Current Evidence for Corynebacterium on the Ocular Surface
Abstract
1. Introduction
2. Corynebacterium Species
2.1. C. macginleyi
2.2. C. accolens
2.3. C. propinquum
2.4. C. amycolatum
2.5. C. jeikeium
2.6. C. mastitidis, C. lowii, and C. oculi
3. Laboratory Examinations
3.1. The Ocular Manifestations of Corynebacterium Species
3.2. Microscopy Examinations
3.3. Culture Tests
3.4. Antimicrobial Susceptibility Testing
4. The Susceptibility of Corynebacterium to Antibiotics
5. Genetic Mutations and Drug Resistance
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inoue, Y.; Usui, M.; Ohashi, Y.; Shiota, H.; Yamazaki, T. Preoperative disinfection of the conjunctival sac with antibiotics and iodine compounds: A prospective randomized multicenter study. Jpn. J. Ophthalmol. 2008, 52, 151–161. [Google Scholar] [CrossRef]
- Davis, C.P. Normal flora. In Medical Microbiology, 4th ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Funke, G.; von Graevenitz, A.; Clarridge, J.E., 3rd; Bernard, K.A. Clinical microbiology of coryneform bacteria. Clin. Microbiol Rev. 1997, 10, 125–159. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.; Oliveira, L.C.; Aburjaile, F.; Benevides, L.; Tiwari, S.; Jamal, S.B.; Silva, A.; Figueiredo, H.C.P.; Ghosh, P.; Portela, R.W.; et al. Insight of Genus Corynebacterium: Ascertaining the Role of Pathogenic and Non-pathogenic Species. Front. Microbiol. 2017, 8, 1937. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, S.; Hashida, M.; Urabe, K. Risk factors for aerobic bacterial conjunctival flora in preoperative cataract patients. Eye 2016, 30, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- Petrillo, F.; Pignataro, D.; Lavano, M.A.; Santella, B.; Folliero, V.; Zannella, C.; Astarita, C.; Gagliano, C.; Franci, G.; Avitabile, T.; et al. Current Evidence on the Ocular Surface Microbiota and Related Diseases. Microorganisms 2020, 8, 1033. [Google Scholar] [CrossRef] [PubMed]
- Funke, G.; Pagano-Niederer, M.; Bernauer, W. Corynebacterium macginleyi has to date been isolated exclusively from conjunctival swabs. J. Clin. Microbiol. 1998, 36, 3670–3673. [Google Scholar] [CrossRef]
- Eguchi, H.; Kuwahara, T.; Miyamoto, T.; Nakayama-Imaohji, H.; Ichimura, M.; Hayashi, T.; Shiota, H. High-level fluoroquinolone resistance in ophthalmic clinical isolates belonging to the species Corynebacterium macginleyi. J. Clin. Microbiol. 2008, 46, 527–532. [Google Scholar] [CrossRef][Green Version]
- Das, S.; Rao, A.S.; Sahu, S.K.; Sharma, S. Corynebacterium spp as causative agents of microbial keratitis. Br. J. Ophthalmol. 2016, 100, 939–943. [Google Scholar] [CrossRef]
- Kuriyan, A.E.; Sridhar, J.; Flynn, H.W., Jr.; Huang, L.C.; Yannuzzi, N.A.; Smiddy, W.E.; Davis, J.L.; Albini, T.A.; Berrocal, A.M.; Miller, D. Endophthalmitis Caused by Corynebacterium Species: Clinical Features, Antibiotic Susceptibility, and Treatment Outcomes. Ophthalmol. Retin. 2017, 1, 200–205. [Google Scholar] [CrossRef]
- Claeys, G.; Vanhouteghem, H.; Riegel, P.; Wauters, G.; Hamerlynck, R.; Dierick, J.; de Witte, J.; Verschraegen, G.; Vaneechoutte, M. Endocarditis of native aortic and mitral valves due to Corynebacterium accolens: Report of a case and application of phenotypic and genotypic techniques for identification. J. Clin. Microbiol. 1996, 34, 1290–1292. [Google Scholar] [CrossRef]
