The Interplay between Campylobacter and the Caecal Microbial Community of Commercial Broiler Chickens over Time
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Design
2.2. Campylobacter Identification from Caecal Swabs
2.3. Metataxonomics
2.3.1. DNA Extraction
2.3.2. 16SrDNA Sequencing
2.3.3. Read Preprocessing and OTU Table Construction
2.3.4. Microbiota Analysis
2.4. Statistical Analysis
3. Results
3.1. Dynamics of Campylobacter Infection over Time
3.2. Effect of C. jejuni Colonization on the Caecal Microbial Community Composition
3.3. Effect of C. jejuni Colonization on Caecal Microbial Community Diversity
3.4. Effect of C. jejuni Colonization on Caecal Microbial Community Interactions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Facciolà, A.; Riso, R.; Avventuroso, E.; Visalli, G.; Delia, S.; Laganà, P. Campylobacter: From microbiology to prevention. J. Prev. Med. Hyg. 2017, 58, E79–E92. [Google Scholar] [PubMed]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union One Health 2018 Zoonoses Report. EFSA J. 2019, 17, 5926. [Google Scholar]
- Skarp, C.; Hanninen, M.; Rautelin, H. Campylobacteriosis: The role of poultry meat. Clin. Microbiol. Infect. 2016, 22, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Sahin, O.; Zhang, Q.; Meitzler, J.C.; Harr, B.S.; Morishita, T.Y.; Mohan, R. Prevalence, Antigenic Specificity, and Bactericidal Activity of Poultry Anti-Campylobacter Maternal Antibodies. Appl. Environ. Microbiol. 2001, 67, 3951–3957. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, S.; Chaloner, G.; Kemmett, K.; Davidson, N.; Williams, N.; Kipar, A.; Humphrey, T.; Wigley, P. Campylobacter jejuni Is Not Merely a Commensal in Commercial Broiler Chickens and Affects Bird Welfare. mBio 2014, 5, e01364-14. [Google Scholar] [CrossRef] [PubMed]
- Chaloner, G.; Wigley, P.; Humphrey, S.; Kemmett, K.; Lacharme-Lora, L.; Humphrey, T.; Williams, N. Dynamics of Dual Infection with Campylobacter jejuni Strains in Chickens Reveals Distinct Strain-to-Strain Variation in Infection Ecology. Appl. Environ. Microbiol. 2014, 80, 6366–6372. [Google Scholar] [CrossRef]
- Awad, W.A.; Molnár, A.; Aschenbach, J.R.; Ghareeb, K.; Khayal, B.; Hess, C.; Liebhart, D.; Dublecz, K.; Hess, M. Campylobacter infection in chickens modulates the intestinal epithelial barrier function. Innate Immun. 2015, 21, 151–160. [Google Scholar] [CrossRef]
- Guerin, M.T.; Sir, C.; Sargeant, J.M.; Waddell, L.A.; O’Connor, A.; Wills, R.W.; Bailey, R.H.; Byrd, J.A. The change in prevalence of Campylobacter on chicken carcasses during processing: A systematic review. Poult. Sci. 2010, 89, 1070–1084. [Google Scholar] [CrossRef]
- Hermans, D.; Pasmans, F.; Messens, W.; Martel, A.; Van Immerseel, F.; Rasschaert, G.; Heyndrickx, M.; Van Deun, K.; Haesebrouck, F. Poultry as a Host for the Zoonotic PathogenCampylobacter jejuni. Vector-Borne Zoonotic Dis. 2012, 12, 89–98. [Google Scholar] [CrossRef]
- Newell, D.G.; Elvers, K.T.; Dopfer, D.; Hansson, I.; Jones, P.; James, S.; Gittins, J.; Stern, N.J.; Davies, R.; Connerton, I.; et al. Biosecurity-Based Interventions and Strategies to Reduce Campylobacter spp. on Poultry Farms. Appl. Environ. Microbiol. 2011, 77, 8605–8614. [Google Scholar] [CrossRef]
