Abstract
Yeast PARK9 (YPK9) shares homology with human ATP13A2, which encodes a polyamine transporter implicated in juvenile forms of Parkinson’s disease. We used YPK9 to gain insight into how ATP13A2 affects cell growth and sensitivity to oxidative stress. Surprisingly, the YPK9 deletion strain from the Saccharomyces cerevisiae deletion collection (YKO) in wildtype BY4741 (mating type a) grew faster and was more resistant to hydrogen peroxide than a commercial, putative parental BY4741 wildtype strain (BY4741COM). In contrast, deleting YPK9 from BY4741COM rendered it very sensitive to hydrogen peroxide, suggesting its background is different from that of the deletion collection. Whole-genome sequencing revealed that BY4741COM and BY4741COM ypk9∆ contain a novel premature stop codon near the 3′ end of WHI2 (WHI2G1324T), whereas the collection’s YPK9 deletion strain contains WHI2, which encodes a 486 amino acid protein, Whi2p. Replacing full-length WHI2 with the sequence coding for the predicted truncation (Whi2pE442*) rendered strains more sensitive to hydrogen peroxide, whereas the converse replacement rendered them more resistant. The sequences of WHI2 in 20 randomly chosen strains from the collection encode the full-length protein, indicating that the putative parental BY4741 WHI2G1324T strain’s genetic background differs from that of the deletion collection. Examination of WHI2 sequences in several commonly used wildtype S. cerevisiae strains and isolates revealed other Whi2p truncations that might yield altered phenotypes. Together, these results demonstrate a novel premature stop codon in WHI2 that renders yeast sensitive to hydrogen peroxide; they also reveal a negative genetic interaction between WHI2 and YPK9 in the presence of hydrogen peroxide in the BY4741 background.
1. Introduction
Lindquist et al. first identified YPK9 as a suppressor of α-synuclein toxicity in yeast [1]. α-synuclein is the major component of Lewy bodies, which are cytoplasmic structures that are the hallmark of Parkinson’s disease. S. cerevisiae YPK9 is 38% identical to human ATP13A2 [2], which encodes a multispanning membrane protein of lysosomes that exports polyamines to the cytosol [3]. Mutations in human ATP13A2 are associated with Kufor–Rakeb syndrome, an early onset form of Parkinson’s disease [4]. Polyamines are organic polycations with diverse functions including binding nucleic acids (RNA and DNA), scavenging reactive oxygen species (ROS), and activating eIF5A (yeast Hyp2p) by hypusination, an essential post-translational modification [5,6]. Thus, the convergence of oxidative stress, protein condensates, and polyamine metabolism on YPK9 may yield insight into the dysregulation of fundamental pathways underlying Parkinson’s disease.
WHI2 was originally identified by Sudbery et al. [7] during a screen for cell-cycle mutants. The whi2 mutant yielded small, mostly budding cells in the stationary phase. In 2008, Hardwick et al. showed that cells lacking FIS1, a gene required for mitochondrial fission, acquired a secondary mutation, a premature stop codon in WHI2, rendering it presumably inactive and allowing the cells to grow in amino-acid-deficient media, in particular leucine-deficient media [8]. WHI2, in essence, acts like a leucine sensor. WHI2 was shown to block TORC1, a complex of proteins that regulates cell metabolism and protein translation in yeast and higher eukaryotes [9]. Thus, under limiting leucine levels, cell survival is enhanced by WHI2 blocking TORC1, thereby attenuating translation and increasing autophagy. These findings extend those of Costanzo et al. [10], who showed in a large-scale screen that WHI2 interacts with certain members of TORC1. Thus, identifying and characterizing novel whi2 mutants may deepen our understanding of core conserved eukaryotic growth signaling pathways.
During our recent investigation into the role of YPK9 in peroxisomal proliferation, we noticed that two YPK9 deletion strains in BY4741 had different sensitivities to hydrogen peroxide: a laboratory-generated deletion was much more sensitive than ypk9∆ from the yeast deletion collection. In the studies described below, we used whole-genome sequencing to identify WHI2 as the gene responsible for the difference in oxidative stress sensitivity and revealed its negative genetic interaction with YPK9.
2. Materials and Methods
2.1. Yeast Strains, Media, and Plasmid
The strains used in this study are described in Table S1. Strains were cultured at 30 °C in YPD (1% yeast extract, 2% peptone, and 2% glucose) or synthetic complete media (CSM (Sunrise Science, Knoxville, TN, USA) supplemented with yeast nitrogen base (YNB) and 2% glucose). For growth on agar plates, YPD medium was supplemented with 2% agar. The yeast deletion collection was obtained from Thermo Scientific (YKO-MATa, #YSC1053). Hygromycin (Hyg), nourseothricin (NTC), and G418 were obtained from Gold Biotechnology and used at 100, 100, and 200 µg/mL, respectively. Hydrogen peroxide (30%) was purchased from Fisher Scientific. pAG32 was a gift from John McCusker (plasmid #35122; Addgene, Watertown, MA, USA).