- Ang, L.M.; Brown, H. Corynebacterium accolens isolated from breast abscess: Possible association with granulomatous mastitis. J. Clin. Microbiol. 2007, 45, 1666–1668. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.S.; Seaward, L.M.; Ho, C.P.; Anderson, T.P.; Lau, E.O.; Amodeo, M.R.; Metcalf, S.C.; Pithie, A.D.; Murdoch, D.R. Corynebacterium accolens-associated pelvic osteomyelitis. J. Clin. Microbiol. 2010, 48, 654–655. [Google Scholar] [CrossRef] [PubMed]
- Badenoch, P.R.; O’Daniel, L.J.; Wise, R.P.; Slattery, J.A.; Mills, R.A. Corynebacterium propinquum Keratitis Identified Using MALDI-TOF. Cornea 2016, 35, 686–687. [Google Scholar] [CrossRef] [PubMed]
- Duignan, E.S.; Farrell, S.; Treacy, M.P.; Fulcher, T.; O’Brien, P.; Power, W.; Murphy, C.C. Corneal inlay implantation complicated by infectious keratitis. Br. J. Ophthalmol. 2016, 100, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Todokoro, D.; Eguchi, H.; Yamada, N.; Sodeyama, H.; Hosoya, R.; Kishi, S. Contact Lens-Related Infectious Keratitis with White Plaque Formation Caused by Corynebacterium propinquum. J. Clin. Microbiol. 2015, 53, 3092–3095. [Google Scholar] [CrossRef]
- Ruoff, K.L.; Toutain-Kidd, C.M.; Srinivasan, M.; Lalitha, P.; Acharya, N.R.; Zegans, M.E.; Schwartzman, J.D. Corynebacterium macginleyi isolated from a corneal ulcer. Infect. Dis. Rep. 2010, 2. [Google Scholar] [CrossRef][Green Version]
- Alsuwaidi, A.R.; Wiebe, D.; Burdz, T.; Ng, B.; Reimer, A.; Singh, C.; Bernard, K. Corynebacterium macginleyi conjunctivitis in Canada. J. Clin. Microbiol. 2010, 48, 3788–3790. [Google Scholar] [CrossRef][Green Version]
- Suzuki, T.; Iihara, H.; Uno, T.; Hara, Y.; Ohkusu, K.; Hata, H.; Shudo, M.; Ohashi, Y. Suture-related keratitis caused by Corynebacterium macginleyi. J. Clin. Microbiol. 2007, 45, 3833–3836. [Google Scholar] [CrossRef]
- Giammanco, G.M.; Di Marco, V.; Priolo, I.; Intrivici, A.; Grimont, F.; Grimont, P.A. Corynebacterium macginleyi isolation from conjunctival swab in Italy. Diagn Microbiol. Infect. Dis. 2002, 44, 205–207. [Google Scholar] [CrossRef]
- Li, A.; Lal, S. Corynebacterium pseudodiphtheriticum keratitis and conjunctivitis: A case report. Clin. Exp. Ophthalmol. 2000, 28, 60–61. [Google Scholar] [CrossRef]
- Deguchi, H.; Kitazawa, K.; Kayukawa, K.; Kondoh, E.; Fukumoto, A.; Yamasaki, T.; Kinoshita, S.; Sotozono, C. The trend of resistance to antibiotics for ocular infection of Staphylococcus aureus, coagulase-negative staphylococci, and Corynebacterium compared with 10-years previous: A retrospective observational study. PLoS ONE 2018, 13, e0203705. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, S.; Todokoro, D.; Sasaki, T. Corynebacterium Species of the Conjunctiva and Nose: Dominant Species and Species-Related Differences of Antibiotic Susceptibility Profiles. Cornea 2020, 11, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- Riegel, P.; Ruimy, R.; de Briel, D.; Prévost, G.; Jehl, F.; Christen, R.; Monteil, H. Genomic diversity and phylogenetic relationships among lipid-requiring diphtheroids from humans and characterization of Corynebacterium macginleyi sp. nov. Int. J. Syst. Bacteriol. 1995, 45, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Heidemann, D.G.; Dunn, S.P.; Diskin, J.A.; Aiken, T.B. Corynebacterium striatus keratitis. Cornea 1991, 10, 81–82. [Google Scholar] [CrossRef] [PubMed]
- Rubinfeld, R.S.; Cohen, E.J.; Arentsen, J.J.; Laibson, P.R. Diphtheroids as ocular pathogens. Am. J. Ophthalmol. 1989, 108, 251–254. [Google Scholar] [CrossRef]
