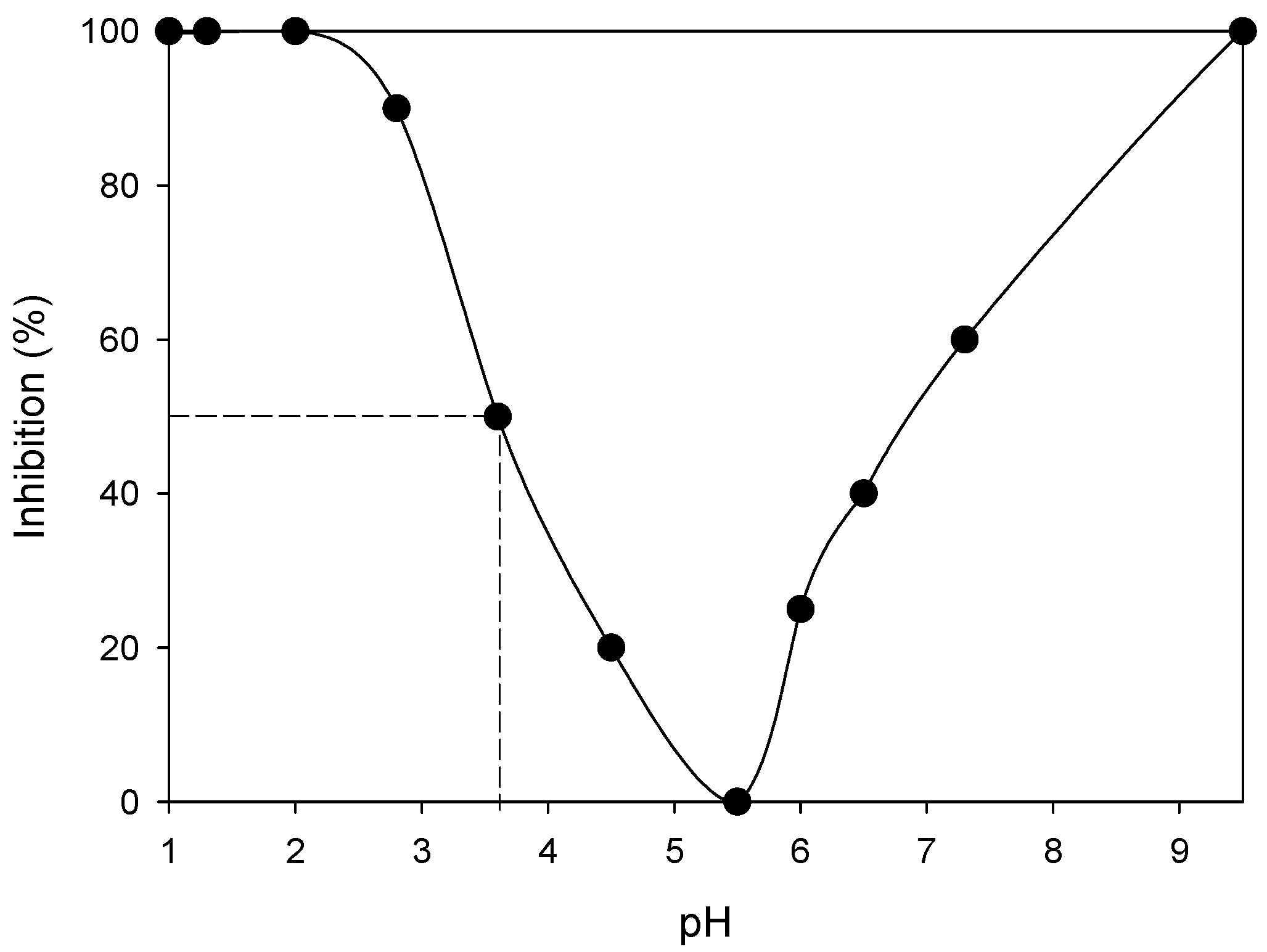
2.2.2. Ecotoxicology Bioassays
2.3. Tailings Samples
2.4. Prediction of AMD Generation
2.4.1. Physicochemical Tests
2.4.2. Biooxidation Tests Using Collection Strains
2.5. Enrichment, Isolation, and Microbial Characterization
2.6. Biooxidation Tests Using Native Consortia
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akcil, A.; Koldas, S. Acid mine drainage (AMD): Causes, treatment and case studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Simate, G.S.; Ndlovu, S. Acid mine drainage: Challenges and opportunities. J. Environ. Chem. Eng. 2014, 2, 1785–1803. [Google Scholar] [CrossRef]
- Carlier, J.D.; Ettamimi, S.; Cox, C.J.; Hammani, K.; Ghazal, H.; Costa, M.C. Prokaryotic diversity in stream sediments affected by acid mine drainage. Extremophiles 2020, 24, 809–819. [Google Scholar] [CrossRef]
- Park, I.; Tabelin, C.B.; Jeon, S.; Li, X.; Seno, K.; Ito, M.; Hiroyoshi, N. A review of recent strategies for acid mine drainage prevention and mine tailings recycling. Chemosphere 2019, 219, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Rambabu, K.; Banat, F.; Pham, Q.M.; Ho, S.H.; Ren, N.Q.; Show, P.L. Biological remediation of acid mine drainage: Review of past trends and current outlook. Environ. Sci. Ecotechnol. 2020, 2, 100024. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Corpuz, R.D.; Igarashi, T.; Villacorte-Tabelin, M.; Diaz-Alorro, R.; Yoo, Q.; Raval, S.; Ito, M.; Hiroyoshi, N. Acid mine drainage formation and arsenic mobility under strongly acidic conditions: Importance of soluble phases, iron oxyhydroxides/oxides and nature of oxidation layer on pyrite. J. Hazard. Mater. 2020, 399, 122844. [Google Scholar] [CrossRef]
- Schrenk, M.; Edwards, K.; Goodman, R.; Hamers, R.; Bandfield, J. Distribution of Thiobacillus ferrooxidans and Leptospirillum ferrooxidans: Implications for generation of acid mine drainage. Science 1998, 279, 1519–1522. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Bellenberg, S.; Castro, L.; Neu, T.R.; Sand, W.; Vera, M. Colonization and biofilm formation of the extremely acidophilic archaeon Ferroplasma Acidiphilum. Hydrometall. 2014, 150, 245–252. [Google Scholar] [CrossRef]
- Nordstrom, D.K.; Blowes, D.W.; Ptacek, C.J. Hydrogeochemistry and microbiology of mine drainage: An update. Appl. Geochem. 2015, 57, 3–16. [Google Scholar] [CrossRef]
- Chen, L.-X.; Huang, L.-N.; Méndez-García, C.; Kuang, J.-L.; Hua, Z.-S.; Liu, J.; Shu, W.-S. Microbial communities, processes and functions in acid mine drainage ecosystems. Curr. Opin. Biotechnol. 2016, 38, 150–158. [Google Scholar] [CrossRef]
- Teng, W.; Kuang, J.; Luo, Z.; Shu, W. Microbial diversity and community assembly across environmental Gradients in Acid Mine Drainage. Minerals 2017, 7, 106. [Google Scholar] [CrossRef] [Green Version]
