Use of Stress Signals of Their Attached Bacteria to Monitor Sympagic Algae Preservation in Canadian Arctic Sediments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sediment Sampling
2.2. Lipid Analysis
2.3. Derivatization
2.4. Determination of Double-Bond Stereochemistry
2.5. 10S-DOX Degradation Estimate
2.6. Gas Chromatography/Tandem Mass Spectrometry
2.7. Gas Chromatography-EI Quadrupole Time of Flight Mass Spectrometry
2.8. Statistical Analysis
3. Results and Discussion
3.1. Contribution of Sympagic Material to Arctic Sediments
3.2. Impact of Stress State of Bacteria on the Degradation of Sympagic Material in Sediments
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poulin, M.; Daugbjerg, N.; Gradinger, R.; Ilyash, L.; Ratkova, T.; von Quillfeldt, C. The pan-Arctic biodiversity of marine pelagic and sea-ice unicellular eukaryotes: A first-attempt assessment. Mar. Biodiv. 2011, 41, 13–28. [Google Scholar] [CrossRef]
- Boetius, A.; Albrecht, S.; Bakker, K.; Bienhold, C.; Felden, J.; Fernández-Méndez, M.; Hendricks, S.; Katlein, C.; Lalande, C.; Krumpen, T.; et al. Export of Algal Biomass from the Melting Arctic Sea Ice. Science 2013, 339, 1430–1432. [Google Scholar] [CrossRef]
- Gosselin, M.; Levasseur, M.; Wheeler, P.A.; Horner, R.A.; Booth, B.C. New measurements of phytoplankton and ice algal production in the Arctic Ocean. Deep-Sea Res. Part II 1997, 44, 1623–1644. [Google Scholar] [CrossRef]
- Wassmann, P.; Duarte, C.M.; AgustÍ, S.; Sejr, M.K. Footprints of climate change in the Arctic marine ecosystem. Glob. Chang. Biol. 2011, 17, 1235–1249. [Google Scholar] [CrossRef]
- Loose, B.; Miller, L.A.; Elliott, S.; Papakyriakou, T. Sea ice biogeochemistry and material transport across the frozen interface. Oceanography 2011, 24, 202–218. [Google Scholar] [CrossRef]
- Fernández-Méndez, M.; Katlein, C.; Rabe, B.; Nicolaus, M.; Peeken, I.; Bakker, K.; Flores, H.; Boetius, A. Photosynthetic production in the central Arctic Ocean during the record sea-ice minimum in 2012. Biogeosciences 2015, 12, 3525–3549. [Google Scholar] [CrossRef] [Green Version]
- Amiraux, R.; Belt, S.T.; Vaultier, F.; Galindo, V.; Gosselin, M.; Bonin, P.; Rontani, J.-F. Monitoring photo-oxidative and salinity-induced bacterial stress in the Canadian Arctic using specific lipid tracers. Mar. Chem. 2017, 194, 89–99. [Google Scholar] [CrossRef]
- Rontani, J.-F.; Amiraux, R.; Lalande, C.; Babin, M.; Kim, H.-R.; Belt, S.T. Use of palmitoleic acid and its oxidation products for monitoring the degradation of ice algae in Arctic waters and bottom sediments. Org. Geochem. 2018, 124, 88–102. [Google Scholar] [CrossRef] [Green Version]
- Yunda-Guarin, G.; Brown, T.A.; Michel, L.N.; Saint-Béat, B.; Amiraux, R.; Nozais, C.; Archambault, P. Reliance of deep-sea benthic macrofauna on ice-derived organic matter highlighted by multiple trophic markers during spring in Baffin Bay, Canadian Arctic. Elementa 2020, 8, 047. [Google Scholar] [CrossRef]
- Moriceau, B.; Garvey, M.; Ragueneau, O.; Passow, U. Evidence for reduced biogenic silica dissolution rates in diatom aggregates. Mar. Ecol. Prog. Ser. 2007, 333, 129–142. [Google Scholar] [CrossRef]
- Riebesell, U.; Schloss, I.; Smetacek, V. Aggregation of algae released from melting sea ice—Implications for seeding and sedimentation. Polar Biol. 1991, 11, 239–248. [Google Scholar] [CrossRef]
- Lürling, M.; Van Donk, E. Zooplankton-induced unicell-colony transformation in Scenedesmus acutus and its effect on growth of herbivore Daphnia. Oecologia 1996, 108, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Bidle, K.D.; Brzezinski, M.A.; Long, R.A.; Jones, J.L.; Azam, F. Diminished efficiency in the oceanic silica pump caused by bacteria-mediated silica dissolution. Limnol. Oceanogr. 2003, 48, 1855–1868. [Google Scholar] [CrossRef] [Green Version]