- Sibanda, N.; McKenna, A.; Richmond, A.; Ricke, S.C.; Callaway, T.; Stratakos, A.C.; Gundogdu, O.; Corcionivoschi, N. A Review of the Effect of Management Practices on Campylobacter Prevalence in Poultry Farms. Front. Microbiol. 2018, 9, 2002. [Google Scholar] [CrossRef] [PubMed]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Willer, T.; Colin, P.; Pielsticker, C.; Rychlik, I.; Velge, P.; Kaspers, B.; Rautenschlein, S. Influence of the Gut Microbiota Composition on Campylobacter jejuni Colonization in Chickens. Infect. Immun. 2017, 85, e00380-17. [Google Scholar] [CrossRef] [PubMed]
- O’Loughlin, J.L.; Samuelson, D.R.; Braundmeier-Fleming, A.G.; White, B.A.; Haldorson, G.J.; Stone, J.B.; Lessmann, J.J.; Eucker, T.P.; Konkel, M.E. The Intestinal Microbiota Influences Campylobacter jejuni Colonization and Extraintestinal Dissemination in Mice. Appl. Environ. Microbiol. 2015, 81, 4642–4650. [Google Scholar] [CrossRef]
- Rouhani, S.; Griffin, N.W.; Yori, P.P.; Olortegui, M.P.; Salas, M.S.; Trigoso, D.R.; Moulton, L.H.; Houpt, E.R.; Barratt, M.J.; Kosek, M.N.; et al. Gut Microbiota Features Associated with Campylobacter Burden and Postnatal Linear Growth Deficits in a Peruvian Birth Cohort. Clin. Infect. Dis. 2020, 71, 1000–1007. [Google Scholar] [CrossRef]
- Couturier, M.R. Revisiting the Roles of Culture and Culture-Independent Detection Tests for Campylobacter. J. Clin. Microbiol. 2016, 54, 1186–1188. [Google Scholar] [CrossRef]
- Yan, W.; Sun, C.; Yuan, J.; Yang, N. Gut metagenomic analysis reveals prominent roles of Lactobacillus and cecal microbiota in chicken feed efficiency. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Evans, S.; Sayers, A. A longitudinal study of campylobacter infection of broiler flocks in Great Britain. Prev. Veter- Med. 2000, 46, 209–223. [Google Scholar] [CrossRef]
- Denis, M.; Soumet, C.; Rivoal, K.; Ermel, G.; Blivet, D.; Salvat, G.; Colin, P. Development of a m-PCR assay for simultaneous identification of Campylobacter jejuni and C. coli. Lett. Appl. Microbiol. 1999, 29, 406–410. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2012, 41, e1. [Google Scholar] [CrossRef]
- Babraham Bioinformatics—FastQC a Quality Control Tool for High Throughput Sequence Data. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 25 January 2019).
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- McDonald, D.; Price, M.N.; Goodrich, J.K.; Nawrocki, E.P.; DeSantis, T.Z.; Probst, A.J.; Andersen, G.L.; Knight, R.; Hugenholtz, P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2011, 6, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Baruzzo, G.; Patuzzi, I.; Camillo, B.D. Beware to Ignore the Rare: How Imputing Zero-Values Can Improve the Quality of 16S rRNA Gene Studies Results. Zenodo, July 2020. Available online: https://zenodo.org/record/3965557#.X2i4tWgzaUl (accessed on 15 August 2020).
- Chen, L.; Reeve, J.; Zhang, L.; Huang, S.; Wang, X.; Chen, J. GMPR: A robust normalization method for zero-inflated count data with application to microbiome sequencing data. PeerJ 2018, 6, e4600. [Google Scholar] [CrossRef] [PubMed]
- Wv, L.; Jj, L. An accurate and robust imputation method scImpute for single-cell RNA-seq data. Nat. Commun. 2018, 9, 997. [Google Scholar]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org (accessed on 11 August 2018).