- Dutta, M.; Islam, N.; Rabha, S.; Narzary, B.; Bordoloi, M.; Saikia, D.; Silva, L.F.O.; Saikia, B.K. Acid mine drainage in an Indian high-sulphur coal mining area: Cytotoxicity assay and remediation study. J. Hazard. Mater. 2020, 389, 121851. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.J.; Banfield, J.F. Microbial communities in acid mine drainage. FEMS Microbiol. Ecol. 2003, 44, 139–152. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.-N.; Kuang, J.-L.; Shu, W.-S. Microbial ecology and evolution in the acid mine drainage model system. Trends Microbiol. 2016, 24, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Castro, C.; Urbieta, S.; Plaza-Cazón, J.; Donati, E.R. Review: Metal biorecovery and bioremediation: Whether or not thermophilic are better than mesophilic microorganisms. Bioresour. Technol. 2019, 279, 317–326. [Google Scholar] [CrossRef]
- Hou, D.; Zhang, P.; Zhang, J.; Zhou, Y.; Yang, Y.; Mao, Q.; Tsang, D.C.W.; Núñez-Delgado, A.; Luo, L. Spatial variation of sediment bacterial community in an acid mine drainage contaminated area and surrounding river basin. J. Environ. Manag. 2019, 251, 109542. [Google Scholar] [CrossRef]
- Chen, H.; Xiao, T.; Ning, Z.; Li, Q.; Xiao, E.; Liu, Y.; Xiao, Q.; Lan, X.; Ma, L.; Lu, F. In-Situ remediation of acid mine drainage from abandoned coal mine by filed pilot scale passive treatment system: Performance and response of microbial communities to low pH and elevated Fe. Bioresour. Technol. 2020, 317, 123985. [Google Scholar] [CrossRef]
- Xin, R.; Banda, J.F.; Hao, C.; Dong, H.; Pei, L.; Guo, D.; Wei, P.; Du, Z.; Zhang, Y.; Dong, H. Contrasting seasonal variations of geochemistry and microbial community in two adjacent acid mine drainage lakes in Anhui Province, China. Environ. Poll. 2020, 268, 115826. [Google Scholar] [CrossRef]
- Ogbughalu, O.T.; Vasileiadis, V.; Schumann, R.C.; Gerson, A.R.; Li, J.; Smart, R.S.C.; Short, M.D. Role of microbial diversity for sustainable pyrite oxidation control in acid and metalliferous drainage prevention. J. Hazard. Mater. 2020, 395, 122338. [Google Scholar] [CrossRef]
- Bernardelli, C.E.; Maza, S.N.; Lecomte, K.L.; Collo, G.; Astini, R.A.; Donati, E.R. Acidophilic microorganisms enhancing geochemical dynamics in an acidic drainage system, Amarillo river in La Rioja, Argentina. Chemosphere 2021, 263, 128098. [Google Scholar] [CrossRef]
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: A review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Xiao, S.; Liu, J. Microbial communities in acid mine drainage and their interaction with pyrite surface. Curr. Microbiol. 2009, 59, 71. [Google Scholar] [CrossRef] [PubMed]
- Méndez-García, C.; Peláez, A.I.; Mesa, V.; Sánchez, J.; Golyshina, O.V.; Ferrer, M. Microbial diversity and metabolic networks in acid mine drainage habitats. Front. Microbiol. 2015, 6, 475. [Google Scholar] [PubMed] [Green Version]