2.2. Strain Genome Sequencing and Analysis
We used a DropSense 96 instrument (PerkinElmer, Waltham, MA, USA) and DropQuant software to quantify and assess the purity of input DNA. We performed whole-genome library preparation according to the on-bead tagmentation protocol (Illumina doc# 1000000025416 v06) using 500 ng of input DNA for each sample. Libraries were sequenced on a NovaSeq 6000 sequencer (Illumina) as 2 × 150 reads with an average number of reads of 16,513,549 (4954 Mb) per sample. Sequencing reads were aligned to the S. cerevisiae reference genome (SacCer3, [11]) using Burrows-Wheeler Aligner (BWA, [12]). Single nucleotide polymorphisms (SNPs) and insertion-deletion (INDEL) variants were visualized with the standalone Integrative Genomics Viewer (IGV) browser (Broad Institute, Cambridge, MA, USA).
2.3. Construction of WHI2 Variants
We used the method of gene replacement to introduce WHI2 or WHI2G1324T in various strains [13]. In the first step, a hygromycin cassette was amplified from pAG32 [14]. The forward primer contained 45 bases of the 3′ terminus of WHI2 and 22 bases of the cassette (WHI2&HYG_fwd: CGTAGAGTTTGGACTTTAGAGTTGAGCGTTATTGGGGTGCAGTGACAGCTGAAGCTTCGTACGCTGC). The reverse primer contained 45 bases immediately downstream of WHI2 and 22 bases of the cassette (WHI2&HYG_rev) TGGCCCGATCTCTTTCCATTTCTTTCTCTAATATATTATATACACGCATAGGCCACTAGTGGATCTG). The amplified product was used to transform BY4741COM, which contained WHI2G1324T and BY4742, which contained WHI2. The WHI2G1324T-HYG and WHI2-HYG fusions were amplified from genomic DNA isolated from the transformants. Genomic DNA was extracted as previously described [15]. The forward primer (WHI2_UP_45) was GATAAAGATAAAGGTTGTCTGAGCTTACACTTATTATAAACAATG and the reverse primer (WHI2_DN_42) was CCCGATCTCTTTCCATTTCTTTCTCTAATATATTATATACAC [16]. The amplified products were used to exchange WHI2G1324T and WHI2 in certain strains. Phusion High-Fidelity DNA Polymerase (New England Biolabs, Ipswich, MA, USA) and Buffer GC were used in amplification reactions following the manufacturer’s instructions. The sequence of mutant and wildtype WHI2 in all strains was verified by DNA sequencing. The forward sequencing primer (WHI2_FWD_710p) was AGACTAAAGCAACAACAGCAAC. The reverse primer (WHI2-SEQ-R-REV: GGGATACCAAGAAACCATACTG) was used to verify the WHI2 sequence in randomly selected strains from the yeast deletion collection.
2.4. Yeast Growth Assays
Frozen stocks of strains were used to prepare fresh streaks on YPD agar plates, samples of which were used to inoculate 5 mL of YPD with or without antibiotics, and the cultures were rotated overnight at 30 °C. Cultures were diluted to 0.05 OD600 and three 200 μL aliquots of each strain were placed in a sterile 96-well microplate. For background correction, certain wells contained only medium. Cultures were incubated in a Sunrise Tecan microplate reader at 25 °C for 2 h with shaking. Hydrogen peroxide (50 µM final concentration) or water was added to the cultures and the incubation was continued for a total of 24 h. The OD600 was recorded in 15 min intervals.
2.5. Calculation of the Strength of Genetic Interactions
The strength of a genetic interaction (ε) was calculated using a multiplicative model [17]. The following equation was used:
where fa and fb are the fitness of single mutants a and b, respectively; and fab is the observed fitness of the double mutant. Fitness was defined as the OD600 after 24 h of growth in the presence of hydrogen peroxide normalized to the wildtype. A positive ε signifies a positive interaction, whereas a negative ε signifies a negative interaction.
3. Results
Based on YPK9’s sequence homology to human ATP13A2, we speculated that a strain lacking YPK9 would have a growth defect and be sensitive to oxidative stress. We found, however, that the YKO collection’s ypk9∆ strain grew slightly better (~15%, based on OD600 at mid-log point) than the commercial strain putatively isogenic to the parental strain, BY4741COM (Figure 1). Because oxidative damage accumulates during ageing, we sought to uncover a sensitivity to oxidative stress by treating YKO’s ypk9∆ with hydrogen peroxide. Hydrogen peroxide reduced the mid-log phase growth of YKO’s ypk9∆ by only 19%, but that of BY4741COM by 32% relative to the corresponding untreated strains, suggesting that loss of YPK9 rendered BY4741 more resistant to oxidative stress (Figure 1). To corroborate this unexpected result, we deleted YPK9 from BY4741COM and measured its growth and hydrogen peroxide sensitivity. The growth of this BY4741COM ypk9∆ deletion strain at mid-log phase was about 14% less than that of the YKO ypk9∆ strain (Figure 1). In the presence of hydrogen peroxide, however, the BY4741COM ypk9∆’s growth in the mid-log phase was severely hampered: about 68% less than YKO ypk9∆’s. At least two explanations are possible for the difference in hydrogen peroxide sensitivity of the two ypk9∆ strains. First, the YKO ypk9∆ strain may have acquired a suppressor of oxidative stress since the collection was constructed [16]. Hardwick et al. estimated that the majority of strains in the YKO collection have acquired a suppressor [18]. Second, the commercial, putative parental BY4741COM may not share the same genetic background as the deletion collection.