- Wickham, H. ggplot2. In Elegant Graphics for Data Analysis, 2nd ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Finotello, F.; Mastrorilli, E.; Di Camillo, B. Measuring the diversity of the human microbiota with targeted next-generation sequencing. Briefings Bioinform. 2016, 19, 679–692. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Shaw, G.T.-W.; Pao, Y.-Y.; Wang, D. MetaMIS: A metagenomic microbial interaction simulator based on microbial community profiles. BMC Bioinform. 2016, 17, 1–12. [Google Scholar] [CrossRef]
- Zou, S.; Gong, L.; Khan, T.A.; Pan, L.; Yan, L.; Li, D.; Cao, L.; Li, Y.; Ding, X.; Yi, G.; et al. Comparative analysis and gut bacterial community assemblages of grass carp and crucian carp in new lineages from the Dongting Lake area. Microbiologyopen 2020, 9, e996. [Google Scholar] [CrossRef]
- Awad, W.A.; Hess, C.; Hess, M. Re-thinking the chicken–Campylobacter jejuni interaction: A review. Avian Pathol. 2018, 47, 352–363. [Google Scholar] [CrossRef]
- Sakaridis, I.; Ellis, R.J.; Cawthraw, S.A.; Van Vliet, A.H.M.; Stekel, D.J.; Penell, J.; Chambers, M.; La Ragione, R.M.; Cook, A.J. Investigating the Association Between the Caecal Microbiomes of Broilers and Campylobacter Burden. Front. Microbiol. 2018, 9, 927. [Google Scholar] [CrossRef]
- Alrubaye, B.; Abraha, M.; Almansour, A.; Bansal, M.; Wang, H.; Kwon, Y.M.; Huang, Y.; Hargis, B.; Sun, X. Microbial metabolite deoxycholic acid shapes microbiota against Campylobacter jejuni chicken colonization. PLoS ONE 2019, 14, e0214705. [Google Scholar] [CrossRef] [PubMed]