- Amiraux, R.; Burot, C.; Bonin, P.; Guasco, S.; Babin, M.; Rontani, J.-F. Stress factors resulting from the Arctic vernal sea ice melt: Impact on the viability of the bacterial communities associated to sympagic algae. Elementa 2020, 8, 076. [Google Scholar] [CrossRef]
- Rieck, A.; Herlemann, D.P.; Jürgens, K.; Grossart, H.-P. Particle-associated differ from free-living bacteria in surface waters of the Baltic Sea. Front. Microbiol. 2015, 6, 1297. [Google Scholar] [CrossRef] [Green Version]
- Alldredge, A.L.; Silver, M.W. Characteristics, dynamics and significance of marine snow. Prog. Oceangr. 1988, 20, 41–82. [Google Scholar] [CrossRef]
- Riedel, A.; Michel, C.; Gosselin, M. Seasonal study of sea-ice exopolymeric substances on the Mackenzie shelf: Implications for transport of sea-ice bacteria and algae. Aquat. Microb. Ecol. 2006, 45, 195–206. [Google Scholar] [CrossRef]
- Junge, K.; Eicken, H.; Deming, J.W. Bacterial activity at −2 to −20 °C in Arctic wintertime sea ice. Appl. Environ. Microbiol. 2004, 70, 550–557. [Google Scholar] [CrossRef] [Green Version]
- Garneau, M.-È.; Vincent, W.F.; Terrado, R.; Lovejoy, C. Importance of particle-associated bacterial heterotrophy in a coastal Arctic ecosystem. J. Mar. Syst. 2009, 75, 185–197. [Google Scholar] [CrossRef]
- Ortega-Retuerta, E.; Jeffrey, W.; Babin, M.; Bélanger, S.; Benner, R.; Marie, D.; Matsuoka, A.; Raimbault, P.; Joux, F. Carbon fluxes in the Canadian Arctic: Patterns and drivers of bacterial abundance, production and respiration on the Beaufort Sea margin. Biogeosciences 2012, 9, 3679–3692. [Google Scholar] [CrossRef] [Green Version]
- Lapoussière, A.; Michel, C.; Starr, M.; Gosselin, M.; Poulin, M. Role of free-living and particle-attached bacteria in the recycling and export of organic material in the Hudson Bay system. J. Mar. Syst. 2011, 88, 434–445. [Google Scholar] [CrossRef]
- Meyer-Reil, L.-A. Bacterial biomass and heterotrophic activity in sediments and overlying waters. In Heterotrophic Activity in the Sea; Springer: Berlin/Heidelberg, Germany, 1984; pp. 523–546. [Google Scholar]
- Thompson, J.; MacLeod, R.A. Functions of Na+ and K+ in the active transport of α-aminoisobutyric acid in a marine pseudomonad. J. Biol. Chem. 1971, 246, 4066–4074. [Google Scholar] [CrossRef]
- Piuri, M.; Sanchez-Rivas, C.; Ruzal, S. Adaptation to high salt in Lactobacillus: Role of peptides and proteolytic enzymes. J. Appl. Microbiol. 2003, 95, 372–379. [Google Scholar] [CrossRef]
- Kim, L.H.; Chong, T.H. Physiological Responses of Salinity-Stressed Vibrio sp. and the Effect on the Biofilm Formation on a Nanofiltration Membrane. Environ. Sci. Technol. 2017, 51, 1249–1258. [Google Scholar] [CrossRef]
- Loffeld, B.; Keweloh, H. Cis/trans isomerization of unsaturated fatty acids as possible control mechanism of membrane fluidity in Pseudomonas putida P8. Lipids 1996, 31, 811–815. [Google Scholar] [CrossRef]
- Heipieper, H.J.; Meinhardt, F.; Segura, A. The cis-trans isomerase of unsaturated fatty acids in Pseudomonas and Vibrio: Biochemistry, molecular biology and physiological function of a unique stress adaptive mechanism. FEMS Microbiol. Lett. 2003, 229, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Guckert, J.; Hood, M.; White, D. Phospholipid ester-linked fatty acid profile changes during nutrient deprivation of Vibrio cholerae: Increases in the trans/cis ratio and proportions of cyclopropyl fatty acids. Appl. Environ. Microbiol. 1986, 52, 794–801. [Google Scholar] [CrossRef] [Green Version]
