Regulation of d-Aspartate Oxidase Gene Expression by Pyruvate Metabolism in the Yeast Cryptococcus humicola
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Strains, Media, and Growth Conditions
2.3. DNA and RNA Preparation
2.4. Cloning and Expression of ChPYC1 Gene
2.5. Pyc Activity Assay
2.6. Disruption of ChPYC1 Gene
2.7. ChDDO Gene Induction Experiment
2.8. DDO Activity Assay
2.9. Intracellular d-Asp and Pyruvate Quantification
2.10. Quantitative Real-Time RT-PCR (qRT-PCR)
2.11. Sequence Analyses
3. Results
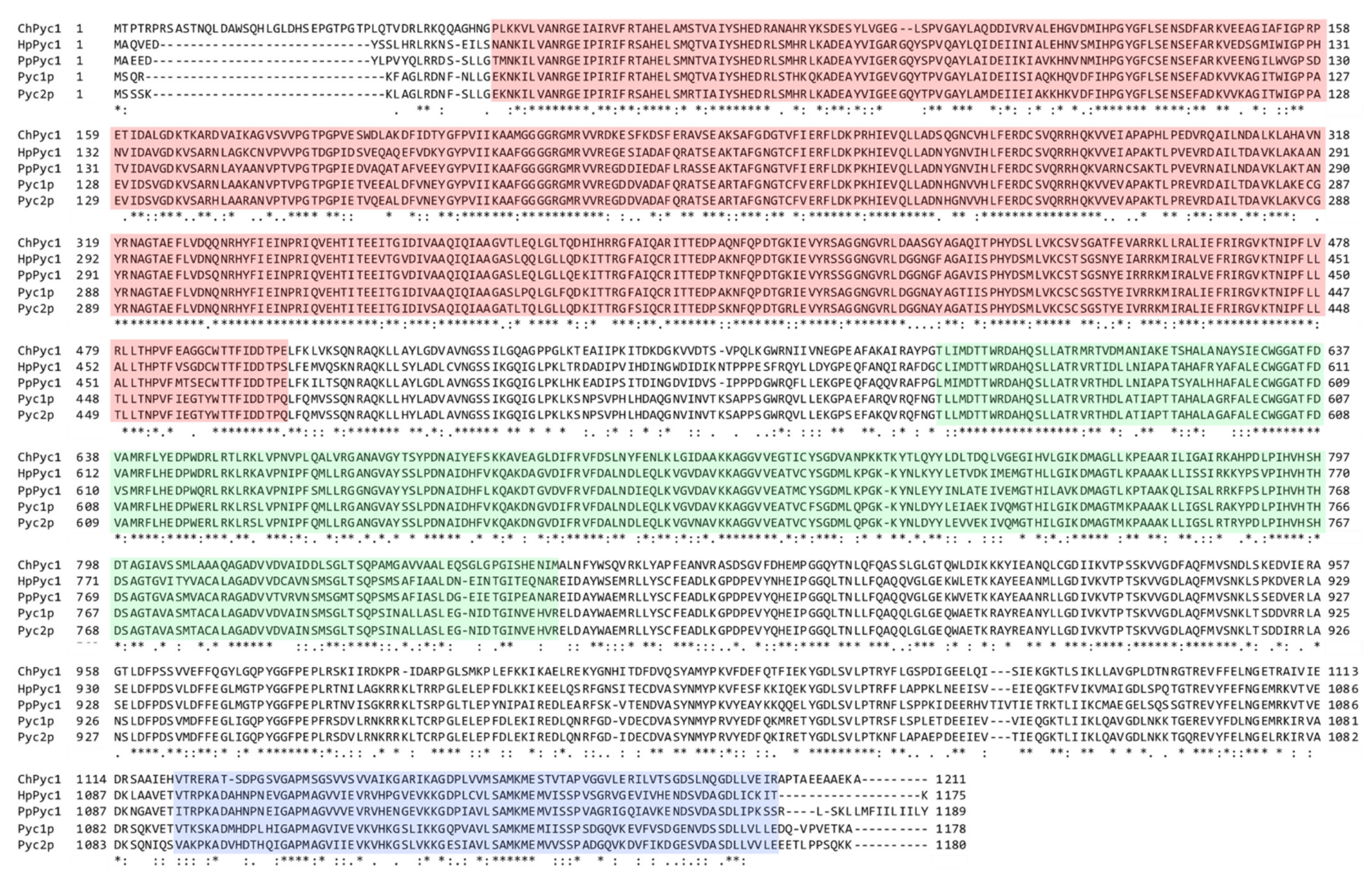
3.1. Identification of Pyc Homolog of C. humicola Strain UJ1
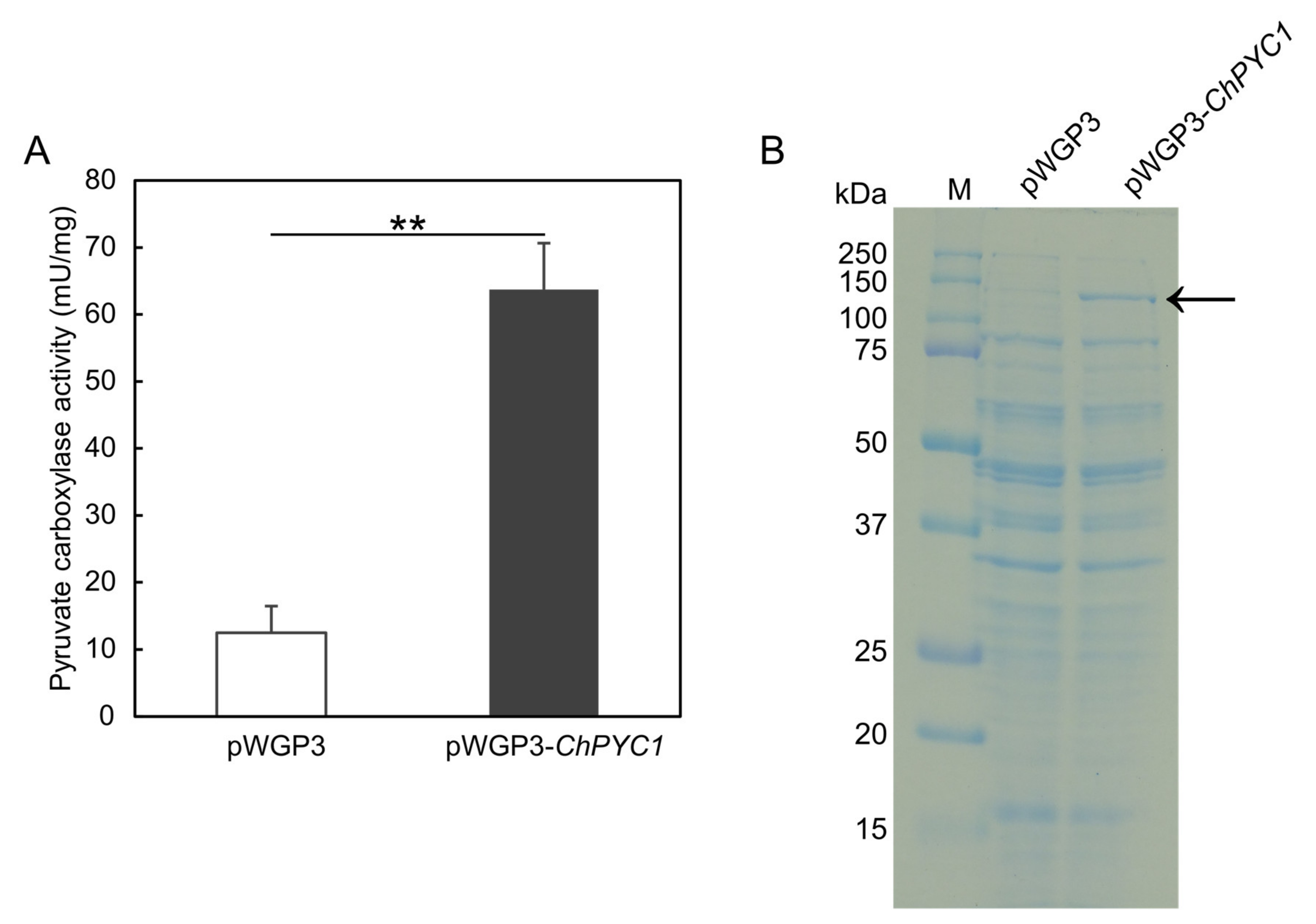
3.2. Expression of ChPYC1 Gene in S. cerevisiae
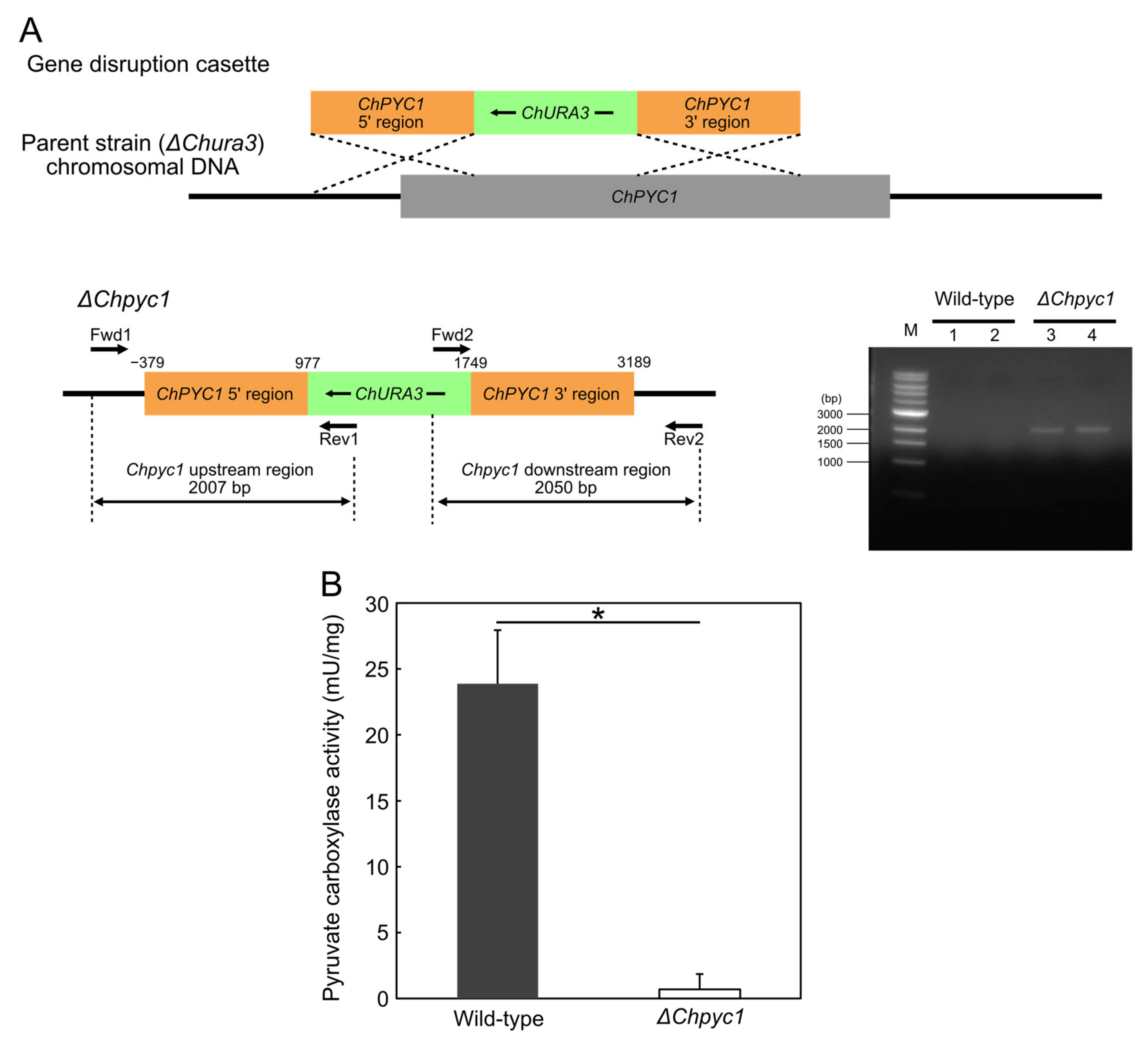
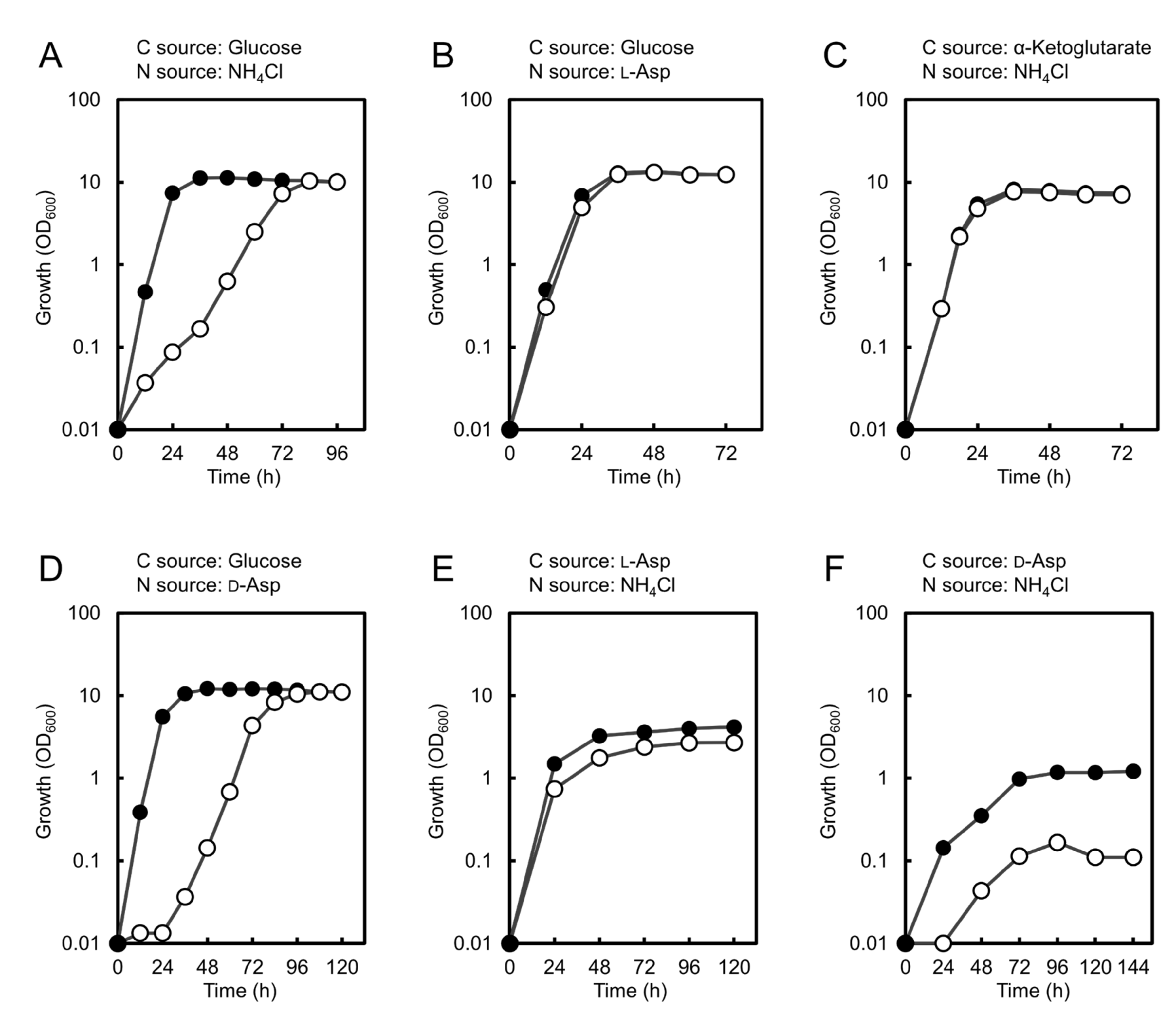
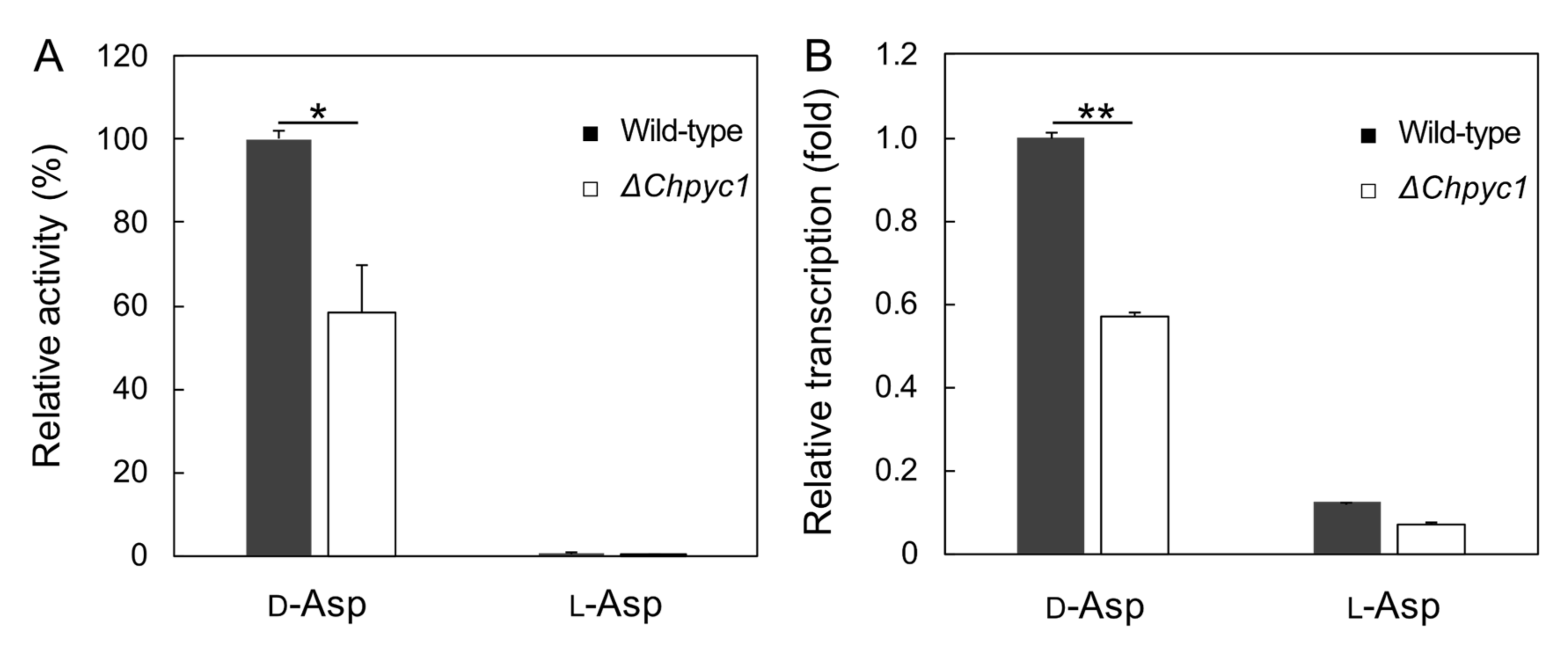
3.3. Growth Characteristics of ΔChpyc1 Strain