- Oakley, B.B.; Vasconcelos, E.J.R.; Diniz, P.P.V.P.; Calloway, K.N.; Richardson, E.; Meinersmann, R.J.A.; Cox, N.; Berrang, M.E. The cecal microbiome of commercial broiler chickens varies significantly by season. Poult. Sci. 2018, 97, 3635–3644. [Google Scholar] [CrossRef] [PubMed]
- Pandit, R.J.; Hinsu, A.T.; Patel, N.V.; Koringa, P.G.; Jakhesara, S.J.; Thakkar, J.R.; Shah, T.M.; Limon, G.; Psifidi, A.; Guitian, J.; et al. Microbial diversity and community composition of caecal microbiota in commercial and indigenous Indian chickens determined using 16s rDNA amplicon sequencing. Microbiome 2018, 6, 115. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Guk, J.H.; Mun, S.H.; An, J.U.; Song, H.; Kim, J.; Ryu, S.; Jeon, B.; Cho, S. Metagenomic analysis of isolation methods of a targeted microbe, Campylobacter jejuni, from chicken feces with high microbial contamination. Microbiome 2019, 7, 67. [Google Scholar] [CrossRef] [PubMed]
- Newell, D.G.; Wagenaar, J.A. Poultry infections and their control at the farm level. In Campylobacter, 2nd ed.; Nachamkin, I., Blaser, M.J., Eds.; American Society for Microbiology: Washington, DC, USA, 2000; Chapter 26; pp. 497–509. [Google Scholar]
- Connerton, P.L.; Richards, P.J.; Lafontaine, G.M.; O’Kane, P.M.; Ghaffar, N.; Cummings, N.J.; Smith, D.L.; Fish, N.M.; Connerton, I.F. The effect of the timing of exposure to Campylobacter jejuni on the gut microbiome and inflammatory responses of broiler chickens. Microbiome 2018, 6, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Rawson, T.; Dawkins, M.S.; Bonsall, M.B. A Mathematical Model of Campylobacter Dynamics Within a Broiler Flock. Front. Microbiol. 2019, 10, 1940. [Google Scholar] [CrossRef]