- Fischer, J.; Schauer, F.; Heipieper, H.J. The trans/cis ratio of unsaturated fatty acids is not applicable as biomarker for environmental stress in case of long-term contaminated habitats. Appl. Microbiol. Biotechnol. 2010, 87, 365–371. [Google Scholar] [CrossRef]
- Eberlein, C.; Baumgarten, T.; Starke, S.; Heipieper, H.J. Immediate response mechanisms of Gram-negative solvent-tolerant bacteria to cope with environmental stress: Cis-trans isomerization of unsaturated fatty acids and outer membrane vesicle secretion. Appl. Microbiol. Biotechnol. 2018, 102, 2583–2593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenway, D.; Dyke, K. Mechanism of the inhibitory action of linoleic acid on the growth of Staphylococcus aureus. Microbiology 1979, 115, 233–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chamberlain, N.R.; Mehrtens, B.; Xiong, Z.; Kapral, F.; Boardman, J.; Rearick, J. Correlation of carotenoid production, decreased membrane fluidity, and resistance to oleic acid killing in Staphylococcus aureus 18Z. Infect. Immun. 1991, 59, 4332–4337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, A.; Casals, I.; Busquets, M.; Leon, Y.; Manresa, A. Oxydation of oleic acid to (E)-10-hydroperoxy-8-octadecenoic and (E)-10-hydroxy-8-octadecenoic acids by Pseudomonas sp. 42A2. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1997, 1347, 75–81. [Google Scholar] [CrossRef]
- Shoja Chaghervand, S. Characterization of the Enzymes Involved in the Diolsynthase Pathway in Pseudomonas aeruginosa. Ph.D. Thesis, Universitat de Barcelona, Barcelona, Spain, 2019. [Google Scholar]
- Amiraux, R.; Rontani, J.-F.; Armougom, F.; Frouin, E.; Babin, M.; Artigue, L.; Bonin, P. Bacterial diversity and lipid biomarkers in sea ice and sinking particulate organic material during the melt season in the Canadian Arctic. Elementa 2021, 9, 1–17. [Google Scholar] [CrossRef]
- Rontani, J.-F.; Smik, L.; Vaultier, F.; Widdicombe, C.; Belt, S.T. Seasonal monitoring of lipid degradation processes in the western English Channel links bacterial 10S-DOX enzyme activity to free fatty acid production by phytoplankton. Mar. Chem. 2021, 230, 103928. [Google Scholar] [CrossRef]
- Rontani, J.; Charriere, B.; Forest, A.; Heussner, S.; Vaultier, F.; Petit, M.; Delsaut, N.; Fortier, L.; Sempéré, R. Intense photooxidative degradation of planktonic and bacterial lipids in sinking particles collected with sediment traps across the Canadian Beaufort Shelf (Arctic Ocean). Biogeosciences 2012, 9, 7743–7781. [Google Scholar] [CrossRef] [Green Version]
- Belt, S.T.; Smik, L.; Köseoğlu, D.; Knies, J.; Husum, K. A novel biomarker-based proxy for the spring phytoplankton bloom in Arctic and sub-arctic settings–HBI T25. Earth Planet. Sci. Lett. 2019, 523, 115703. [Google Scholar] [CrossRef]
- Smik, L.; Cabedo-Sanz, P.; Belt, S.T. Semi-quantitative estimates of paleo Arctic sea ice concentration based on source-specific highly branched isoprenoid alkenes: A further development of the PIP25 index. Org. Geochem. 2016, 92, 63–69. [Google Scholar] [CrossRef]
- Brown, T.A.; Yurkowski, D.J.; Ferguson, S.H.; Alexander, C.; Belt, S.T. H-Print: A new chemical fingerprinting approach for distinguishing primary production sources in Arctic ecosystems. Environ. Chem. Lett. 2014, 12, 387–392. [Google Scholar] [CrossRef]
- Brown, T.A.; Belt, S.T.; Gosselin, M.; Levasseur, M.; Poulin, M.; Mundy, C.J. Quantitative estimates of sinking sea ice particulate organic carbon based on the biomarker IP25. Mar. Ecol. Prog. Ser. 2016, 546, 17–29. [Google Scholar] [CrossRef] [Green Version]