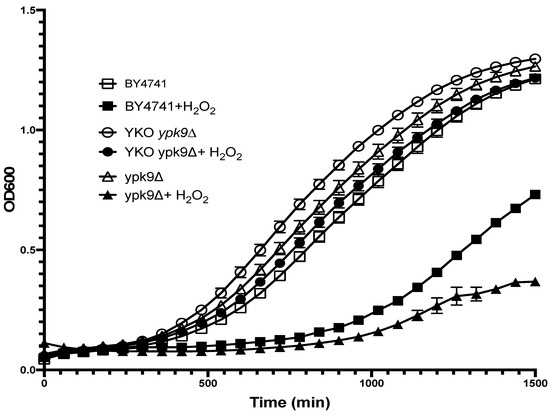
Figure 1.
H2O2 sensitivity of the YKO collection and laboratory YPK9 deletion strains. The growth curves of BY4741COM (squares), the YKO collection’s ypk9∆ in BY4741 (circles), and our ypk9∆ in BY4741COM (triangles) were compared in the presence (solid symbols) or absence (open symbols) of 50 µM H2O2. Differences in growth (see text) were based on OD600 at mid-log point. Error bars represent the SEM, N = 3.
To identify genomic differences that may explain these results, we sequenced the genomes of BY4741COM, BY4742, YKO ypk9∆, and BY4741COM ypk9∆. The four strains had a total of 290 sequence changes relative to the SacCer3 reference genome (Table S2). Of those, 202 were common to all four strains and could not provide insight into the phenotypic differences among them. Most of the remaining variants (88) were short single-nucleotide insertions, deletions, or SNPs located between ORFs and, therefore, of uncertain significance. However, we detected variants in four genes (ADH7, DNF2, RAX1, and WHI2) that were shared between BY4741COM and our ypk9∆ BY4741COM strain but not with the YKO ypk9∆ (Table 1).

Table 1.
SNPs within ORFs determined by whole-genome sequencing.
Alcohol dehydrogenase 7 (ADH7) is an NADP-dependent alcohol dehydrogenase and member of the cinnamyl alcohol dehydrogenase family [19]. We identified a G-to-A SNP at position 309,566 in chr III predicted to change Gly-166 to Asp. This sequence change was characterized as a passenger mutation and is therefore unlikely to affect growth [20]. DNF2 is an aminophospholipid translocase (flippase) [21,22]. We identified a G-to-C SNP at position 632,116 on chr IV predicted to change Gly-279 to Arg. This SNP in DNF2 was not found among the gene variants listed on the Saccharomyces Genome Database (SGD [23]). RAX1 is involved in bud site selection [24,25]. We identified a C-to-A SNP at position 881,528 predicted to change a Cys-188 to a stop codon. The full-length protein has 435 amino acid residues. WHI2 is a negative regulator of TORC1 and is required for the activation of the general stress response in yeast. We identified a G-to-T SNP at position 412,193 in chr XV predicted to change a Glu-442 codon to a stop codon yielding Whi2pE442*. Full-length Whi2p has 486 amino acid residues. Neither the RAX1 nor WHI2 variant described here was found in the SGD [23].
We focused our attention on WHI2 because it plays a key role in the general stress response [26,27,28], regulates TORC1 [9], and is one of five genes most commonly mutated in strains containing a suppressor [20]. Full-length WHI2 is found in YKO’s BY4741 ypk9∆ and BY4742, whereas WHI2G1324T is found in BY4741COM and BY4741COM ypk9∆ (Figure 2).
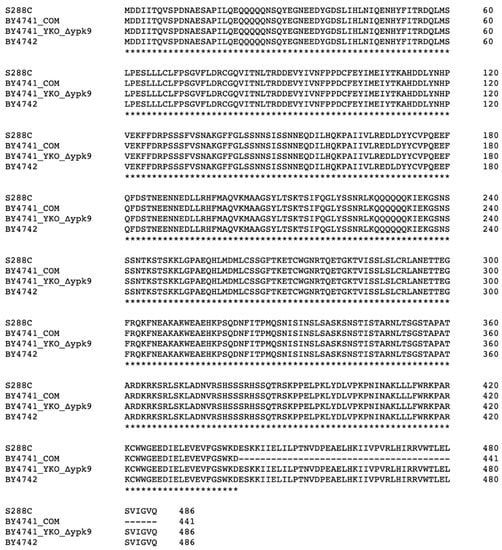
Figure 2.