- Panda, S.; Mishra, S.; Akcil, A. Bioremediation of acidic mine effluents and the role of sulfidogenic biosystems: A mini-review. Euro-Mediterr. J. Environ. Integr. 2016, 1, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Kirschbaum, A.; Murray, J.; Arnosio, M.; Tonda, R.; Cacciabue, L. Pasivos ambientales mineros en el noroeste de Argentina: Aspectos mineralógicos, geoquímicos y consecuencias ambientales. Rev. Mex. Cienc. Geol. 2012, 29, 248–264. [Google Scholar]
- Plaza-Cazón, J.; Benítez, L.; Murray, J.; Kirschbaum, A.; Kirschbaum, P.; Donati, E. Environmental impact on soil, water and plants from the abandoned Pan de Azúcar Mine. Adv. Mater. Res. 2013, 825, 88–91. [Google Scholar] [CrossRef]
- Murray, J.; Kirschbaum, A.; Dold, B.; Mendes Guimaraes, E.; Pannunzio Miner, E. Jarosite versus soluble iron-sulfate formation and their role in acid mine drainage formation at the Pan de Azúcar Mine Tailings (Zn-Pb-Ag), NW Argentina. Minerals 2014, 4, 477–502. [Google Scholar] [CrossRef]
- Sarkar, D.; Haldar, A. Physical and Chemical Methods in Soil Analysis, 1st ed.; New Age International (P) Limited, Publishers: New Delhi, India, 2005. [Google Scholar]
- Sánchez-Meza, J.C.; Pacheco-Salazar, V.F.; Pavón-Silva, T.B.; Gutiérrez-García, V.G.; De Jesús Avila-González, C.; Guerrero-García, P. Toxicity assessment of a complex industrial wastewater using aquatic and terrestrial bioassays Daphnia pulex and Lactuca sativa. J. Environ. Sci. Health A 2007, 42, 1425–1431. [Google Scholar] [CrossRef]
- Lottermoser, B.G. Environmental Indicators in Metal Mining, 1st ed.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Bruynesteyn, A.; Hackl, R.P. Evaluation of acid production potential of mining waste materials. Miner. Environ. 1982, 4, 5–8. [Google Scholar] [CrossRef]
- Urbieta, M.S.; Gonzalez Toril, E.; Aguilera, A.; Giaveno, M.A.; Donati, E.R. First prokaryotic biodiversity assessment using molecular techniques of an acidic river in Neuquén, Argentina. Microb. Ecol. 2012, 64, 91–104. [Google Scholar] [CrossRef]
- Urbieta, M.S.; González Toril, E.; Aguilera, A.; Giaveno, M.A.; Donati, E. Comparison of the microbial communities of hot springs waters and the microbial biofilms in the acidic geothermal area of Copahue (Neuquen, Argentina). Extremophiles 2015, 19, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Muyzer, G.; Smalla, K. Application of denaturing gradient gel electrophoresis (DGGE) and temperature gradient gel electrophoresis (TGGE) in microbial ecology. Antonie Leeuwenhoek 1998, 73, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.A.; Urbieta, M.S.; Donati, E. Arsenic-tolerant microbial consortia from sediments of Copahue geothermal system with potential applications in bioremediation. J. Basic Microbiol. 2019, 59, 680–691. [Google Scholar] [CrossRef] [PubMed]
- U.S. EPA (Environmental Protection Agency). 2018 Edition of the Drinking Water Standards and Health Advisories Tables; Office of Water U.S. Environmental Protection Agency: Washington, DC, USA, 2018.