- Wakeham, S.G. Reduction of stenols to stanols in particulate matter at oxic–anoxic boundaries in sea water. Nature 1989, 342, 787–790. [Google Scholar] [CrossRef]
- De Leeuw, J.; Baas, M. Early-stage diagenesis of steroids. Methods Geochem. Geophys. 1986, 24, 101–123. [Google Scholar]
- Bergeron, M.; Tremblay, J.É. Shifts in biological productivity inferred from nutrient drawdown in the southern Beaufort Sea (2003–2011) and northern Baffin Bay (1997–2011), Canadian Arctic. Geophys. Res. Lett. 2014, 41, 3979–3987. [Google Scholar] [CrossRef]
- Rachold, V.; Eicken, H.; Gordeev, V.; Grigoriev, M.N.; Hubberten, H.-W.; Lisitzin, A.P.; Shevchenko, V.; Schirrmeister, L. Modern terrigenous organic carbon input to the Arctic Ocean. In The Organic Carbon Cycle in the Arctic Ocean; Springer: Berlin/Heidelberg, Germany, 2004; pp. 33–55. [Google Scholar]
- Macdonald, R.; Solomon, S.; Cranston, R.; Welch, H.; Yunker, M.; Gobeil, C. A sediment and organic carbon budget for the Canadian Beaufort Shelf. Mar. Geol. 1998, 144, 255–273. [Google Scholar] [CrossRef]
- Magen, C.; Chaillou, G.; Crowe, S.A.; Mucci, A.; Sundby, B.; Gao, A.; Makabe, R.; Sasaki, H. Origin and fate of particulate organic matter in the southern Beaufort Sea—Amundsen Gulf region, Canadian Arctic. Estuar. Coast. Shelf Sci. 2010, 86, 31–41. [Google Scholar] [CrossRef]
- Myers, P.G.; Kulan, N.; Ribergaard, M.H. Irminger water variability in the West Greenland Current. Geophys. Res. Lett. 2007, 34, 1–6. [Google Scholar] [CrossRef]
- Richerol, T.; Rochon, A.; Blasco, S.; Scott, D.B.; Schell, T.M.; Bennett, R.J. Evolution of paleo sea-surface conditions over the last 600 years in the Mackenzie Trough, Beaufort Sea (Canada). Mar. Micropaleontol. 2008, 68, 6–20. [Google Scholar] [CrossRef]
- Kuzyk, Z.Z.A.; Gobeil, C.; Macdonald, R.W. 210Pb and 137Cs in margin sediments of the Arctic Ocean: Controls on boundary scavenging. Glob. Biogeochem. Cycles 2013, 27, 422–439. [Google Scholar] [CrossRef]
- Comiso, J.C.; Hall, D.K. Climate trends in the Arctic as observed from space. Wiley Interdiscip. Rev. Clim. Chang. 2014, 5, 389–409. [Google Scholar] [CrossRef]
- O’Brien, M.C.; Macdonald, R.W.; Melling, H.; Iseki, K. Particle fluxes and geochemistry on the Canadian Beaufort Shelf: Implications for sediment transport and deposition. Cont. Shelf Res. 2006, 26, 41–81. [Google Scholar] [CrossRef]
- Forest, A.; Sampei, M.; Hattori, H.; Makabe, R.; Sasaki, H.; Fukuchi, M.; Wassmann, P.; Fortier, L. Particulate organic carbon fluxes on the slope of the Mackenzie Shelf (Beaufort Sea): Physical and biological forcing of shelf-basin exchanges. J. Mar. Syst. 2007, 68, 39–54. [Google Scholar] [CrossRef]
- Lalande, C.; Forest, A.; Barber, D.G.; Gratton, Y.; Fortier, L. Variability in the annual cycle of vertical particulate organic carbon export on Arctic shelves: Contrasting the Laptev Sea, Northern Baffin Bay and the Beaufort Sea. Cont. Shelf Res. 2009, 29, 2157–2165. [Google Scholar] [CrossRef]
- Forest, A.; Bélanger, S.; Sampei, M.; Sasaki, H.; Lalande, C.; Fortier, L. Three-year assessment of particulate organic carbon fluxes in Amundsen Gulf (Beaufort Sea): Satellite observations and sediment trap measurements. Deep Sea Res. Part I 2010, 57, 125–142. [Google Scholar] [CrossRef]
- Hargrave, B.; Walsh, I.; Murray, D. Seasonal and spatial patterns in mass and organic matter sedimentation in the North Water. Deep Sea Res. Part II 2002, 49, 5227–5244. [Google Scholar] [CrossRef]