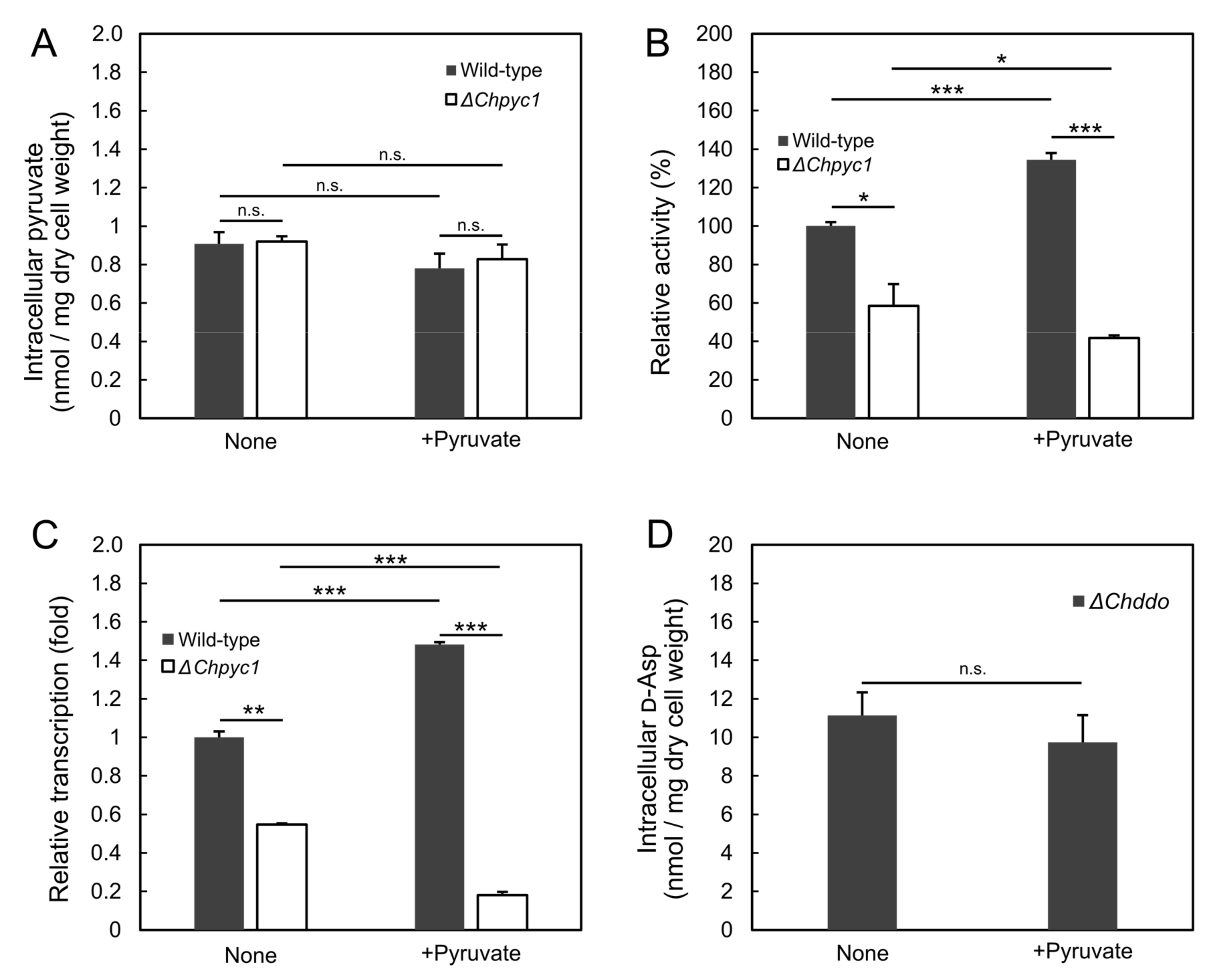
3.4. Effect of Pyruvate on the Induction of ChDDO Gene by d-Asp
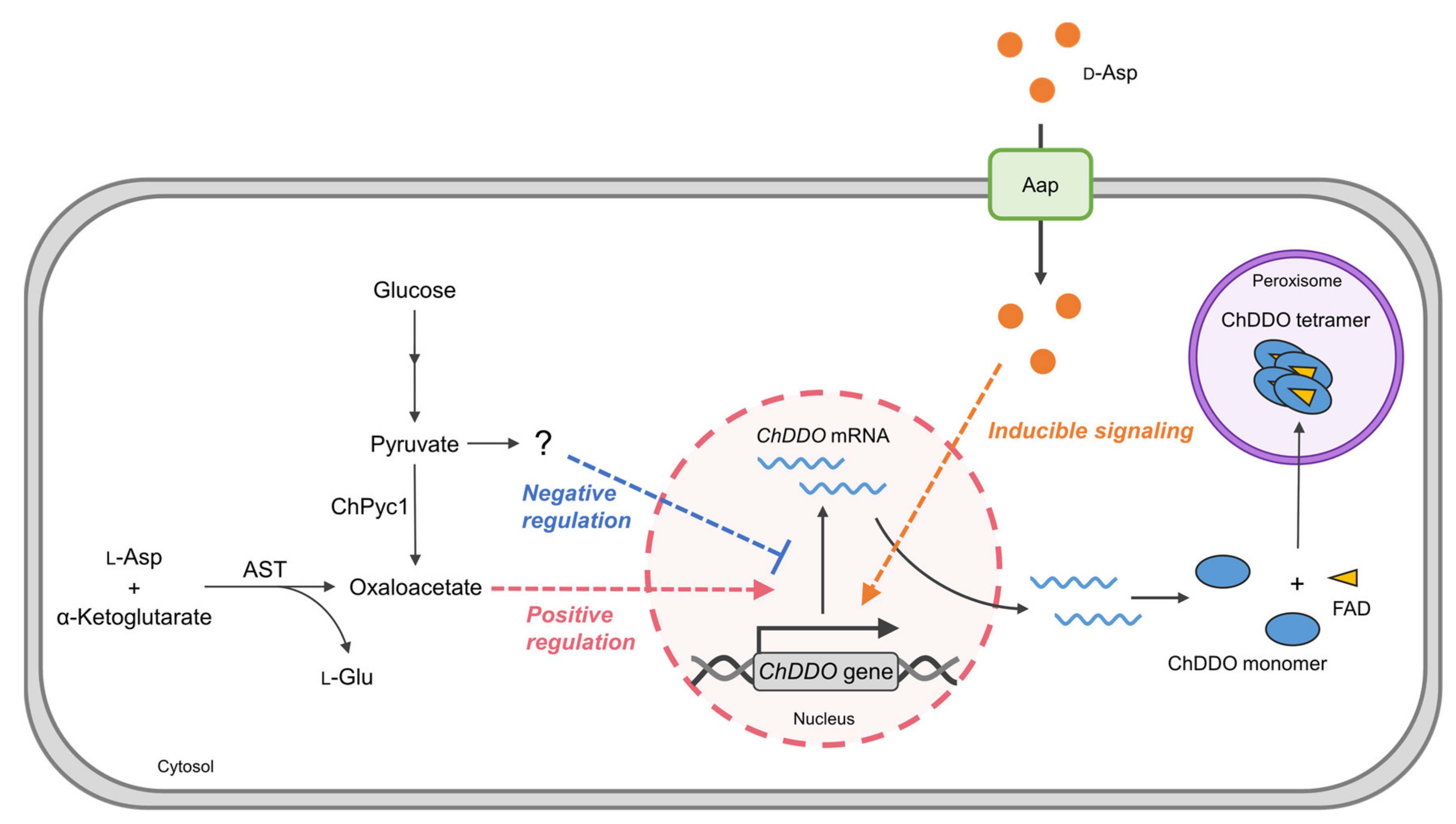
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Negri, A.; Ceciliani, F.; Tedeschi, G.; Simonic, T.; Ronchi, S. The primary structure of the flavoprotein d-aspartate oxidase from beef kidney. J. Biol. Chem. 1992, 267, 11865–11871. [Google Scholar] [CrossRef]