- Bagur-González, M.G.; Estepa-Molina, C.; Martín-Peinado, F.; Morales-Ruano, S. Toxicity assessment using Lactuca sativa L. bioassay of the metal(loid)s As, Cu, Mn, Pb and Zn in soluble-in-water saturated soil extracts from an abandoned mining site. J. Soils Sediments 2011, 11, 281–289. [Google Scholar]
- Yucel, D.S.; Baba, A. Prediction of acid mine drainage generation potential of various lithologies using static tests: Etili coal mine (NW Turkey) as a case study. Environ. Monit. Assess. 2016, 188, 473. [Google Scholar] [CrossRef] [Green Version]
- Oh, C.; Ji, S.; Yim, G.; Cheong, Y. Evaluation of net acid generation pH as a single indicator for acid forming potential of rocks using geochemical properties. Environ. Monit. Assess. 2017, 189, 165. [Google Scholar] [CrossRef]
- Karlsson, T.; Räisänen, M.L.; Lehtonen, M.; Alakangas, L. Comparison of static and mineralogical ARD prediction methods in the Nordic environment. Environ. Monit. Assess. 2018, 190, 719. [Google Scholar] [CrossRef] [Green Version]
- Sand, W. Ferric iron reduction by Thiobacillus ferrooxidans at extremely low pH-values. Biogeochemistry 1989, 7, 195–201. [Google Scholar] [CrossRef]
- White, W.W., III; Lapakko, K.A.; Cox, R.L. Static-test methods most commonly used to predict acid-mine drainage: Practical guidelines for use and interpretation. In The Environmental Geochemistry of Mineral Deposits: Part A: Processes, Techniques, and Health Issues. Reviews in Economic Geology; Plumlee, G.S., Logsdon, M.J., Filipek, L.F., Eds.; Society of Economic Geologists: Littleton, CO, USA, 1999; pp. 225–338. [Google Scholar]
- Bouzahzah, H.; Benzaazoua, M.; Bussiere, B.; Plante, B. Prediction of acid mine drainage: Importance of mineralogy and the test protocols for static and kinetic tests. Mine Water Environ. 2014, 33, 54–65. [Google Scholar] [CrossRef]
- Balci, N.; Demirel, C. Prediction of acid mine drainage (AMD) and metal release sources at the Küre copper mine site, Kastamonu, NW Turkey. Mine Water Environ. 2018, 37, 56–74. [Google Scholar] [CrossRef]