- Danzeisen, J.L.; Kim, H.B.; Isaacson, R.E.; Tu, Z.J.; Johnson, T.J. Modulations of the Chicken Cecal Microbiome and Metagenome in Response to Anticoccidial and Growth Promoter Treatment. PLoS ONE 2011, 6, e27949. [Google Scholar] [CrossRef]
- Dicksved, J.; Ellström, P.; Engstrand, L.; Rautelin, H. Susceptibility to Campylobacter Infection Is Associated with the Species Composition of the Human Fecal Microbiota. mBio 2014, 5, e01212-14. [Google Scholar] [CrossRef]
- Bereswill, S.; Fischer, A.; Plickert, R.; Haag, L.M.; Otto, B.; Kühl, A.A.; Dasti, J.I.; Zautner, A.E.; Muñoz, M.; Loddenkemper, C.; et al. Novel Murine Infection Models Provide Deep Insights into the “Ménage à Trois” of Campylobacter jejuni, Microbiota and Host Innate Immunity. PLoS ONE 2011, 6, e20953. [Google Scholar] [CrossRef]
- Haag, L.-M.; Fischer, A.; Otto, B.; Plickert, R.; Kühl, A.A.; Göbel, U.B.; Bereswill, S.; Heimesaat, M.M. Intestinal Microbiota Shifts towards Elevated Commensal Escherichia coli Loads Abrogate Colonization Resistance against Campylobacter jejuni in Mice. PLoS ONE 2012, 7, e35988. [Google Scholar] [CrossRef]
- Lu, J.; Idris, U.; Harmon, B.; Hofacre, C.; Maurer, J.J.; Lee, M.D. Diversity and Succession of the Intestinal Bacterial Community of the Maturing Broiler Chicken. Appl. Environ. Microbiol. 2003, 69, 6816–6824. [Google Scholar] [CrossRef] [PubMed]