- Buser, H.R.; Arn, H.; Guerin, P.; Rauscher, S. Determination of double bond position in mono-unsaturated acetates by mass spectrometry of dimethyl disulfide adducts. Anal. Chem. 1983, 55, 818–822. [Google Scholar] [CrossRef]
- Frankel, E.N. Lipid Oxidation; The Oily Press: Dundee, Scotland, 1998. [Google Scholar]
- Marchand, D.; Rontani, J.-F. Characterisation of photo-oxidation and autoxidation products of phytoplanktonic monounsaturated fatty acids in marine particulate matter and recent sediments. Org. Geochem. 2001, 32, 287–304. [Google Scholar] [CrossRef]
- Porter, N.A.; Caldwell, S.E.; Mills, K.A. Mechanisms of free radical oxidation of unsaturated lipids. Lipids 1995, 30, 277–290. [Google Scholar] [CrossRef]
- Galeron, M.-A.; Radakovitch, O.; Charriere, B.; Vaultier, F.; Volkman, J.K.; Bianchi, T.S.; Ward, N.D.; Medeiros, P.M.; Sawakuchi, H.O.; Tank, S. Lipoxygenase-induced autoxidative degradation of terrestrial particulate organic matter in estuaries: A widespread process enhanced at high and low latitude. Org. Geochem. 2018, 115, 78–92. [Google Scholar] [CrossRef] [Green Version]
- Belicka, L.L.; Macdonald, R.W.; Yunker, M.B.; Harvey, H.R. The role of depositional regime on carbon transport and preservation in Arctic Ocean sediments. Mar. Chem. 2004, 86, 65–88. [Google Scholar] [CrossRef]
- Fahl, K.; Kattner, G. Lipid content and fatty acid composition of algal communities in sea-ice and water from the Weddell Sea (Antarctica). Polar Biol. 1993, 13, 405–409. [Google Scholar] [CrossRef]
- Falk-Petersen, S.; Sargent, J.R.; Henderson, J.; Hegseth, E.N.; Hop, H.; Okolodkov, Y.B. Lipids and fatty acids in ice algae and phytoplankton from the Marginal Ice Zone in the Barents Sea. Polar Biol. 1998, 20, 41–47. [Google Scholar] [CrossRef]
- Kolattukudy, P. Cutin, suberin, and waxes. In Lipids: Structure and Function; Elsevier: Amsterdam, The Netherlands, 1980; pp. 571–645. [Google Scholar]
- Otto, A.; Simoneit, B.R. Chemosystematics and diagenesis of terpenoids in fossil conifer species and sediment from the Eocene Zeitz formation, Saxony, Germany. Geochim. Cosmochim. Acta 2001, 65, 3505–3527. [Google Scholar] [CrossRef]
- Rontani, J.-F.; Charrìère, B.; Petit, M.; Vaultier, F.; Heipieper, H.; Link, H.; Chaillou, G.; Sempere, R. Degradation state of organic matter in surface sediments from the Southern Beaufort Sea: A lipid approach. Biogeosciences 2012, 9, 3513–3530. [Google Scholar] [CrossRef] [Green Version]
- Morata, N.; Poulin, M.; Renaud, P.E. A multiple biomarker approach to tracking the fate of an ice algal bloom to the sea floor. Polar Biol. 2011, 34, 101–112. [Google Scholar] [CrossRef] [Green Version]
- Brown, T.A.; Belt, S.T.; Philippe, B.; Mundy, C.J.; Massé, G.; Poulin, M.; Gosselin, M. Temporal and vertical variations of lipid biomarkers during a bottom ice diatom bloom in the Canadian Beaufort Sea: Further evidence for the use of the IP25 biomarker as a proxy for spring Arctic sea ice. Polar Biol. 2011, 34, 1857–1868. [Google Scholar] [CrossRef]
- Juul-Pedersen, T.; Michel, C.; Gosselin, M.; Seuthe, L. Seasonal changes in the sinking export of particulate material under first-year sea ice on the Mackenzie Shelf (western Canadian Arctic). Mar. Ecol. Prog. Ser. 2008, 353, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Nadaï, G.; Nöthig, E.-M.; Fortier, L.; Lalande, C. Early snowmelt and sea ice breakup enhance algal export in the Beaufort Sea. Prog. Oceangr. 2021, 190, 102479. [Google Scholar] [CrossRef]
- Saunders, P.; Deibel, D.; Stevens, C.; Rivkin, R.B.; Lee, S.; Klein, B. Copepod herbivory rate in a large arctic polynya and its relationship to seasonal and spatial variation in copepod and phytoplankton biomass. Mar. Ecol. Prog. Ser. 2003, 261, 183–199. [Google Scholar] [CrossRef] [Green Version]