| AMD-1 | AMD-2 | Cincel River | Guideline Values (US EPA) [36] | |
|---|---|---|---|---|
| pH | 0.58 | 2.25 | 8.16 | 6.5–9.5 |
| Fe (g·L−1) | 97.40 | 0.860 | <3.10−5 | 2.10−4 |
| Zn (g·L−1) | 25.10 | 0.214 | <5.10−6 | 5.10−3 |
| Pb (mg·L−1) | 1.30 | <0.2 | <5.10−2 | 1.10−2 |
| Cd (mg·L−1) | 161 | 1.4 | <2.10−2 | 5.10−3 |
| Ca (mg·L−1) | <2 | 9.2 | 11.4 | N.P. |
| Ni (mg·L−1) | 5 | 0 | <5.10−2 | 2.10−2 |
| Mg (mg·L−1) | 320 | 23.2 | 4.4 | N.P. |
| Na (mg·L−1) | 517 | 2.0 | 25.7 | 20 |
| K (mg·L−1) | <1 | 1.3 | 0.9 | N.P. |
| SO4−2 (g·L−1) | 42.30 | 3.98 | N.D. | 0.250 |
| DC2-1 | DC2-2 | DC2-3 | DC2-4 | DC2-5 | |
|---|---|---|---|---|---|
| Depth (cm) | 0–14 | 14–26 | 26–50 | 50–66 | >66 |
| Estimated area (m3) | 3668 | 3144 | 6288 | 4192 | 35111 |
| Estimated mass (tons) | 7.6 | 8.6 | 17.6 | 12.1 | 92.0 |
| pH | 2.69 | 2.43 | 2.00 | 2.12 | 1.90 |