Alignment of Whi2p variants. The reference strain (S288C), BY4742, and YKO collection’s ypk9∆ contain the sequence for full-length Whi2p (486 amino acids). In contrast, BY4741COM’s WHI2 sequence has a premature stop codon yielding a truncated Whi2p of 441 amino acids residues. Clustal Omega was used to align sequences [29].
We tested the hypothesis that WHI2G1324T renders BY4741COM and BY4741COM ypk9∆ sensitive to hydrogen peroxide by systematically exchanging WHI2 and WHI2G1324T in our strains (Figure 3) and then measuring their sensitivity to hydrogen peroxide (Figure 4). Hydrogen peroxide reduced the growth of BY4741YKO ypk9∆ by about 8% compared to its untreated control (Figure 4A). Replacing WHI2 with WHI2G1324T in ypk9∆ BY4741YKO rendered it very sensitive to hydrogen peroxide reducing growth by 67% (Figure 4A).
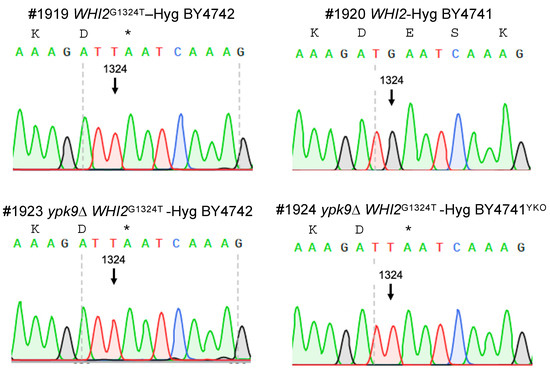
Figure 3.
Sequence confirmation of engineered WHI2 variants. A hygromycin cassette was fused to WHI2 or its variant containing a stop codon at position 1324 (WHI2G1324T) and used to replace the endogenous version of WHI2 in BY4741COM, BY4742, and YKO collection’s ypk9∆ in BY4741YKO. DNA sequencing was used to confirm the replacement. The asterisk represents the stop codon. Numbers with a hashtag represent the laboratory strain identification number.
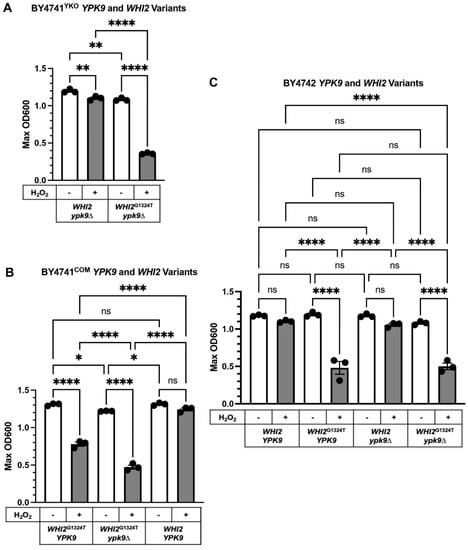
Figure 4.
Effect H2O2 on the growth of WHI2 and YPK9 variants in different background strains. (A) WHI2 variants in the YKO collection’s ypk9∆ in BY4741YKO. (B) WHI2 variants in BY4741COM. (C) WHI2 and YPK9 variants in BY4742. Hygromycin (Hyg) was used to select for gene replacement. The OD shown is the maximum recorded during 25 h of growth. Results were analyzed using one-way ANOVA and Tukey’s post hoc test. Error bars represent the SEM, n = 3. Asterisks represent p-values: * 0.03332, ** 0.0021, **** < 0.0001; ns, not significant.
We extended the analysis by replacing WHI2G1324T with WHI2 in BY4741COM and comparing its hydrogen peroxide sensitivity to that of BY4741COM WHI2G1324T. As expected, BY4741COM WHI2 was mostly resistant to hydrogen peroxide (only a 5% growth reduction), whereas BY4741COM WHI2G1324T was sensitive (40% growth reduction, Figure 4B). Deleting YPK9 from BY4741COM WHI2G1324T rendered the strain very sensitive to hydrogen peroxide (61% growth reduction) and revealed a negative genetic interaction (ε = −0.24, p-value < 0.0001) between ypk9∆ and WHI2G1324T under these conditions.
We next examined how the changes in YPK9 and WHI2 affect the hydrogen peroxide sensitivity of BY4742, the α mating type. BY4742, which has full-length WHI2, was not sensitive to hydrogen peroxide (Figure 4C). However, introducing WHI2’s premature stop codon into BY4742 yielded a strain (BY4742 WHI2G1324T) that was very sensitive to hydrogen peroxide (Figure 4C, 60% growth reduction). We deleted YPK9 from BY4742 WHI2 and showed that the resulting strain (BY4742 ypk9∆ WHI2) was not sensitive to hydrogen peroxide (Figure 4C). The combination of WHI2G1324T and ypk9∆ in BY4742 was very sensitive to hydrogen peroxide but indistinguishable from that of BY4742 WHI2G1324T (Figure 4C, 54% and 60% growth reduction, respectively). Thus, loss of YPK9 from BY4742 WHI2 provided no additional sensitivity to hydrogen peroxide. Together, these results support the idea that WHI2 is a key regulator of oxidative stress in S. cerevisiae [27,28]. YPK9’s role in regulating oxidative stress is revealed in BY4741COM but obscured in BY4742.