- Inata, K.; Maeda, I.; Ikeda, Y.; Miyazaki, D.; Inoue, K.; Eguchi, H.; Shiota, H.; Kuwahara, T. A Case of lnfectious Keratitis Caused by Corynebacterium. J. Eye 2009, 26, 1105–1107. [Google Scholar]
- Joussen, A.M.; Funke, G.; Joussen, F.; Herbertz, G. Corynebacterium macginleyi: A conjunctiva specific pathogen. Br. J. Ophthalmol. 2000, 84, 1420–1422. [Google Scholar] [CrossRef][Green Version]
- Ferrer, C.; Ruiz-Moreno, J.M.; Rodríguez, A.; Montero, J.; Alió, J.L. Postoperative Corynebacterium macginleyi endophthalmitis. J. Cataract. Refract. Surg. 2004, 30, 2441–2444. [Google Scholar] [CrossRef]
- Tabuenca Del Barrio, L.; Mozo Cuadrado, M.; Borque Rodríguez-Maimón, E.; Zubicoa Eneriz, A.; Garralda Luquín, A. Decreased painful visual acuity. Corynebacterium macginleyi blebitis-endophthalmitis infection. Rev. Esp. Quim. 2020, 33, 80–82. [Google Scholar] [CrossRef]
- Qin, V.; Laurent, T.; Ledoux, A. Corynebacterium macginleyi-associated Blebitis: A Case Report. J. Glaucoma 2018, 27, e174–e176. [Google Scholar] [CrossRef]
- Eguchi, H. Ocular infections caused by Corynebacterium species. In Infection Control; IntechOpen: London, UK, 2013; pp. 75–82. [Google Scholar]
- Villanueva, J.L.; Domínguez, A.; Ríos, M.J.; Iglesias, C. Corynebacterium macginleyi isolated from urine in a patient with a permanent bladder catheter. Scand. J. Infect. Dis 2002, 34, 699–700. [Google Scholar] [CrossRef] [PubMed]
- Mosele, M.; Veronese, N.; Bolzetta, F.; Manzato, E.; Sergi, G. A rare case of sepsis due to Corynebacterium macginleyi from central venous catheter in an elderly woman. New Microbiol. 2012, 35, 89–91. [Google Scholar] [PubMed]
- Dobler, G.; Braveny, I. Highly resistant Corynebacterium macginleyi as cause of intravenous catheter-related infection. Eur. J. Clin. Microbiol. Infect. Dis. 2003, 22, 72–73. [Google Scholar] [CrossRef] [PubMed]
- Villamil-Cajoto, I.; Rodríguez-Otero, L.; García-Zabarte, M.A.; Aguilera-Guirao, A.; García-Riestra, C.; Regueiro, B.J.; Villacián-Vicedo, M.J. Septicemia caused by Corynebacterium macginleyi: A rare form of extraocular infection. Int. J. Infect. Dis. 2008, 12, 333–335. [Google Scholar] [CrossRef][Green Version]
- Neubauer, M.; Šourek, J.; Rýc, M.; Boháček, J.; Mára, M.; Mňuková, J. Corynebacterium accolens sp. nov., a Gram-Positive Rod Exhibiting Satellitism, from Clinical Material. Syst. Appl. Microbiol. 1991, 14, 46–51. [Google Scholar] [CrossRef]
- Riegel, P.; de Briel, D.; Prévost, G.; Jehl, F.; Monteil, H. Proposal of Corynebacterium propinquum sp. nov. for Corynebacterium group ANF-3 strains. FEMS Microbiol. Lett. 1993, 113, 229–234. [Google Scholar] [CrossRef][Green Version]
- Bernard, K.; Pacheco, A.L.; Cunningham, I.; Gill, N.; Burdz, T.; Wiebe, D. Emendation of the description of the species Corynebacterium propinquum to include strains which produce urease. Int. J. Syst. Evol. Microbiol. 2013, 63, 2146–2154. [Google Scholar] [CrossRef]
- Yang, K.; Kruse, R.L.; Lin, W.V.; Musher, D.M. Corynebacteria as a cause of pulmonary infection: A case series and literature review. Pneumonia (Nathan) 2018, 10, 10. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Matsubara, K.; Ishihara, H.; Nigami, H.; Iwata, A.; Kawaguchi, K.; Fukaya, T.; Kawamura, Y.; Kikuchi, K. Corynebacterium propinquum as the first cause of infective endocarditis in childhood. J. Infect. Chemother. 2014, 20, 317–319. [Google Scholar] [CrossRef]