- Ranjitkar, S.; Lawley, B.; Tannock, G.; Engberg, R.M. Bacterial Succession in the Broiler Gastrointestinal Tract. Appl. Environ. Microbiol. 2016, 82, 2399–2410. [Google Scholar] [CrossRef] [PubMed]
- Videnska, P.; Sedlar, K.; Lukac, M.; Faldynova, M.; Gerzova, L.; Cejkova, D.; Sisak, F.; Rychlik, I. Succession and Replacement of Bacterial Populations in the Caecum of Egg Laying Hens over Their Whole Life. PLoS ONE 2014, 9, e115142. [Google Scholar] [CrossRef] [PubMed]
- Baffoni, L.; Gaggìa, F.; Di Gioia, D.; Santini, C.; Mogna, L.; Biavati, B. A Bifidobacterium-based synbiotic product to reduce the transmission of C. jejuni along the poultry food chain. Int. J. Food Microbiol. 2012, 157, 156–161. [Google Scholar] [CrossRef]
- Abramov, V.M.; Kosarev, I.V.; Priputnevich, T.V.; Machulin, A.V.; Khlebnikov, V.S.; Pchelintsev, S.Y.; Vasilenko, R.N.; Sakulin, V.K.; Suzina, N.E.; Chikileva, I.O.; et al. S-layer protein 2 of Lactobacillus crispatus 2029, its structural and immunomodulatory characteristics and roles in protective potential of the whole bacteria against foodborne pathogens. Int. J. Biol. Macromol. 2020, 150, 400–412. [Google Scholar] [CrossRef]
- Chen, X.; Xu, J.; Shuai, J.; Chen, J.; Zhang, Z.; Fang, W. The S-layer proteins of Lactobacillus crispatus strain ZJ001 is responsible for competitive exclusion against Escherichia coli O157:H7 and Salmonella typhimurium. Int. J. Food Microbiol. 2007, 115, 307–312. [Google Scholar] [CrossRef]