To determine if the WHI2G1324T mutation is common in the yeast deletion collection or is unique to the commercial BY4741 isolate, we sequenced the WHI2 gene in 20 randomly chosen strains from the yeast BY4741 deletion collection. All 20 strains contained full-length WHI2, indicating that the parental strain lacked the WHI2G1324T mutation (Figure S1).
The differences in the genetic backgrounds of BY4741COM and BY4741YKO, especially the SNP in WHI2 (Table 1 and Figure 2), prompted us to sequence WHI2 in several S. cerevisiae wildtype strains and compare them to previously reported WHI2 sequences (Figure S2). The genomes of S288C, BY4741 (Stanford), BY4741 (Toronto), BY4741 (Euroscarf), and BY4742 (Euroscarf) encode full-length Whi2p. The genomes of BY4741 (Open Biosystems), BY4741COM (GE Healthcare, Figure 2), and BY4741COM ypk9∆ (this study, Figure S2) encode a truncated Whi2p of 441 amino acid residues. The genome of BY4742 (Toronto) encodes a truncated Whi2p of 369 amino acid residues, whereas that of BY4742 (Stanford) encodes a truncated Whi2p of 423 amino acid residues. Thus, BY4741 and BY4742 isolates have, respectively, at least two and three alleles of WHI2. The WHI2 alleles can cause dramatic phenotypes, for example, the sensitivity to oxidative stress reported here. The different WHI2 alleles found in commonly used S. cerevisiae wildtype isolates, including the a (BY4741) and α (BY4742) mating types, underscore the need for genomic sequence verification of wildtype isolates and heightened awareness within the yeast community.
4. Discussion
We showed above that much of the hydrogen peroxide sensitivity of BY4741COM can be attributed to a G-to-T SNP at position 412,193 chr XV (WHI2) yielding a 441 amino acid-truncated version of Whi2p (Whi2pE442*). To the best of our knowledge, this truncation has not previously been reported. Strains lacking YPK9 but containing full-length WHI2 in the BY4741 background were only modestly sensitive to hydrogen peroxide (~10% growth reduction).
The transcription factors Msn2 and Msn4 were identified as master regulators of the general stress response, an umbrella term that includes nutrient starvation, heat shock, and oxidative stress [30]. Kaida et al. [27] showed that Psr1p/Psr2p phosphatases, Msn2p, and Whi2p physically interact and can be found together at the cell membrane. Upon oxidative stress, Msn2p is released from Psr1p and Whi2p. Msn2p enters the nucleus, where it activates transcription of stress response genes including catalase and superoxide dismutase. Whi2p binds Psr1p, and both are required for full activation of Msn2p [27]. Our results are consistent with the hypothesis that the C-terminal 45-amino-acid residues of Whi2p mediate this important role in regulating Msn2p activation during the oxidative stress response.
The putative parental wildtype strain BY4741COM used in our studies has WHI2G1324T, whereas all twenty of the YKO collection BY4741 strains we sampled encoded full-length Whi2p. Thus, this BY4741COM isolate is not isogenic with the parental strain used to construct the deletion collection. The genomes of the Stanford, Toronto, and Euroscarf BY4741 isolates encode full-length Whi2p, whereas that of Open Biosystem encodes the truncated Whi2p of 441 amino acid residues. In the case of BY4742, the genomes of the Toronto and Stanford isolates encode, respectively, the truncated Whi2p of 369- and 423-amino-acid residues. The genomes of the BY4742 isolate used in our studies and that of Euroscarf’s encode full-length Whi2p. The presence of variants in established wildtype strains and their potentially profound effect on phenotype, for example, the oxidative stress reported here, underscore the need for whole-genome sequencing of commonly used strains [31]. Not only will this resolve the interpretation of conflicting results, but also reveal the biological significance of fortuitous variants.
Other groups have reported informative mutations in WHI2 [20,32,33]. About 30% of the strains sequenced during the construction of a global suppressor network contained at least one mutation in a group of six genes, one of which was WHI2 [20]. Comyn et al. [32] identified WHI2 secondary mutations in 13 strains from the deletion collection during a screen for genes important for protein quality control. The mutant strains were defective in degrading misfolded proteins resulting, perhaps, from disruption of the interaction between Whi2p and Psr1p/Msn2p and its effect on downstream factors [32]. Of the 13 strains, 11 had a premature stop codon in WHI2. The stop codons were evenly distributed throughout WHI2 sequence. Cheng et al. [33] reported truncated versions of Whi2p of 27, 68, and 152 amino acid residues in length. Thus, WHI2 is a member of a small group of genes for whom loss-of-function, or partial truncation, mutations appear to be frequently advantageous.