- Neemuchwala, A.; Soares, D.; Ravirajan, V.; Marchand-Austin, A.; Kus, J.V.; Patel, S.N. In Vitro Antibiotic Susceptibility Pattern of Non-diphtheriae Corynebacterium Isolates in Ontario, Canada, from 2011 to 2016. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Collins, M.D.; Burton, R.A.; Jones, D. Corynebacterium amycolatum sp. nov. a new mycolic acid-less Corynebacterium species from human skin. FEMS Microbiol. Lett. 1988, 49, 349–352. [Google Scholar] [CrossRef]
- Toribio, J.A.; Marrodan, T.; Fernandez-Natal, I. Orbital implant infection by Corynebacterium amycolatum. Orbit 2017, 36, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Walter, S.; Rademacher, F.; Kobinger, N.; Simanski, M.; Gläser, R.; Harder, J. RNase 7 participates in cutaneous innate control of Corynebacterium amycolatum. Sci. Rep. 2017, 7, 13862. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.G.; Ferguson, W.P. Corynebacterium jeikeium (group JK) resistance to ciprofloxacin emerging during therapy. J. Antimicrob. Chemother. 1987, 20, 922–923. [Google Scholar] [CrossRef]
- Ortiz-Pérez, A.; Martín-de-Hijas, N.Z.; Esteban, J.; Fernández-Natal, M.I.; García-Cía, J.I.; Fernández-Roblas, R. High frequency of macrolide resistance mechanisms in clinical isolates of Corynebacterium species. Microb. Drug Resist. 2010, 16, 273–277. [Google Scholar] [CrossRef]
- Philippon, A.; Bimet, F. In vitro susceptibility of Corynebacterium group D2 and Corynebacterium jeikeium to twelve antibiotics. Eur. J. Clin. Microbiol. Infect. Dis. 1990, 9, 892–895. [Google Scholar] [CrossRef]
- Soriano, F.; Zapardiel, J.; Nieto, E. Antimicrobial susceptibilities of Corynebacterium species and other non-spore-forming gram-positive bacilli to 18 antimicrobial agents. Antimicrob. Agents Chemother. 1995, 39, 208–214. [Google Scholar] [CrossRef]
- Fernandez-Garayzabal, J.F.; Collins, M.D.; Hutson, R.A.; Fernandez, E.; Monasterio, R.; Marco, J.; Dominguez, L. Corynebacterium mastitidis sp. nov., isolated from milk of sheep with subclinical mastitis. Int. J. Syst. Bacteriol. 1997, 47, 1082–1085. [Google Scholar] [CrossRef]
- St Leger, A.J.; Caspi, R.R. Visions of Eye Commensals: The Known and the Unknown About How the Microbiome Affects Eye Disease. Bioessays 2018, 40, e1800046. [Google Scholar] [CrossRef]
- Bernard, K.A.; Pacheco, A.L.; Loomer, C.; Burdz, T.; Wiebe, D.; Huynh, C.; Kaplen, B.; Olson, A.B.; Cnockaert, M.; Eguchi, H.; et al. Corynebacterium lowii sp. nov. and Corynebacterium oculi sp. nov., derived from human clinical disease and an emended description of Corynebacterium mastitidis. Int. J. Syst. Evol. Microbiol. 2016, 66, 2803–2812. [Google Scholar] [CrossRef]
- Fukumoto, A.; Sotozono, C.; Hieda, O.; Kinoshita, S. Infectious keratitis caused by fluoroquinolone-resistant Corynebacterium. Jpn. J. Ophthalmol. 2011, 55, 579–580. [Google Scholar] [CrossRef] [PubMed]
- Sano, H.; Eguchi, H.; Miyamaoto, T.; Hotta, F.; Mitamura, S.; Mitamura, Y. Quinolone-resistant Corynebacterium Keratitis Successfully Treated with 1.5% Levofloxacin Ophthalmic Solution. J. Eye 2014, 31, 1683–1686. [Google Scholar]