- Seuthe, L.; Darnis, G.; Riser, C.W.; Wassmann, P.; Fortier, L. Winter–spring feeding and metabolism of Arctic copepods: Insights from faecal pellet production and respiration measurements in the southeastern Beaufort Sea. Polar Biol. 2007, 30, 427–436. [Google Scholar] [CrossRef]
- Sherr, E.B.; Sherr, B.F.; Hartz, A.J. Microzooplankton grazing impact in the Western Arctic Ocean. Deep Sea Res. Part II 2009, 56, 1264–1273. [Google Scholar] [CrossRef]
- Michel, C.; Legendre, L.; Ingram, R.; Gosselin, M.; Levasseur, M. Carbon budget of sea-ice algae in spring: Evidence of a significant transfer to zooplankton grazers. J. Geophys. Res. Ocean. 1996, 101, 18345–18360. [Google Scholar] [CrossRef]
- Paffenhöfer, G.; Knowles, S.C. Ecological implications of fecal pellet size, production and consumption by copepods. J. Mar. Res. 1979, 37, 35–49. [Google Scholar]
- Gowing, M.; Silver, M. Origins and microenvironments of bacteria mediating fecal pellet decomposition in the sea. Mar. Biol. 1983, 73, 7–16. [Google Scholar] [CrossRef]
- Smetacek, V.S. Role of sinking in diatom life-history cycles: Ecological, evolutionary and geological significance. Mar. Biol. 1985, 84, 239–251. [Google Scholar] [CrossRef]
- Forest, A.; Tremblay, J.-É.; Gratton, Y.; Martin, J.; Gagnon, J.; Darnis, G.; Sampei, M.; Fortier, L.; Ardyna, M.; Gosselin, M.; et al. Biogenic carbon flows through the planktonic food web of the Amundsen Gulf (Arctic Ocean): A synthesis of field measurements and inverse modeling analyses. Prog. Oceangr. 2011, 91, 410–436. [Google Scholar] [CrossRef]
- Morata, N.; Renaud, P.E. Sedimentary pigments in the western Barents Sea: A reflection of pelagic-benthic coupling? Deep Sea Res. Part II 2008, 55, 2381–2389. [Google Scholar] [CrossRef]
- Tang, C.C.L.; Ross, C.K.; Yao, T.; Petrie, B.; DeTracey, B.M.; Dunlap, E. The circulation, water masses and sea-ice of Baffin Bay. Prog. Oceangr. 2004, 63, 183–228. [Google Scholar] [CrossRef]
- Saint-Béat, B.; Fath, B.D.; Aubry, C.; Colombet, J.; Dinasquet, J.; Fortier, L.; Galindo, V.; Grondin, P.-L.; Joux, F.; Lalande, C. Contrasting pelagic ecosystem functioning in eastern and western Baffin Bay revealed by trophic network modeling. Elementa 2020, 8. [Google Scholar] [CrossRef]
- Martínez, E.; Hamberg, M.; Busquets, M.; Díaz, P.; Manresa, A.; Oliw, E.H. Biochemical characterization of the oxygenation of unsaturated fatty acids by the dioxygenase and hydroperoxide isomerase of Pseudomonas aeruginosa 42A2. J. Biol. Chem. 2010, 285, 9339–9345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monfort, P.; Demers, S.; Levasseur, M. Bacterial dynamics in first year sea ice and underlying seawater of Saroma-ko Lagoon (Sea of Okhotsk, Japan) and Resolute Passage (High Canadian Arctic): Inhibitory effects of ice algae on bacterial dynamics. Can. J. Microbiol. 2000, 46, 623–632. [Google Scholar] [CrossRef]
- Rapp, J.Z.; Fernández-Méndez, M.; Bienhold, C.; Boetius, A. Effects of ice-algal aggregate export on the connectivity of bacterial communities in the central Arctic Ocean. Front. Microbiol. 2018, 9, 1035. [Google Scholar] [CrossRef]
- Tamburini, C.; Boutrif, M.; Garel, M.; Colwell, R.R.; Deming, J.W. Prokaryotic responses to hydrostatic pressure in the ocean–A review. Environ. Microbiol. 2013, 15, 1262–1274. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Hwang, C.Y.; Cho, B.C. Arcobacter marinus sp. nov. Int. J. Syst. Evol. 2010, 60, 531–536. [Google Scholar] [CrossRef]