Although neither a genetic nor physical interaction between YPK9 and WHI2 has been previously reported, a comparison of their respective known interactors converges on eIF5A (Hyp2p). In S. cerevisiae, polyamines scavenge reactive oxygen species (ROS) and play a critical role in hypusination [34], a post-translational modification required for the activity of the essential eIF5A (Hyp2p). Whi2p physically interacts with Hyp2p, and YPK9 and HYP2 have a negative genetic interaction based on whole-gene knockout data [10,35]. Thus, Whi2p may block translation [9], in part through its interaction with eIF5A (Hyp2p). We showed a negative genetic interaction between YPK9 and WHI2 in the BY4741COM background in the presence of hydrogen peroxide. When S. cerevisiae is treated with hydrogen peroxide, Tpo1p exports spermine and spermidine, thereby lowering their cytosolic concentrations [36]. Thus, it is tempting to speculate that in the presence of hydrogen peroxide, cell survival and hypusination are more dependent on Ypk9p to deliver polyamines to the cytosol. Although members of the mammalian ATP13A3-5 family cannot suppress the Mn2+ toxicity of ypk9∆ strains [37], whether they complement growth defects during oxidative stress in these strains warrants further investigation.
In S. cerevisiae, cytosolic polyamines, which scavenge ROS, are derived from at least three routes: biosynthesis from ornithine [5], transport into the cell via polyamine transporters (e.g., Tpo1p [36]), and putative export from the vacuole via Ypk9p. The ability of S. cerevisiae to obtain polyamines from the three routes may explain the modest growth reduction of ypk9∆ strains in the presence of hydrogen peroxide, and this genetic redundancy may also reflect the necessity of controlling the cytosolic concentration of polyamines. The ability of spermidine to enhance longevity in yeast, flies, worms, human cells, and mice by inducing autophagy [38]; the interest in spermidine’s effect on cognition [39]; and the link between the polyamine pathway and Parkinson’s disease [40] underscore the need to further investigate polyamines and their regulators, such as YPK9 and WHI2.
This fortuitous discovery of a negative, condition-dependent genetic interaction highlights the importance of multicondition mapping of genetic interactions in multiple backgrounds. While high-throughput reverse-genetics methods are becoming more precise at delivering particular modifications across the genome, unbiased forward genetics is still an important strategy for generating and dissecting unanticipated modes and complexity of genetic variation.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/microorganisms9122584/s1, Figure S1. Chromatograms of WHI2 sequencing reactions; Figure S2. Whi2p amino acid sequence from select wildtype S. cerevisiae strains and isolates; Table S1. List of strains; Table S2. Summary of variants in (A) BY4741COM, (B) BY4741COM ypk9∆, (C) BY4741YKO ypk9∆, and (D) BY4742.
Author Contributions
Conceptualization, W.J.C.; methodology, F.J., D.M., O.V.E., and W.J.C.; validation, F.J., O.V.E., and W.J.C.; formal analysis, F.J., D.M., and W.J.C.; investigation, F.J., O.V.E., and W.J.C.; resources, F.J., D.M., O.V.E., and W.J.C.; data curation, O.V.E., and W.J.C.; writing—original draft preparation, F.J., O.V.E., and W.J.C.; writing—review and editing, F.J., D.M., O.V.E., and W.J.C.; Visualization, F.J., and W.J.C.; supervision, D.M., O.V.E., and W.J.C.; project administration, W.J.C.; funding acquisition, W.J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the School of Graduate Studies at SUNY Downstate and a grant from the SUNY Downstate Dean’s Initiative in Research Investment.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article and supplementary material.
Acknowledgments
We thank David Gresham (New York University) for generously providing BY4742, Peter Lipke (Brooklyn College) for many helpful discussions, and Gabrielle Suppa for technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gitler, A.D.; Chesi, A.; Geddie, M.L.; Strathearn, K.E.; Hamamichi, S.; Hill, K.J.; Caldwell, K.A.; Caldwell, G.A.; Cooper, A.A.; Rochet, J.-C.; et al. Alpha-Synuclein is part of a diverse and highly conserved interaction network that includes PARK9 and manganese toxicity. Nat. Genet. 2009, 41, 308–315. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, K.; Wolfe, D.M.; Stiller, B.; Pearce, D.A. Cd2+, Mn2+, Ni2+ and Se2+ toxicity to Saccharomyces cerevisiae lacking YPK9p the orthologue of human ATP13A2. Biochem. Biophys. Res. Commun. 2009, 383, 198–202. [Google Scholar] [CrossRef] [Green Version]