- Gaudreau, C.; Gilbert, H. Comparison of disc diffusion and agar dilution methods for antibiotic susceptibility testing of Campylobacter jejuni subsp. jejuni and Campylobacter coli. J. Antimicrob. Chemother. 1997, 39, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Haldorsen, B.; Giske, C.G.; Hansen, D.S.; Helgason, K.O.; Kahlmeter, G.; Löhr, I.H.; Matuschek, E.; Österblad, M.; Rantakokko-Jalava, K.; Wang, M.; et al. Performance of the EUCAST disc diffusion method and two MIC methods in detection of Enterobacteriaceae with reduced susceptibility to meropenem: The NordicAST CPE study. J. Antimicrob. Chemother. 2018, 73, 2738–2747. [Google Scholar] [CrossRef] [PubMed]
- Ly, C.N.; Pham, J.N.; Badenoch, P.R.; Bell, S.M.; Hawkins, G.; Rafferty, D.L.; McClellan, K.A. Bacteria commonly isolated from keratitis specimens retain antibiotic susceptibility to fluoroquinolones and gentamicin plus cephalothin. Clin. Exp. Ophthalmol. 2006, 34, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.; Nirmalkar, K.; Mathai, A.; Sharma, S. Clinical features, microbiological profile and treatment outcome of patients with Corynebacterium endophthalmitis: Review of a decade from a tertiary eye care centre in southern India. Br. J. Ophthalmol. 2016, 100, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Bharathi, M.J.; Ramakrishnan, R.; Shivakumar, C.; Meenakshi, R.; Lionalraj, D. Etiology and antibacterial susceptibility pattern of community-acquired bacterial ocular infections in a tertiary eye care hospital in south India. Indian J. Ophthalmol. 2010, 58, 497–507. [Google Scholar] [CrossRef]
- Watson, S.; Cabrera-Aguas, M.; Khoo, P.; Pratama, R.; Gatus, B.J.; Gulholm, T.; El-Nasser, J.; Lahra, M.M. Keratitis antimicrobial resistance surveillance program, Sydney, Australia: 2016 Annual Report. Clin. Exp. Ophthalmol. 2019, 47, 20–25. [Google Scholar] [CrossRef]
- Hagihara, K.; Kitagawa, K.; Koyama, Y.; Tanimura, N.; Iinuma, Y.; Sasaki, H. Five Cases with Ocular Surface Infections in which Multidrug Resistant Coryneform Bacteria was Detected by the Disk Diffusion Method. J. Eye 2020, 37, 619–623. [Google Scholar]
- Kishimoto, R.; Tagawa, Y.; Ohno, S. A case of corneal ulcer due to Corynebacterium speciesc resistant to multiple antibiotics. Jpn. J. Clin. Ophthalmol. 2004, 58, 1341–1344. [Google Scholar]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Swick, M.C.; Morgan-Linnell, S.K.; Carlson, K.M.; Zechiedrich, L. Expression of multidrug efflux pump genes acrAB-tolC, mdfA, and norE in Escherichia coli clinical isolates as a function of fluoroquinolone and multidrug resistance. Antimicrob. Agents Chemother. 2011, 55, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Touzé, T.; Eswaran, J.; Bokma, E.; Koronakis, E.; Hughes, C.; Koronakis, V. Interactions underlying assembly of the Escherichia coli AcrAB–TolC multidrug efflux system. Mol. Microbiol. 2004, 53, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Masuda, N.; Sakagawa, E.; Ohya, S.; Gotoh, N.; Tsujimoto, H.; Nishino, T. Substrate specificities of MexAB-OprM, MexCD-OprJ, and MexXY-oprM efflux pumps in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2000, 44, 3322–3327. [Google Scholar] [CrossRef]
- Hooper, D.C. Emerging mechanisms of fluoroquinolone resistance. Emerg. Infect. Dis. 2001, 7, 337–341. [Google Scholar] [CrossRef]
- Hooper, D.C. Bacterial topoisomerases, anti-topoisomerases, and anti-topoisomerase resistance. Clin. Infect. Dis. 1998, 27 (Suppl. 1), S54–S63. [Google Scholar] [CrossRef]
- Schmutz, E.; Hennig, S.; Li, S.M.; Heide, L. Identification of a topoisomerase IV in actinobacteria: Purification and characterization of ParYR and GyrBR from the coumermycin A1 producer Streptomyces rishiriensis DSM 40489. Microbiology (Reading) 2004, 150, 641–647. [Google Scholar] [CrossRef]