| Apparent density (g·cm−3) | 1.53 | 1.09 | 1.24 | 1.44 | 1.58 |
| Real density (g·cm−3) | 2.07 | 2.73 | 2.80 | 2.88 | 2.62 |
| Porosity | 26.1 | 60.1 | 55.7 | 50.0 | 39.7 |
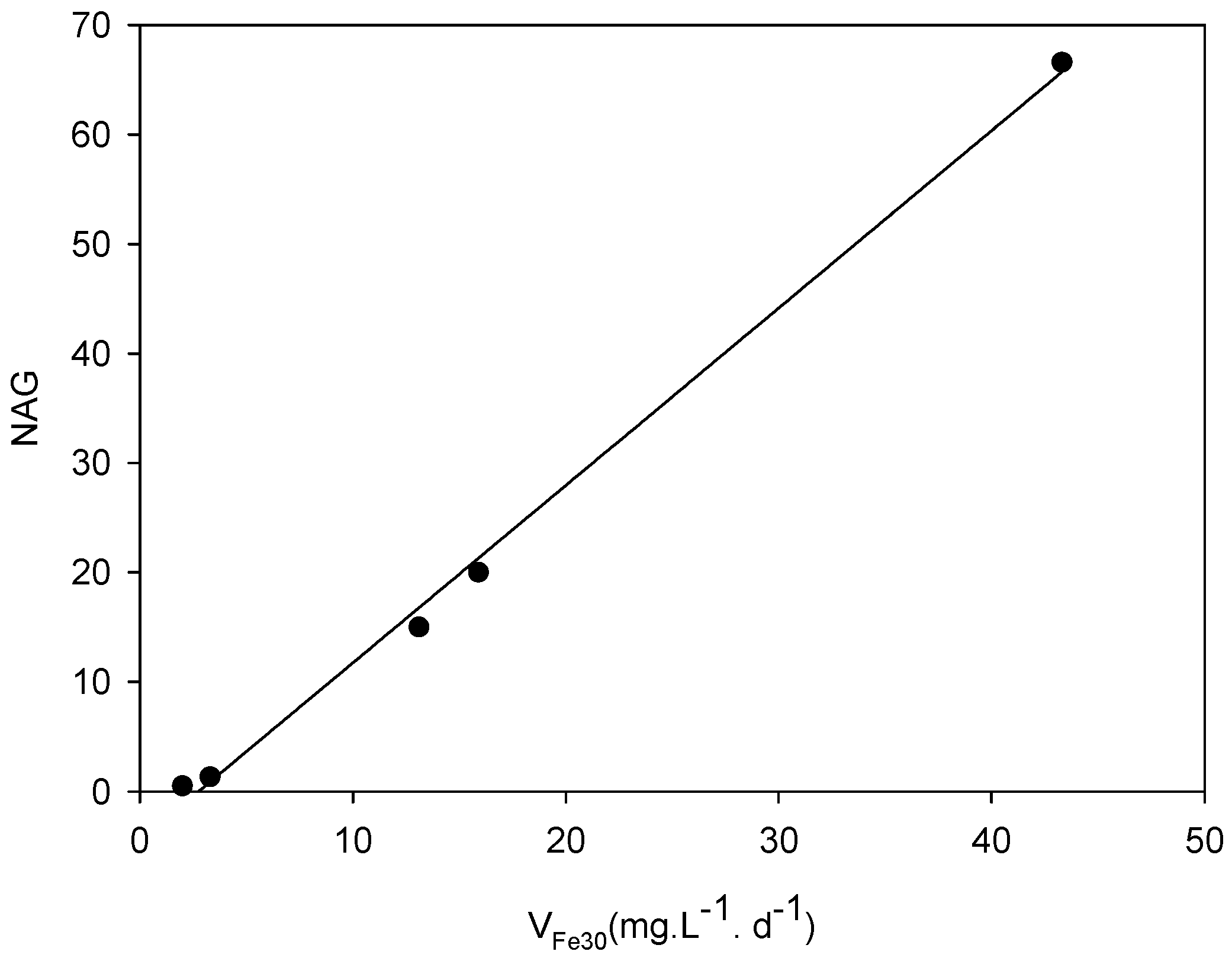
| Sample | NAGpH | NAG * | Type |
|---|---|---|---|
| DC2-1 | 2.01 | 13.1 | Potentially acid forming (PAF) |
| DC2-2 | 2.62 | 3.3 | Potentially acid forming-low capacity (PAF-LC) |
| DC2-3 | 2.66 | 2.0 | Potentially acid forming-low capacity (PAF-LC) |
| DC2-4 | 3.90 | 15.9 | Potentially acid forming (PAF) |
| DC2-5 | 1.74 | 43.3 | Potentially acid forming (PAF) |
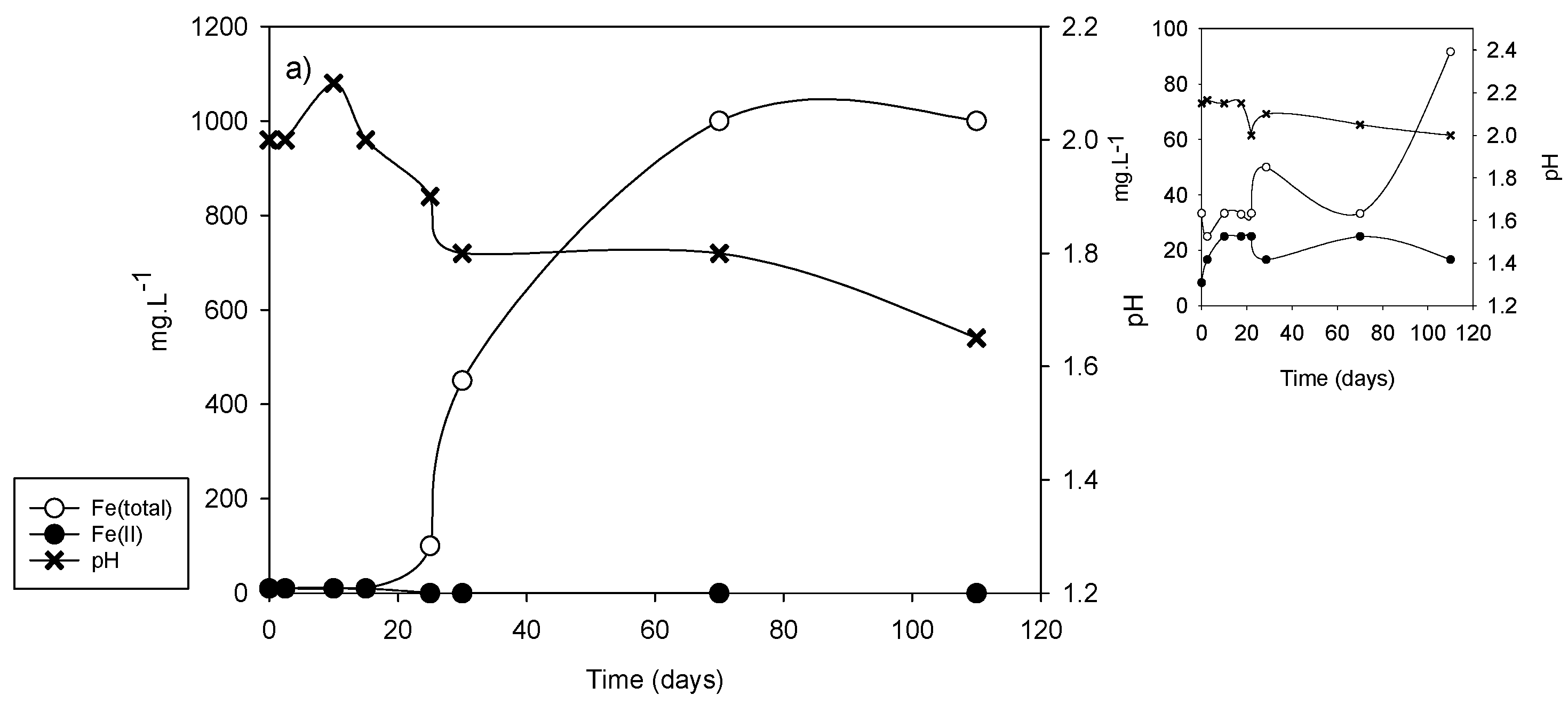
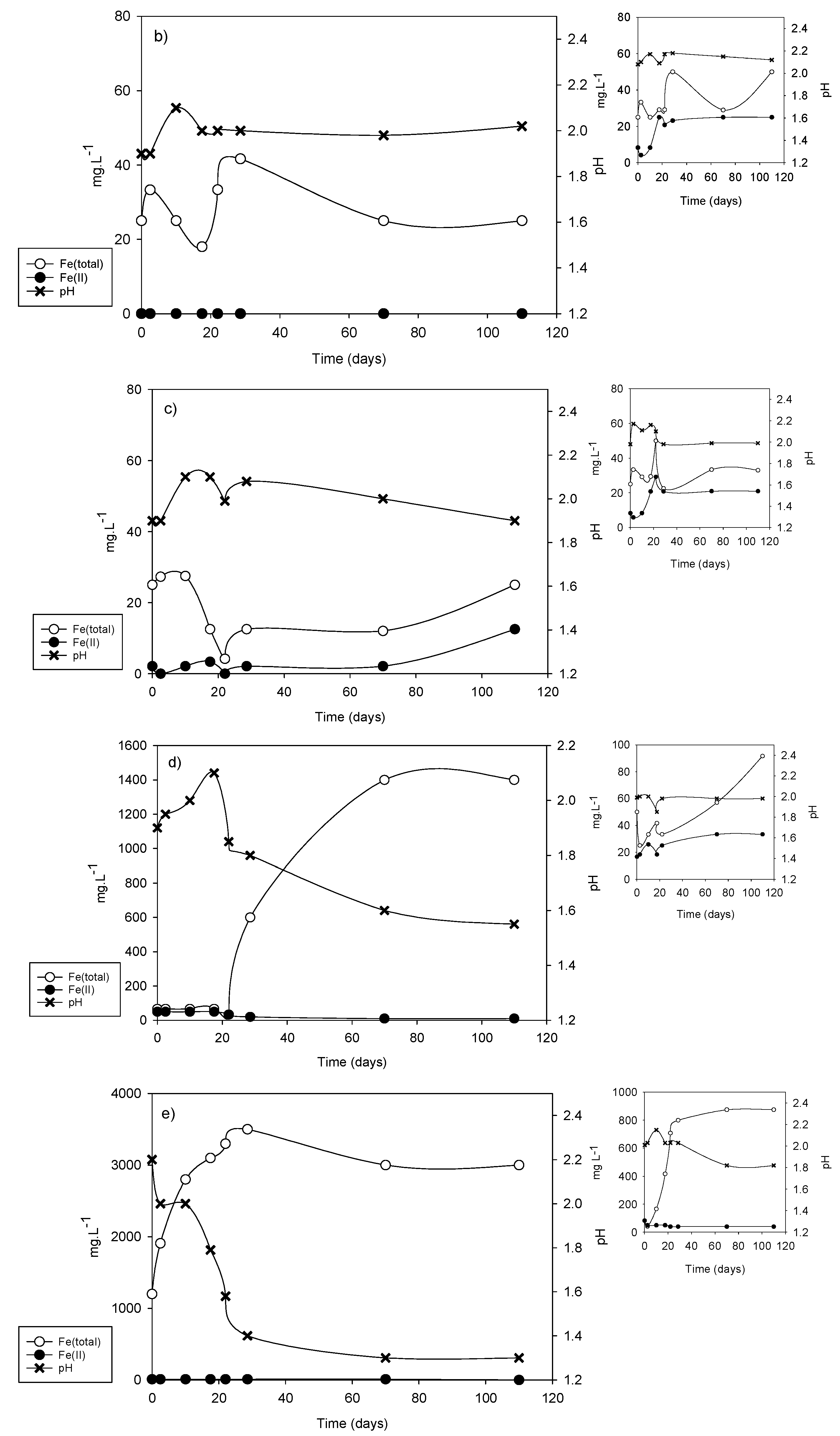
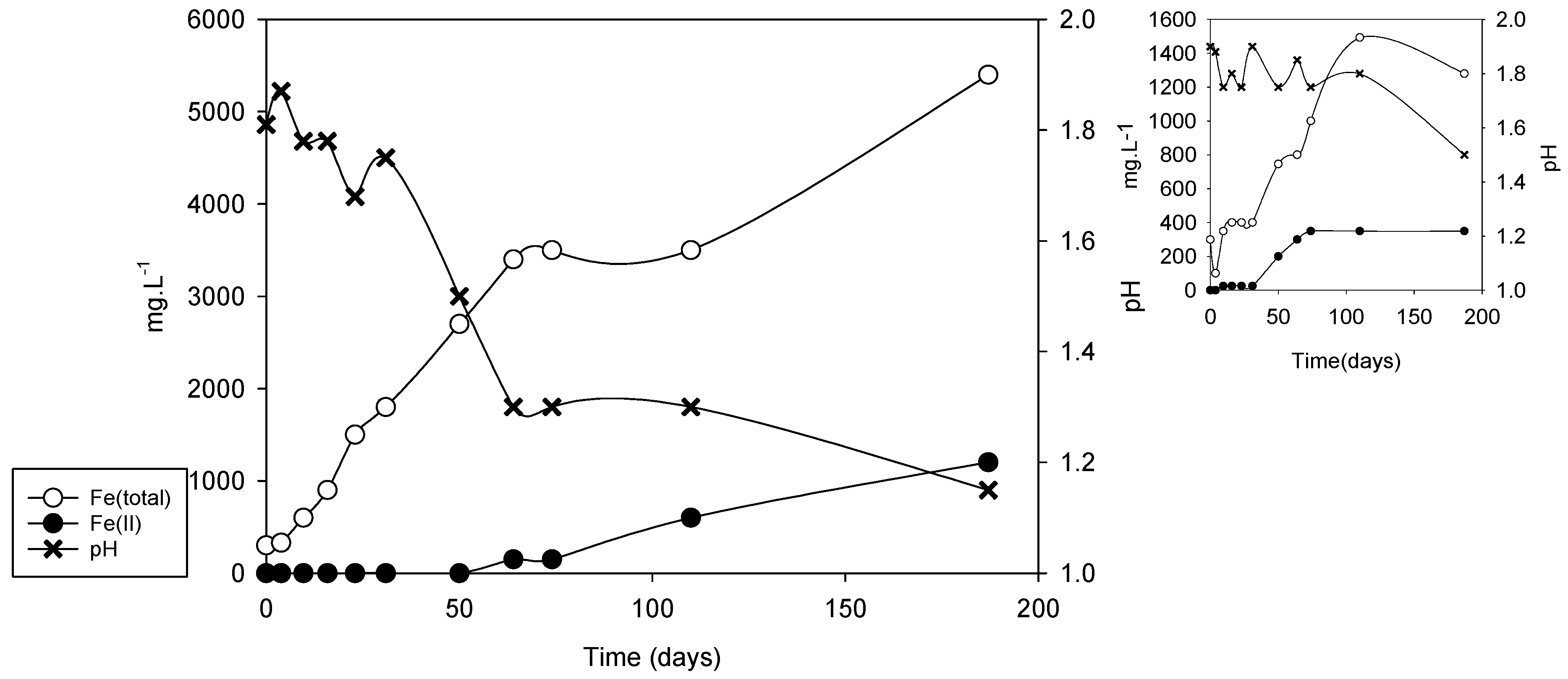
| Sample | vFe30 (mg·L−1·d−1) | pH30 | vFe70 (mg·L−1·d−1) | pH70 | vFe110 (mg·L−1·d−1) | pH110 |
|---|---|---|---|---|---|---|
| DC2-1 | 15.0 | 1.80 | 14.28 | 1.65 | 9.1 | 1.60 |
| DC2-2 | 1.33 | 2.05 | 0.35 | 2.05 | 0.22 | 2.10 |
| DC2-3 | 0.5 | 2.00 | 0.34 | 2.00 | 0.22 | 2.00 |
| DC2-4 | 20.0 | 1.75 | 20.0 | 1.60 | 12.72 | 1.60 |
| DC2-5 | 66.6 | 1.50 | 27.87 | 1.30 | 17.72 | 1.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plaza-Cazón, J.; Benítez, L.; Murray, J.; Kirschbaum, P.; Donati, E. Influence of Extremophiles on the Generation of Acid Mine Drainage at the Abandoned Pan de Azúcar Mine (Argentina). Microorganisms 2021, 9, 281. https://doi.org/10.3390/microorganisms9020281
Plaza-Cazón J, Benítez L, Murray J, Kirschbaum P, Donati E. Influence of Extremophiles on the Generation of Acid Mine Drainage at the Abandoned Pan de Azúcar Mine (Argentina). Microorganisms. 2021; 9(2):281. https://doi.org/10.3390/microorganisms9020281
Chicago/Turabian StylePlaza-Cazón, Josefina, Leonardo Benítez, Jésica Murray, Pablo Kirschbaum, and Edgardo Donati. 2021. "Influence of Extremophiles on the Generation of Acid Mine Drainage at the Abandoned Pan de Azúcar Mine (Argentina)" Microorganisms 9, no. 2: 281. https://doi.org/10.3390/microorganisms9020281