- Horie, M.; Ishiyama, A.; Fujihira-Ueki, Y.; Sillanpää, J.; Korhonen, T.K.; Toba, T. Inhibition of the adherence of Escherichia coli strains to basement membrane by Lactobacillus crispatus expressing an S-layer. J. Appl. Microbiol. 2002, 92, 396–403. [Google Scholar] [CrossRef]
- Thibodeau, A.; Fravalo, P.; Yergeau, É.; Arsenault, J.; Lahaye, L.; Letellier, A. Chicken Caecal Microbiome Modifications Induced by Campylobacter jejuni Colonization and by a Non-Antibiotic Feed Additive. PLoS ONE 2015, 10, e0131978. [Google Scholar] [CrossRef]
- Duncan, S.H.; Holtrop, G.; Lobley, G.E.; Calder, A.G.; Stewart, C.S.; Flint, H.J. Contribution of acetate to butyrate formation by human faecal bacteria. Br. J. Nutr. 2004, 91, 915–923. [Google Scholar] [CrossRef]
- Duncan, S.H.; Hold, G.L.; Harmsen, H.J.M.; Stewart, C.S.; Flint, H.J. Growth requirements and fermentation products of Fusobacterium prausnitzii, and a proposal to reclassify it as Faecalibacterium prausnitzii gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 2002, 52, 2141–2146. [Google Scholar]
- Deun, K.V.; Pasmans, F.; Van Immerseel, F.; Ducatelle, R.; Haesebrouck, F. Butyrate protects Caco-2 cells from Campylobacter jejuni invasion and translocation. Br. J. Nutr. 2008, 100, 480–484. [Google Scholar] [CrossRef] [PubMed]
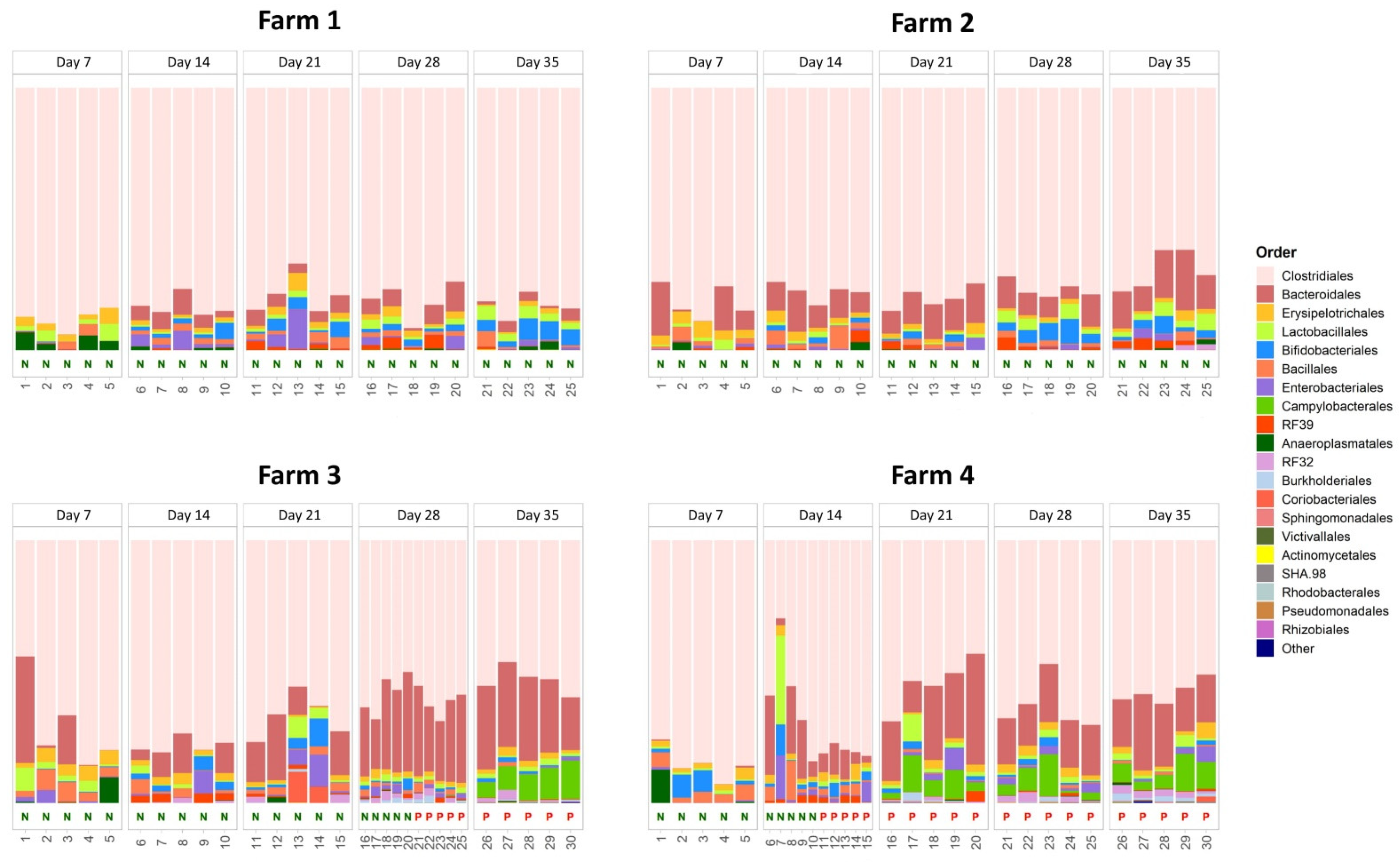
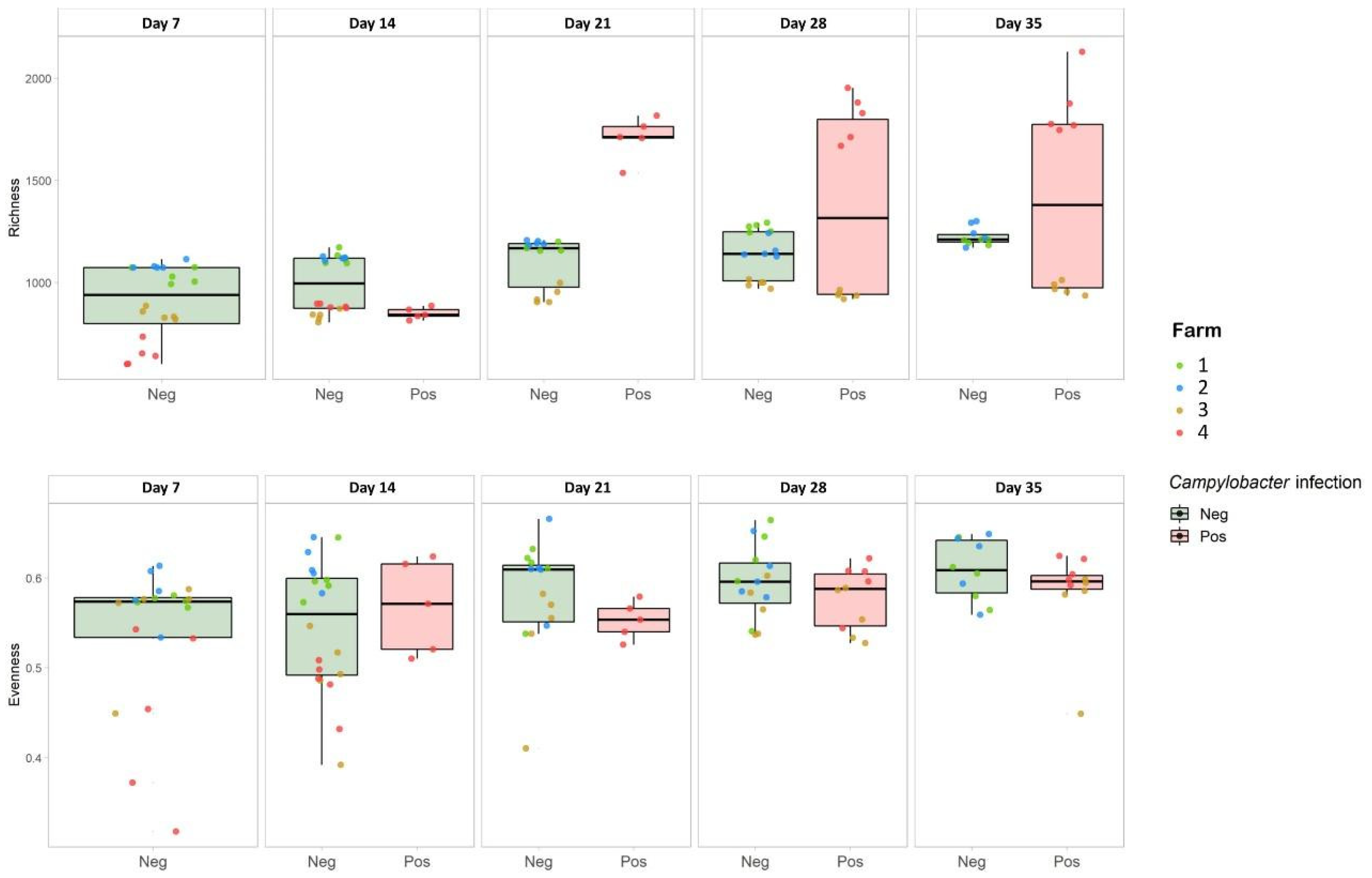

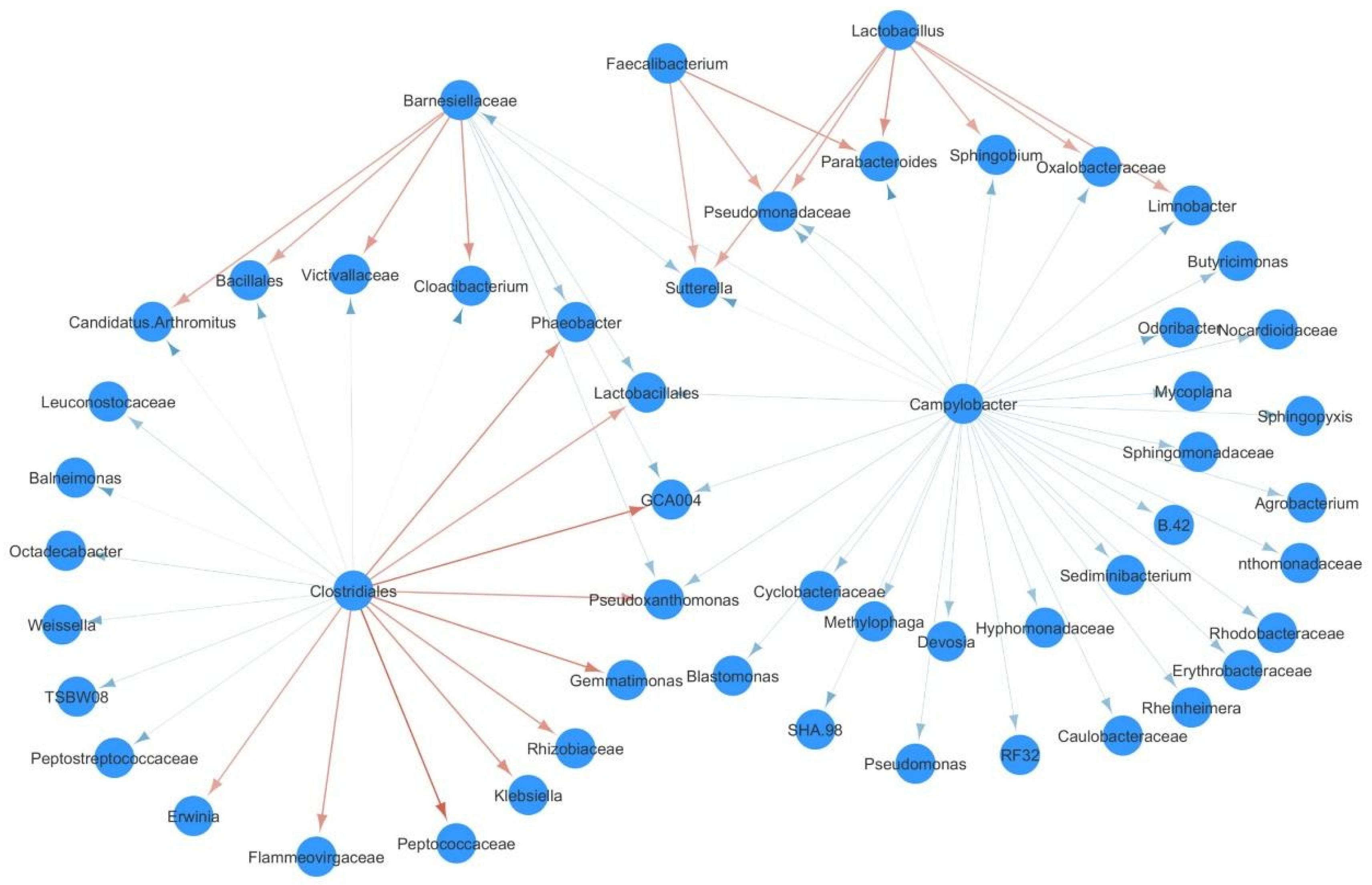
| Farm | Day 7 | Day 14 | Day 21 | Day 28 | Day 35 |
|---|---|---|---|---|---|
| 1 | 0/16 | 0/16 | 0/16 | 0/16 | 0/16 |