- Hooper, D.C.; Jacoby, G.A. Topoisomerase Inhibitors: Fluoroquinolone Mechanisms of Action and Resistance. Cold Spring Harb. Perspect Med. 2016, 6. [Google Scholar] [CrossRef]
- Wohlkonig, A.; Chan, P.F.; Fosberry, A.P.; Homes, P.; Huang, J.; Kranz, M.; Leydon, V.R.; Miles, T.J.; Pearson, N.D.; Perera, R.L.; et al. Structural basis of quinolone inhibition of type IIA topoisomerases and target-mediated resistance. Nat. Struct Mol. Biol. 2010, 17, 1152–1153. [Google Scholar] [CrossRef]
- Sierra, J.M.; Martinez-Martinez, L.; Vazquez, F.; Giralt, E.; Vila, J. Relationship between mutations in the gyrA gene and quinolone resistance in clinical isolates of Corynebacterium striatum and Corynebacterium amycolatum. Antimicrob. Agent Chemother. 2005, 49, 1714–1719. [Google Scholar] [CrossRef]
- Alibi, S.; Ferjani, A.; Boukadida, J.; Cano, M.E.; Fernández-Martínez, M.; Martínez-Martínez, L.; Navas, J. Occurrence of Corynebacterium striatum as an emerging antibiotic-resistant nosocomial pathogen in a Tunisian hospital. Sci. Rep. 2017, 7, 9704. [Google Scholar] [CrossRef] [PubMed]
- Ramos, J.N.; Valadão, T.B.; Baio, P.V.P.; Mattos-Guaraldi, A.L.; Vieira, V.V. Novel mutations in the QRDR region gyrA gene in multidrug-resistance Corynebacterium spp. isolates from intravenous sites. Antonie Van Leeuwenhoek 2020, 113, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Dobinson, H.C.; Anderson, T.P.; Chambers, S.T.; Doogue, M.P.; Seaward, L.; Werno, A.M. Antimicrobial Treatment Options for Granulomatous Mastitis Caused by Corynebacterium Species. J. Clin. Microbiol. 2015, 53, 2895–2899. [Google Scholar] [CrossRef] [PubMed]
- Nudel, K.; Zhao, X.; Basu, S.; Dong, X.; Hoffmann, M.; Feldgarden, M.; Allard, M.; Klompas, M.; Bry, L. Genomics of Corynebacterium striatum, an emerging multidrug-resistant pathogen of immunocompromised patients. Clin. Microbiol. Infect. 2018, 24, 1016.e7–1016.e13. [Google Scholar] [CrossRef] [PubMed]
- Oliva, A.; Belvisi, V.; Iannetta, M.; Andreoni, C.; Mascellino, M.T.; Lichtner, M.; Vullo, V.; Mastroianni, C.M. Pacemaker lead endocarditis due to multidrug-resistant Corynebacterium striatum detected with sonication of the device. J. Clin. Microbiol. 2010, 48, 4669–4671. [Google Scholar] [CrossRef]
- Chatzopoulou, M.; Koufakis, T.; Voulgaridi, I.; Gabranis, I.; Tsiakalou, M. A case of fatal sepsis due to multidrug-resistant Corynebacterium striatum. Hippokratia 2016, 20, 67–69. [Google Scholar]
- Goldner, N.K.; Bulow, C.; Cho, K.; Wallace, M.; Hsu, F.F.; Patti, G.J.; Burnham, C.A.; Schlesinger, P.; Dantas, G. Mechanism of High-Level Daptomycin Resistance in Corynebacterium striatum. mSphere 2018, 3. [Google Scholar] [CrossRef]
- Ramos, J.N.; Souza, C.; Faria, Y.V.; da Silva, E.C.; Veras, J.F.C.; Baio, P.V.P.; Seabra, S.H.; de Oliveira Moreira, L.; Hirata Júnior, R.; Mattos-Guaraldi, A.L.; et al. Bloodstream and catheter-related infections due to different clones of multidrug-resistant and biofilm producer Corynebacterium striatum. BMC Infect. Dis. 2019, 19, 672. [Google Scholar] [CrossRef]
- Abdel-Meguid, A.A.E.; Gabr, A.F.; Said, M.M.; Nassef, M.; Elmenofy, T.M.I. Comparative Study Between Topical Gatifloxacin 0.5% and Moxifloxacin 0.5% as a Prophylactic Measure Before Intraocular Surgery. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2019, 35, 315–318. [Google Scholar] [CrossRef]