- Kim, H.S.; Hyun, D.-W.; Lee, J.-Y.; Kim, P.S.; Whon, T.W.; Kang, W.; Bae, J.-W. Sedimentitalea todarodis sp. nov., isolated from the intestinal tract of a Japanese flying squid. Int. J. Syst. Evol. 2016, 66, 3293–3298. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-H.; Seo, H.-S.; Lee, J.-H.; Kim, S.-J.; Kwon, K.K. Neptunomonas acidivorans sp. nov., isolated from sediment, and emended description of the genus Neptunomonas. Int. J. Syst. Evol. 2014, 64, 3650–3654. [Google Scholar] [CrossRef] [Green Version]
- Wakeham, S.G.; Canuel, E.A. Degradation and preservation of organic matter in marine sediments. Mar. Org. Matter Biomark. Isot. DNA 2006, 2, 295–321. [Google Scholar] [CrossRef]
- Deng, L.; Bölsterli, D.; Kristensen, E.; Meile, C.; Su, C.-C.; Bernasconi, S.M.; Seidenkrantz, M.-S.; Glombitza, C.; Lagostina, L.; Han, X. Macrofaunal control of microbial community structure in continental margin sediments. Proc. Natl. Acad. Sci. USA 2020, 117, 15911–15922. [Google Scholar] [CrossRef]
- Keaveney, E.M.; Radbourne, A.D.; McGowan, S.; Ryves, D.B.; Reimer, P.J. Source and quantity of carbon influence its sequestration in Rostherne Mere (UK) sediment: A novel application of stepped combustion radiocarbon analysis. J. Paleolimnol. 2020, 64, 347–363. [Google Scholar] [CrossRef]
- Lacey, J.H.; Leng, M.J.; Vane, C.H.; Radbourne, A.D.; Yang, H.; Ryves, D.B. Assessing human impact on Rostherne Mere, UK, using the geochemistry of organic matter. Anthropocene 2018, 21, 52–65. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, M. Geochemical characteristics of the high reduction zone of stenols in Suwa sediments and the environmental factors controlling the conversion of stenols into stanols. Geochim. Cosmochim. Acta 1978, 42, 349–357. [Google Scholar] [CrossRef]
- Gagosian, R.B.; Smith, S.O.; Lee, C.; Farrington, J.W.; Frew, N.M. Steroid transformations in recent marine sediments. Phys. Chem. Earth 1980, 12, 407–419. [Google Scholar] [CrossRef]
- Belt, S.T.; Brown, T.A.; Smik, L.; Assmy, P.; Mundy, C. Sterol identification in floating Arctic sea ice algal aggregates and the Antarctic sea ice diatom Berkeleya adeliensis. Org. Geochem. 2018, 118, 1–3. [Google Scholar] [CrossRef]
- Volkman, J. Sterols in microorganisms. Appl. Microbiol. Biotechnol. 2003, 60, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Gõni, M.A.; Yunker, M.B.; Macdonald, R.W.; Eglinton, T.I. Distribution and sources of organic biomarkers in Arctic sediments from the Mackenzie River and Beaufort Shelf. Mar. Chem. 2000, 71, 23–51. [Google Scholar] [CrossRef]
- Wakeham, S.G.; Hedges, J.I.; Lee, C.; Peterson, M.L.; Hernes, P.J. Compositions and transport of lipid biomarkers through the water column and surficial sediments of the equatorial Pacific Ocean. Deep Sea Res. Part II 1997, 44, 2131–2162. [Google Scholar] [CrossRef]
- Killops, S.D.; Killops, V.J. Introduction to Organic Geochemistry; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Gõni, M.A.; Yunker, M.B.; Macdonald, R.W.; Eglinton, T.I. The supply and preservation of ancient and modern components of organic carbon in the Canadian Beaufort Shelf of the Arctic Ocean. Mar. Chem. 2005, 93, 53–73. [Google Scholar] [CrossRef]
- Bianchi, T.S. The role of terrestrially derived organic carbon in the coastal ocean: A changing paradigm and the priming effect. Proc. Natl. Acad. Sci. USA 2011, 108, 19473–19481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianchi, T.S.; Cui, X.; Blair, N.E.; Burdige, D.J.; Eglinton, T.I.; Galy, V. Centers of organic carbon burial and oxidation at the land-ocean interface. Org. Geochem. 2018, 115, 138–155. [Google Scholar] [CrossRef]