| 2 | 0/16 | 0/16 | 0/16 | 0/16 | 0/16 |
| 3 | 0/16 | 0/16 | 0/16 | 7/16 | 16/16 |
| 4 | 0/16 | 5/16 | 16/16 | 16/16 | 16/16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patuzzi, I.; Orsini, M.; Cibin, V.; Petrin, S.; Mastrorilli, E.; Tiengo, A.; Gobbo, F.; Catania, S.; Barco, L.; Ricci, A.; et al. The Interplay between Campylobacter and the Caecal Microbial Community of Commercial Broiler Chickens over Time. Microorganisms 2021, 9, 221. https://doi.org/10.3390/microorganisms9020221
Patuzzi I, Orsini M, Cibin V, Petrin S, Mastrorilli E, Tiengo A, Gobbo F, Catania S, Barco L, Ricci A, et al. The Interplay between Campylobacter and the Caecal Microbial Community of Commercial Broiler Chickens over Time. Microorganisms. 2021; 9(2):221. https://doi.org/10.3390/microorganisms9020221
Chicago/Turabian StylePatuzzi, Ilaria, Massimiliano Orsini, Veronica Cibin, Sara Petrin, Eleonora Mastrorilli, Alessia Tiengo, Federica Gobbo, Salvatore Catania, Lisa Barco, Antonia Ricci, and et al. 2021. "The Interplay between Campylobacter and the Caecal Microbial Community of Commercial Broiler Chickens over Time" Microorganisms 9, no. 2: 221. https://doi.org/10.3390/microorganisms9020221
APA StylePatuzzi, I., Orsini, M., Cibin, V., Petrin, S., Mastrorilli, E., Tiengo, A., Gobbo, F., Catania, S., Barco, L., Ricci, A., & Losasso, C. (2021). The Interplay between Campylobacter and the Caecal Microbial Community of Commercial Broiler Chickens over Time. Microorganisms, 9(2), 221. https://doi.org/10.3390/microorganisms9020221