- Pinna, A.; Donadu, M.G.; Usai, D.; Dore, S.; Boscia, F.; Zanetti, S. In Vitro Antimicrobial Activity of a New Ophthalmic Solution Containing Hexamidine Diisethionate 0.05% (Keratosept). Cornea 2020, 39, 1415–1418. [Google Scholar] [CrossRef]
- Carrijo-Carvalho, L.C.; Sant’ana, V.P.; Foronda, A.S.; de Freitas, D.; de Souza Carvalho, F.R. Therapeutic agents and biocides for ocular infections by free-living amoebae of Acanthamoeba genus. Surv. Ophthalmol. 2017, 62, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, S.; Shimizu, K.; Nejima, R.; Kagaya, F.; Aihara, M.; Iwasaki, T.; Shoji, N.; Miyata, K. Conjunctival Bacteria Flora of Glaucoma Patients During Long-Term Administration of Prostaglandin Analog Drops. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3991–3996. [Google Scholar] [CrossRef] [PubMed]
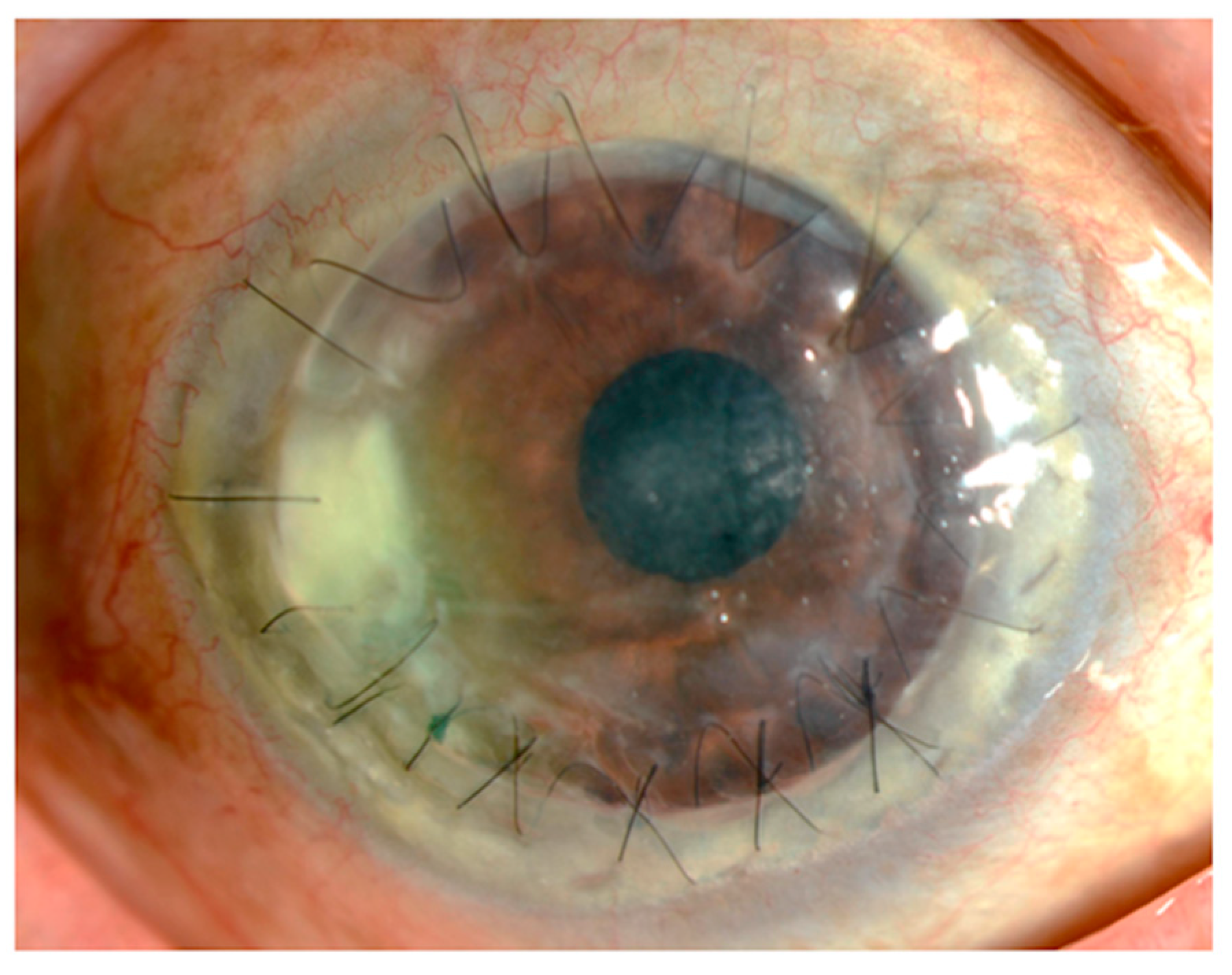
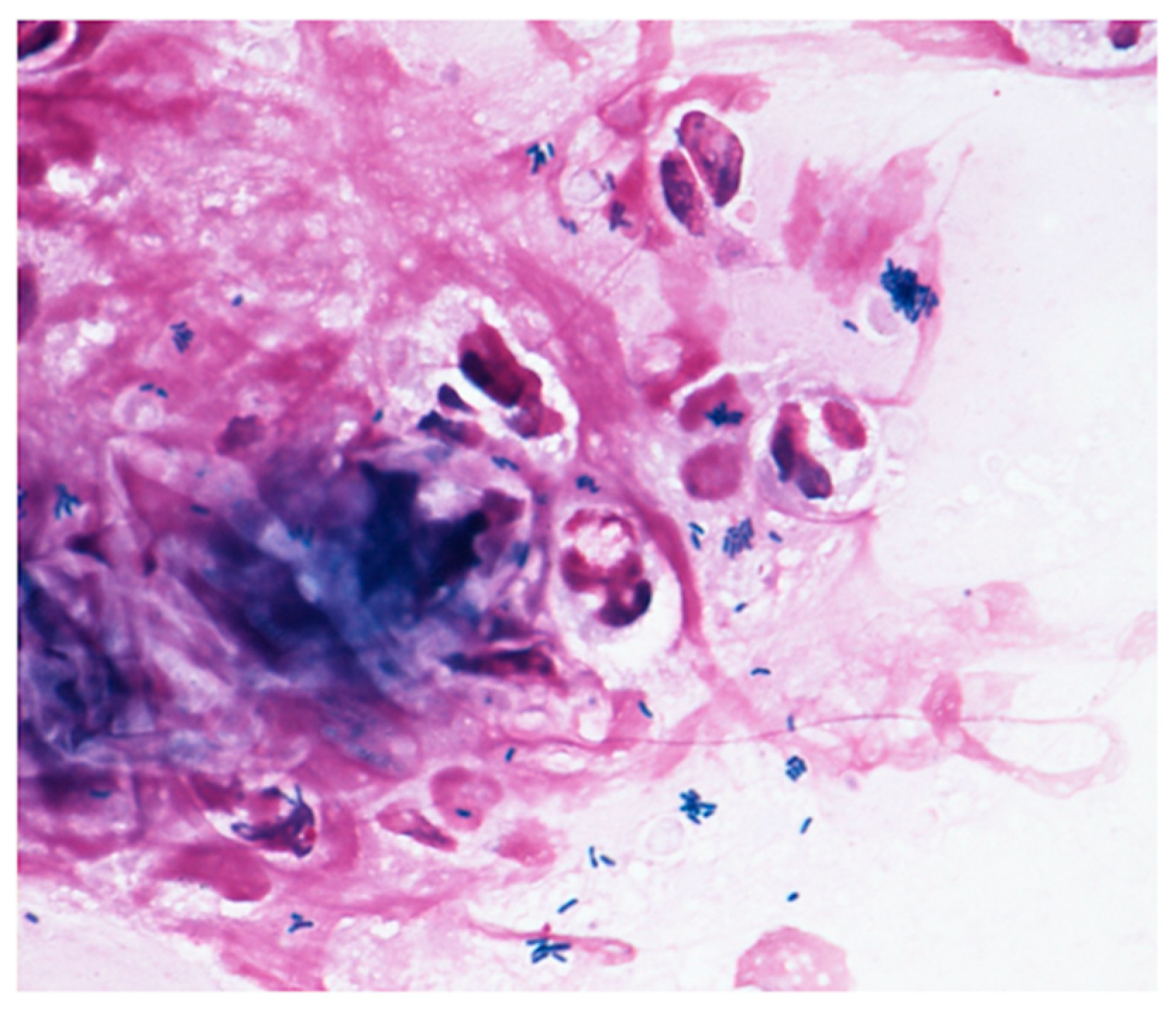
| Authors (Ref. Number) | Year | Country | Age | Disease | Type of Strains | Lipophilic or Non-Lipophilic | Past History | Antibiotic Susceptibilities of Corynebacterium Species (Minimum Inhibitory Concentration; (μg/mL)) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCs | EM | CEPs | LVFX | CPFX | GM/TOB | VCM | CP | ||||||||
| Badenoch PR et al. [14] | 2016 | Australia | 94 | Suture-related keratitis | C. propinquum | Non-lipophilic | Diabetes, Corneal transplantation | 0.02 | >256 (CLDM) | - | - | 0.38 | - | 0.5 | - |
| Duignan ES et al. [15] | 2016 | Ireland | 52 | Keratitis | C. pseudodiphtheriticum | Non-lipophilic | Corneal inlay | - | - | - | S (MFLX) | - | - | - | S |
| Todokoro D et al. [16] | 2015 | Japan | 44 | Contact-related keratitis | C. propinquum | Non-lipophilic | Contact lens wear, Proliferative diabetic retinopathy | - | >256 | 0.125 | 1 | 2 | 0.5 | 1 | - |
| Ruoff KL et al. [17] | 2010 | USA | 84 | Keratitis | C. macginleyi | Lipophilic | Fuchs’ endothelial dystrophy | 0.016 | - | - | - | 0.032 | 0.064 | 0.5 | - |
| Alsuwaidi AR et al. [18] | 2010 | Canada | 54 | Conjunctivitis | C. macginleyi | Lipophilic | Health care worker | S | R | S | S | S | S | S | S |
| Inata K et al. [27] | 2009 | Japan | 23 | Contact-related keratitis | C. mastidis | Lipophilic | Contact lens wear | - | <0.016 | 0.125 | 0.064 | 0.125 | <0.064 | 0.5 | 4 |
| Eguchi et al. [8] | 2008 | Japan | 58 | Conjunctivitis | C. macginleyi | Lipophilic | - | - | - | - | >32 | >32 | - | - | - |
| Eguchi et al. [8] | 2008 | Japan | 72 | Conjunctivitis | C. macginleyi | Lipophilic | - | - | - | - | >32 | >32 | - | - | - |
| Eguchi et al. [8] | 2008 | Japan | 58 | Conjunctivitis | C. macginleyi | Lipophilic | - | - | - | - | >32 | >32 | - | - | - |
| Eguchi et al. [8] | 2008 | Japan | 78 | Conjunctivitis | C. macginleyi | Lipophilic | - | - | - | - | >32 | >32 | - | - | - |
| Suzuki T et al. [19] | 2007 | Japan | 74 | Suture-related keratitis | C. macginleyi | Lipophilic | Corneal transplantation | 16 | 2 | 0.5 | >128 | 128 | <0.13 | 0.5 | - |
| Suzuki T et al. [19] | 2007 | Japan | 49 | Suture-related keratitis | C. macginleyi | Lipophilic | Corneal transplantation | 16 | <0.13 | 0.25 | 64 | 8 | <0.13 | 0.5 | - |
| Giammanco G. M et al. [20] | 2002 | Italy | 65 | Corneal ulcers | C. macginleyi | Lipophilic | Trauma | S | S | S | - | S (ENX) | S | S | S |
| Li A et al. [21] | 2000 | China | 86 | Keratitis | C. pseudodiphtheriticum | Non-lipophilic | Pneumonia, Trichiasis, Lagophthalmos | R | - | R * | - | - | - | S | R |