- Bonin, P.; Prime, A.-H.; Galeron, M.-A.; Guasco, S.; Rontani, J.-F. Enhanced biotic degradation of terrestrial POM in an estuarine salinity gradient: Interactive effects of organic matter pools and changes of bacterial communities. Aquat. Microb. Ecol. 2019, 83, 147–159. [Google Scholar] [CrossRef]
- Zimov, S.; Davydov, S.; Zimova, G.; Davydova, A.; Schuur, E.; Dutta, K.; Chapin, F., III. Permafrost carbon: Stock and decomposability of a globally significant carbon pool. Geophys. Res. Lett. 2006, 33. [Google Scholar] [CrossRef] [Green Version]
- Lalande, C.; Grebmeier, J.M.; Hopcroft, R.R.; Danielson, S.L. Annual cycle of export fluxes of biogenic matter near Hanna Shoal in the northeast Chukchi Sea. Deep Sea Res. II 2021, 177, 104730. [Google Scholar] [CrossRef]
- Lalande, C.; Nöthig, E.-M.; Fortier, L. Algal export in the Arctic Ocean in times of global warming. Geophys. Res. Lett. 2019, 46, 5959–5967. [Google Scholar] [CrossRef] [Green Version]
- Tremblay, C.; Runge, J.A.; Legendre, L. Grazing and sedimentation of ice algae during and immediately after a bloom at the ice-water interface. Mar. Ecol. Prog. Ser. 1989, 56, 291–300. [Google Scholar] [CrossRef]
- Koch, C.W.; Cooper, L.W.; Lalande, C.; Brown, T.A.; Frey, K.E.; Grebmeier, J.M. Seasonal and latitudinal variations in sea ice algae deposition in the Northern Bering and Chukchi Seas determined by algal biomarkers. PLoS ONE 2020, 15, e0231178. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, M.; Amakasu, K.; Kikuchi, T.; Nishino, S. Seasonal dynamics of zooplankton in the southern Chukchi Sea revealed from acoustic backscattering strength. Cont. Shelf Res. 2017, 133, 47–58. [Google Scholar] [CrossRef]
- Grebmeier, J.M.; McRoy, C.P.; Feder, H.M. Pelagic-benthic coupling on the shelf of the northern Bering and Chukchi Seas. I. Food supply source and benthic biomass. Mar. Ecol. Prog. Ser. 1988, 48, 57–67. [Google Scholar] [CrossRef]
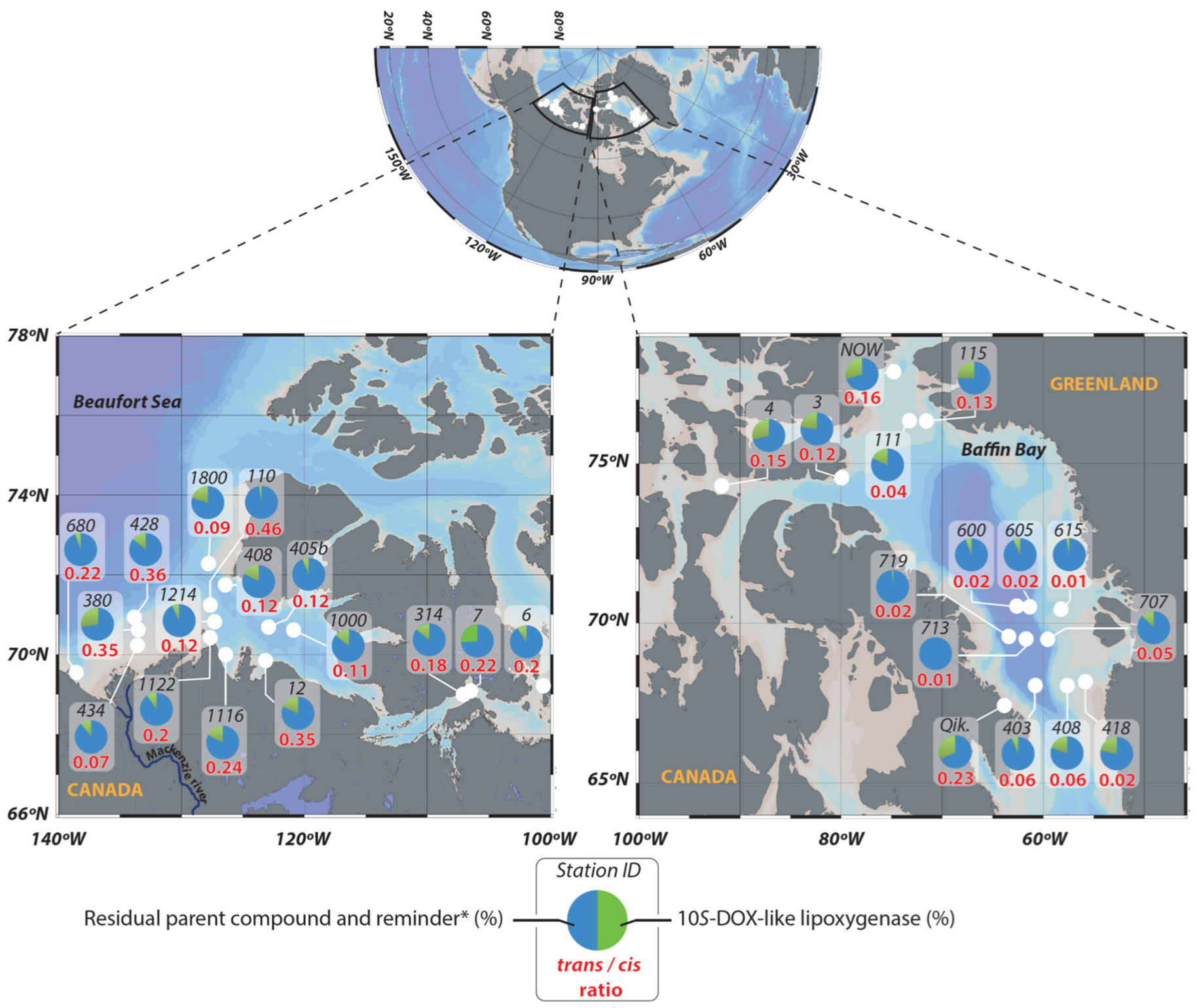

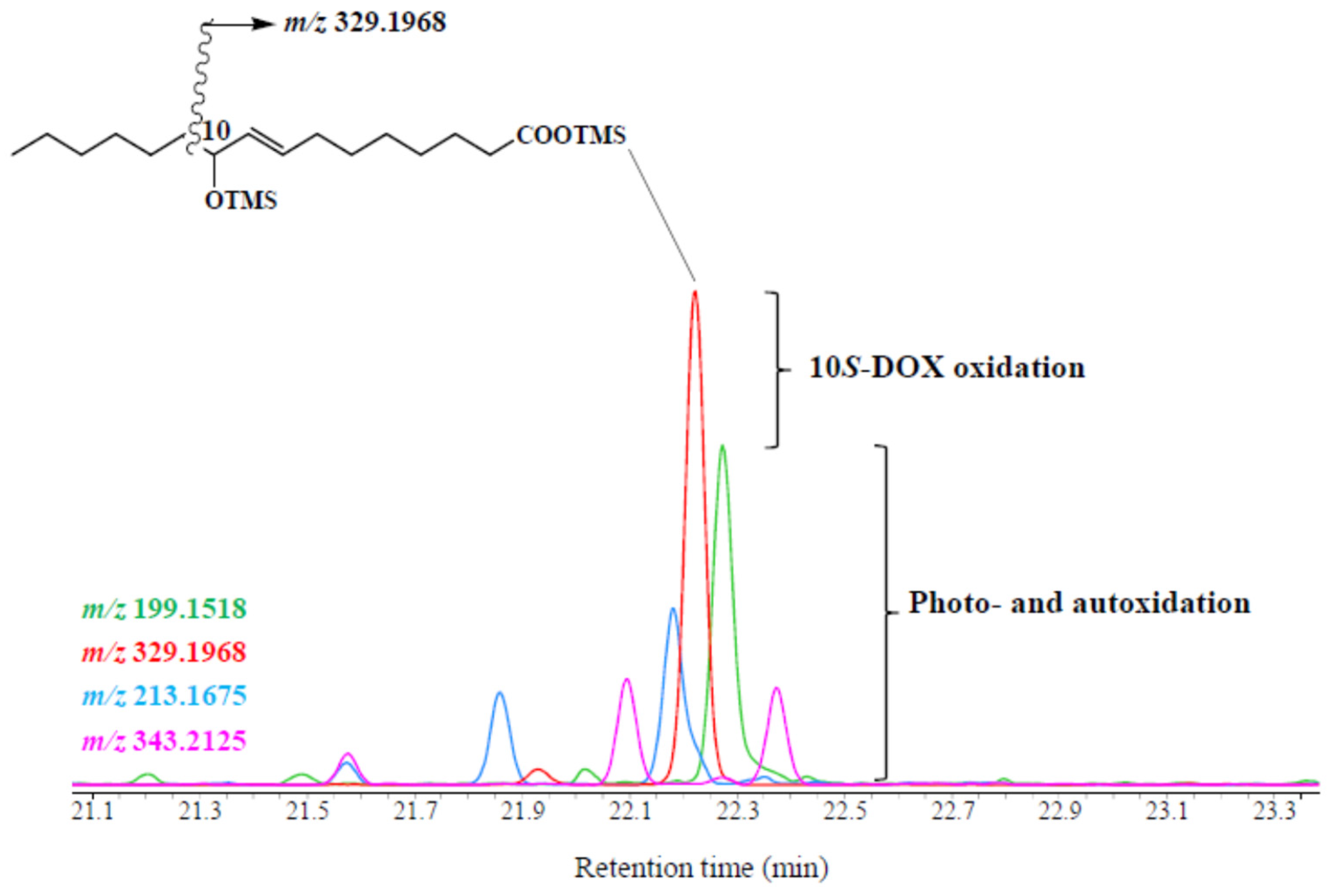
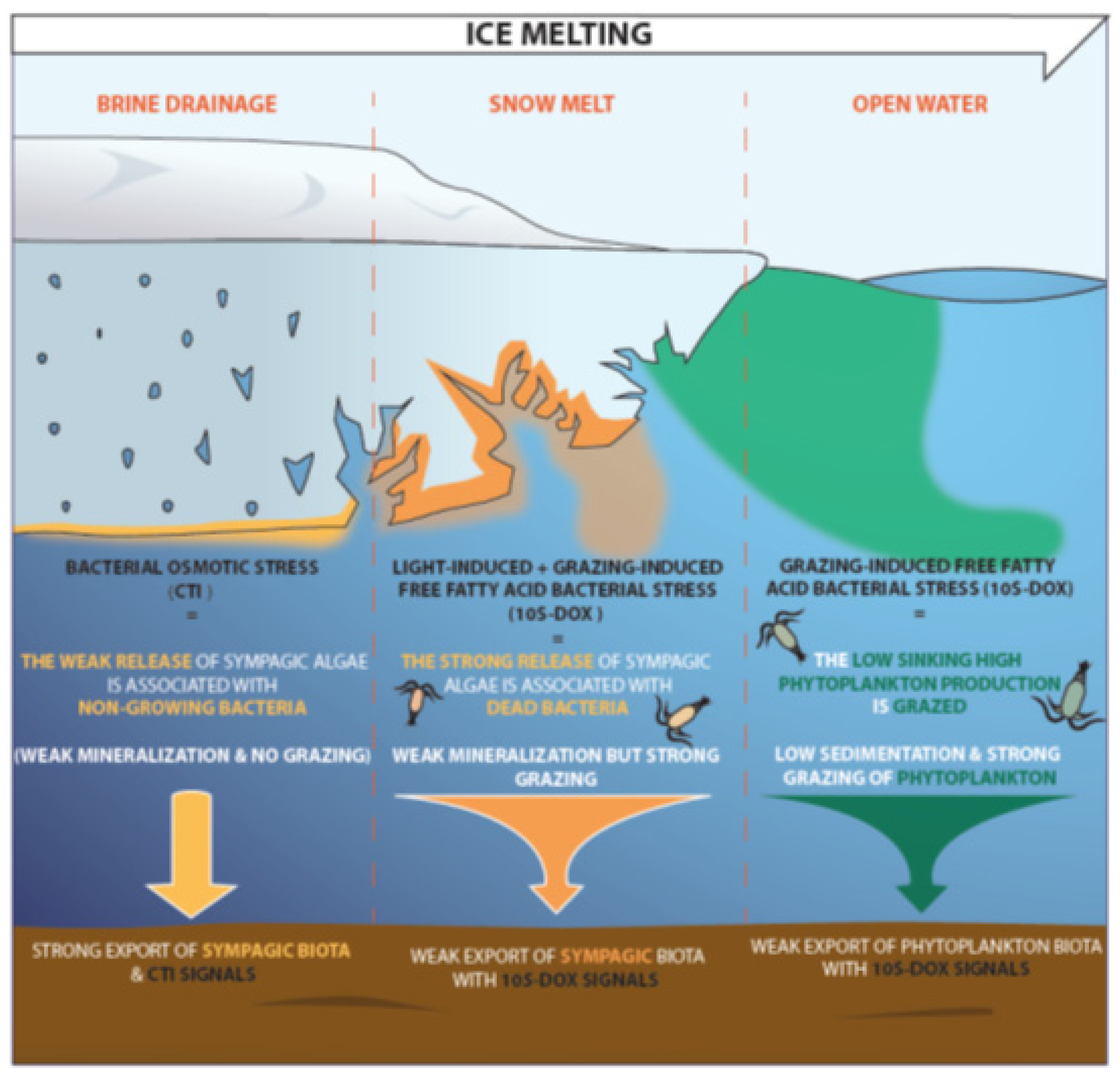
| Beaufort Sea Mean ± SE (Range) | Baffin Bay Mean ± SE (Range) | Overall Mean ± SE (Range) | |
|---|---|---|---|
| Trans/cis ratio (g:g) | 0.20 ± 0.03 a (0.46–0.07) | 0.06 ± 0.02 b (0.23–0.01) | 0.14 ± 0.02 c (0.46–0.01) |
| 10S-DOX (%) d | 14.34 ± 1.83 (29.00–2.20) | 13.56 ± 2.91 (29.70–0) | 14.01 ± 1.61 (29.70–0.00) |
| Sitosterol Mean ± SE (Range) | Brassicasterol Mean ± SE (Range) | |
|---|---|---|
| Stanol/stenol ratio (%) | 52.8 ± 5.9 a (84.6–14.3) | 18.2 ± 1.9 a (35.8–5.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amiraux, R.; Patricia, B.; Christopher, B.; Jean-François, R. Use of Stress Signals of Their Attached Bacteria to Monitor Sympagic Algae Preservation in Canadian Arctic Sediments. Microorganisms 2021, 9, 2626. https://doi.org/10.3390/microorganisms9122626
Amiraux R, Patricia B, Christopher B, Jean-François R. Use of Stress Signals of Their Attached Bacteria to Monitor Sympagic Algae Preservation in Canadian Arctic Sediments. Microorganisms. 2021; 9(12):2626. https://doi.org/10.3390/microorganisms9122626
Chicago/Turabian StyleAmiraux, Rémi, Bonin Patricia, Burot Christopher, and Rontani Jean-François. 2021. "Use of Stress Signals of Their Attached Bacteria to Monitor Sympagic Algae Preservation in Canadian Arctic Sediments" Microorganisms 9, no. 12: 2626. https://doi.org/10.3390/microorganisms9122626
APA StyleAmiraux, R., Patricia, B., Christopher, B., & Jean-François, R. (2021). Use of Stress Signals of Their Attached Bacteria to Monitor Sympagic Algae Preservation in Canadian Arctic Sediments. Microorganisms, 9(12), 2626. https://doi.org/10.3390/microorganisms9122626