- van Veen, S.; Martin, S.; Van den Haute, C.; Benoy, V.; Lyons, J.; Vanhoutte, R.; Kahler, J.P.; Decuypere, J.-P.; Gelders, G.; Lambie, E.; et al. ATP13A2 deficiency disrupts lysosomal polyamine export. Nature 2020, 578, 419–424. [Google Scholar] [CrossRef]
- Ramirez, A.; Heimbach, A.; Gründemann, J.; Stiller, B.; Hampshire, D.; Cid, L.P.; Goebel, I.; Mubaidin, A.F.; Wriekat, A.-L.; Roeper, J.; et al. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat. Genet. 2006, 38, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Tabor, C.W.; Tabor, H. Polyamines in microorganisms. Microbiol. Rev. 1985, 49, 81–99. [Google Scholar] [CrossRef]
- Pegg, A.E. Mammalian polyamine metabolism and function. IUBMB Life 2009, 61, 880–894. [Google Scholar] [CrossRef] [PubMed]
- Sudbery, P.E.; Goodey, A.R.; Carter, B.L.A. Genes which control cell proliferation in the yeast Saccharomyces cerevisiae. Nature 1980, 288, 401–404. [Google Scholar] [CrossRef]
- Teng, X.; Yau, E.; Sing, C.; Hardwick, J.M. Whi2 signals low leucine availability to halt yeast growth and cell death. FEMS Yeast Res. 2018, 18. [Google Scholar] [CrossRef]
- Chen, X.; Wang, G.; Zhang, Y.; Dayhoff-Brannigan, M.; Diny, N.L.; Zhao, M.; He, G.; Sing, C.N.; Metz, K.A.; Stolp, Z.D.; et al. Whi2 is a conserved negative regulator of TORC1 in response to low amino acids. PLoS Genet. 2018, 14, e1007592. [Google Scholar] [CrossRef]
- Costanzo, M.; Baryshnikova, A.; Bellay, J.; Kim, Y.; Spear, E.D.; Sevier, C.S.; Ding, H.; Koh, J.L.; Toufighi, K.; Mostafavi, S.; et al. The Genetic Landscape of a Cell. Science 2010, 327, 425–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engel, S.R.; Dietrich, F.S.; Fisk, D.G.; Binkley, G.; Balakrishnan, R.; Costanzo, M.C.; Dwight, S.S.; Hitz, B.C.; Karra, K.; Nash, R.S.; et al. The Reference Genome Sequence of Saccharomyces cerevisiae: Then and Now. G3: Genes Genomes Genet. 2014, 4, 389–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gietz, R.D.; Schiestl, R.H. High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat. Protoc. 2007, 2, 31–34. [Google Scholar] [CrossRef]
- Goldstein, A.L.; McCusker, J.H. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast 1999, 15, 1541–1553. [Google Scholar] [CrossRef]
- Hoffman, C.S.; Winston, F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformaion of Escherichia coli. Gene 1987, 57, 267–272. [Google Scholar] [CrossRef]
- Giaever, G.; Chu, A.M.; Ni, L.; Connelly, C.; Riles, L.; Véronneau, S.; Dow, S.; Lucau-Danila, A.; Anderson, K.; André, B.; et al. Functional profiling of the Saccharomyces cerevisiae genome. Nature 2002, 418, 387–391. [Google Scholar] [CrossRef]
- Koh, J.L.Y.; Ding, H.; Costanzo, M.; Baryshnikova, A.; Toufighi, K.; Bader, G.; Myers, C.L.; Andrews, B.J.; Boone, C. DRYGIN: A database of quantitative genetic interaction networks in yeast. Nucleic Acids Res. 2009, 38 (Suppl. 1), D502–D507. [Google Scholar] [CrossRef] [Green Version]
- Teng, X.; Hardwick, J.M. Quantification of Genetically Controlled Cell Death in Budding Yeast. Springer Protoc. Handb. 2013, 1004, 161–170. [Google Scholar] [CrossRef] [Green Version]
- Larroy, C.; Pares, X.; Biosca, J.A. Characterization of a Saccharomyces cerevisiae NADP(H)-dependent alcohol dehydrogenase (ADHVII), a member of the cinnamyl alcohol dehydrogenase family. Eur. J. Biochem. 2002, 269, 5738–5745. [Google Scholar] [CrossRef]
- van Leeuwen, J.; Pons, C.; Mellor, J.C.; Yamaguchi, T.N.; Friesen, H.; Koschwanez, J.; Ušaj, M.M.; Pechlaner, M.; Takar, M.; Ušaj, M.; et al. Exploring genetic suppression interactions on a global scale. Science 2016, 354. [Google Scholar] [CrossRef] [Green Version]
- Hua, Z.; Fatheddin, P.; Graham, T.R. An Essential Subfamily of Drs2p-related P-Type ATPases Is Required for Protein Trafficking between Golgi Complex and Endosomal/Vacuolar System. Mol. Biol. Cell 2002, 13, 3162–3177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pomorski, T.G.; Lombardi, R.; Riezman, H.; Devaux, P.F.; van Meer, G.; Holthuis, J.C.M. Drs2p-related P-type ATPases Dnf1p and Dnf2p Are Required for Phospholipid Translocation across the Yeast Plasma Membrane and Serve a Role in Endocytosis. Mol. Biol. Cell 2003, 14, 1240–1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherry, J.M.; Hong, E.L.; Amundsen, C.; Balakrishnan, R.; Binkley, G.; Chan, E.; Christie, K.; Costanzo, M.; Dwight, S.S.; Engel, S.; et al. Saccharomyces Genome Database: The genomics resource of budding yeast. Nucleic Acids Res. 2011, 40, D700–D705. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Hiroko, T.; Chaudhuri, A.; Inose, F.; Lord, M.; Tanaka, S.; Chant, J.; Fujita, A. Multigenerational Cortical Inheritance of the Rax2 Protein in Orienting Polarity and Division in Yeast. Science 2000, 290, 1975–1978. [Google Scholar] [CrossRef] [PubMed]