| Heidemann DG et al. [25] | 1991 | USA | 80 | Keratitis | C. striatum | Non-lipophilic | Proliferative diabetic retinopathy | S | S | S * | - | - | - | S | - |
| Rubinfeld RS et al. [26] | 1989 | USA | 81 | Keratitis | C. striatum | Non-lipophilic | Contact lens wear, Aphakia | S | S | S * | - | - | - | S | - |
| Rubinfeld RS et al. [26] | 1989 | USA | 11 | Suture related keratitis | C. xerosis | Non-lipophilic | Post corneal laceration | - | S | S * | - | - | S | S | S |
| Funke et al. [7] | 1998 | Switzerland | 47 a | Corneal ulcer (n = 3), Conjunctivitis (n = 12) | C. macginleyi | Lipophilic | Contact lens wear, Eyelid closure problems | <0.01–0.125 b | <0.03–>64 b | 0.5–16 b | 0.125–1 (Ofloxacin)b | 0.06–0.125 b | <0.06–0.5 b | 0.5–1 b | 2–4 b |
| Authors (Ref. Number) | Year | Country | Samples | No. of Strains | Type of Strains | % of Susceptibilities to Antibiotics in Corynebacterium Species | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCs | EM | CEPs | LVFX | CPFX | GM | VCM | CP | ||||||
| Hoshi et al. [23] | 2020 | Japan | conjunctiva (n = 46), nose (n = 4) | 50 | - | 100 | 100 | - | 64 | - | 98 (TOB) | - | - |
| Deguchi et al. [22] | 2018 | Japan | conjunctiva | 77 | - | - | 28 | 100 | 0 | - | - | 100 | 88 |
| Watson et al. [60] | 2016 | Australia | cornea | 8 | - | - | - | 60 | - | 86 | - | 100 | 75 |
| Das et al. [9] | 2015 | India | cornea | 22 | C. propinquum (n = 3), C. pseudodiphtheriticum (n = 1), C. striatum (n = 1), C. bovis (n = 1) | - | - | 80 | - | 50 | - | 89 | 56 |
| Eguchi et al. [32] | 2013 | Japan | ocular surface | 20 | - | - | 45 | 100 | 25 | 25 | 95 | 100 | 55 |
| Bharathi et al. [59] | 2010 | India | ocular surface, vitreous humor | 207 | - | - | - | 91 | - | 62 | 49 | 90 | 49 |
| Eguchi et al. [8] | 2008 | Japan | ocular surface | 21 | C. macginleyi (n = 16), C. mastitidis (n = 4), C. accolens (n = 1) | - | - | - | 43 | 43 | - | - | - |
| Cameron et al. [57] | 2006 | Australia | cornea | 8 | C. macginleyi (n = 4) | - | - | 88 | - | 100 | 75 | - | 88 |
| Joussen et al. [28] | 2000 | Germany | conjunctiva | 10 | C. macginleyi (n = 10) | 100 | 70 | - | 80 | - | 100 | - | 67 |
| Samples | No. of Strains | % of Susceptibilities to Antibiotics in Corynebacterium Species | ||||||
|---|---|---|---|---|---|---|---|---|
| EM | CMX | LVFX | ABK | FOM | VCM | CP | ||
| Conjunctiva | 77 | 40 | 96 | 45 | 95 | 1 | 100 | 73 |
| Nose | 176 | 69 | 94 | 74 | 94 | 3 | 100 | 72 |
| Total | 253 | 40 | 95 | 65 | 94 | 3 | 100 | 72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aoki, T.; Kitazawa, K.; Deguchi, H.; Sotozono, C. Current Evidence for Corynebacterium on the Ocular Surface. Microorganisms 2021, 9, 254. https://doi.org/10.3390/microorganisms9020254
Aoki T, Kitazawa K, Deguchi H, Sotozono C. Current Evidence for Corynebacterium on the Ocular Surface. Microorganisms. 2021; 9(2):254. https://doi.org/10.3390/microorganisms9020254
Chicago/Turabian StyleAoki, Takanori, Koji Kitazawa, Hideto Deguchi, and Chie Sotozono. 2021. "Current Evidence for Corynebacterium on the Ocular Surface" Microorganisms 9, no. 2: 254. https://doi.org/10.3390/microorganisms9020254
APA StyleAoki, T., Kitazawa, K., Deguchi, H., & Sotozono, C. (2021). Current Evidence for Corynebacterium on the Ocular Surface. Microorganisms, 9(2), 254. https://doi.org/10.3390/microorganisms9020254