- Fujita, A.; Lord, M.; Hiroko, T.; Hiroko, F.; Chen, T.; Oka, C.; Misumi, Y.; Chant, J. Rax1, a protein required for the establishment of the bipolar budding pattern in yeast. Gene 2004, 327, 161–169. [Google Scholar] [CrossRef]
- Teng, X.; Hardwick, J.M. Whi2: A new player in amino acid sensing. Curr. Genet. 2019, 65, 701–709. [Google Scholar] [CrossRef]
- Kaida, D.; Yashiroda, H.; Toh-e, A.; Kikuchi, Y. Yeast Whi2 and Psr1-phosphatase form a complex and regulate STRE-mediated gene expression. Genes Cells 2002, 7, 543–552. [Google Scholar] [CrossRef]
- Sadeh, A.; Movshovich, N.; Volokh, M.; Gheber, L.; Aharoni, A. Fine-tuning of the Msn2/4–mediated yeast stress responses as revealed by systematic deletion of Msn2/4 partners. Mol. Biol. Cell 2011, 22, 3127–3138. [Google Scholar] [CrossRef] [PubMed]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; López, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef]
- Martinez-Pastor, M.T.; Marchler, G.; Schuller, C.; Marchler-Bauer, A.; Ruis, H.; Estruch, F. The Saccharomyces cerevisiae zinc finger proteins Msn2p and Msn4p are required for transcriptional induction through the stress response element (STRE). EMBO J. 1996, 15, 2227–2235. [Google Scholar] [CrossRef] [PubMed]
- Gallegos, J.E.; Hayrynen, S.; Adames, N.R.; Peccoud, J. Challenges and opportunities for strain verification by whole-genome sequencing. Sci. Rep. 2020, 10, 5873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comyn, S.A.; Flibotte, S.; Mayor, T. Recurrent background mutations in WHI2 impair proteostasis and degradation of misfolded cytosolic proteins in Saccharomyces cerevisiae. Sci. Rep. 2017, 7, 4183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, W.-C.; Teng, X.; Park, H.K.; Tucker, C.M.; Dunham, M.; Hardwick, J.M. Fis1 deficiency selects for compensatory mutations responsible for cell death and growth control defects. Cell Death Differ. 2008, 15, 1838–1846. [Google Scholar] [CrossRef]
- Pegg, A.E. Functions of Polyamines in Mammals. J. Biol. Chem. 2016, 291, 14904–14912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, Y.; Gruhler, A.; Heilbut, A.; Bader, G.D.; Moore, L.; Adams, S.-L.; Millar, A.; Taylor, P.; Bennett, K.L.; Boutilier, K.; et al. Systematic identification of protein complexes in Saccharomyces cerevisiae by mass spectrometry. Nature 2002, 415, 180–183. [Google Scholar] [CrossRef] [Green Version]
- Krüger, A.; Vowinckel, J.; Mülleder, M.; Grote, P.; Capuano, F.; Bluemlein, K.; Ralser, M. Tpo1-mediated spermine and spermidine export controls cell cycle delay and times antioxidant protein expression during the oxidative stress response. EMBO Rep. 2013, 14, 1113–1119. [Google Scholar] [CrossRef]
- Sørensen, D.M.; Holemans, T.; Van Veen, S.; Martin, S.; Arslan, T.; Haagendahl, I.W.; Holen, H.W.; Hamouda, N.N.; Eggermont, J.; Palmgren, M.; et al. Parkinson disease related ATP13A2 evolved early in animal evolution. PLoS ONE 2018, 13, e0193228. [Google Scholar] [CrossRef] [Green Version]
- Eisenberg, T.; Knauer, H.; Schauer, A.; Büttner, S.; Ruckenstuhl, C.; Carmona-Gutierrez, D.; Ring, J.; Schroeder, S.; Magnes, C.; Antonacci, L.; et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 2009, 11, 1305–1314. [Google Scholar] [CrossRef]
- Wirth, M.; Schwarz, C.; Benson, G.; Horn, N.; Buchert, R.; Lange, C.; Köbe, T.; Hetzer, S.; Maglione, M.; Michael, E.; et al. Effects of spermidine supplementation on cognition and biomarkers in older adults with subjective cognitive decline (SmartAge)—study protocol for a randomized controlled trial. Alzheimer’s Res. Ther. 2019, 11, 1–17. [Google Scholar] [CrossRef]
- Lewandowski, N.M.; Ju, S.; Verbitsky, M.; Ross, B.; Geddie, M.L.; Rockenstein, E.; Adame, A.; Muhammad, A.; Vonsattel, J.P.; Ringe, D.; et al. Polyamine pathway contributes to the pathogenesis of Parkinson disease. Proc. Natl. Acad. Sci. USA 2010, 107, 16970–16975. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).