- Takahashi, S.; Takahashi, T.; Kera, Y.; Matsunaga, R.; Shibuya, H.; Yamada, R.H. Cloning and expression in Escherichia coli of the d-aspartate oxidase gene from the yeast Cryptococcus humicola and characterization of the recombinant enzyme. J. Biochem. 2004, 135, 533–540. [Google Scholar] [CrossRef]
- D’Aniello, A.; Palescandolo, R.; Scardi, B. The distribution of the d-aspartate oxidase activity in Cephalopoda. Comp. Biochem. Physiol. B 1975, 50, 209–210. [Google Scholar] [CrossRef]
- Yamada, R.; Nagasaki, H.; Wakabayashi, Y.; Iwashima, A. Presence of d-aspartate oxidase in rat liver and mouse tissues. Biochim. Biophys. Acta 1988, 965, 202–205. [Google Scholar] [CrossRef]
- Kera, Y.; Nagasaki, H.; Iwashima, A.; Yamada, R. Presence of d-aspartate oxidase and free d-aspartate in amphibian (Xenopus laevis, Cynops pyrrhogaster) tissues. Comp. Biochem. Physiol. B 1992, 103, 345–348. [Google Scholar] [CrossRef]
- Kera, Y.; Aoyama, H.; Watanabe, N.; Yamada, R.H. Distribution of d-aspartate oxidase and free d-glutamate and d-aspartate in chicken and pigeon tissues. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1996, 115, 121–126. [Google Scholar] [CrossRef]
- Yamada, R.; Ujiie, H.; Kera, Y.; Nakase, T.; Kitagawa, K.; Imasaka, T.; Arimoto, K.; Takahashi, M.; Matsumura, Y. Purification and properties of d-aspartate oxidase from Cryptococcus humicolus UJ1. Biochim. Biophys. Acta 1996, 1294, 153–158. [Google Scholar] [CrossRef]
- Takahashi, S.; Kakuichi, T.; Fujii, K.; Kera, Y.; Yamada, R.H. Physiological role of d-aspartate oxidase in the assimilation and detoxification of d-aspartate in the yeast Cryptococcus humicola. Yeast 2005, 22, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Katane, M.; Saitoh, Y.; Seida, Y.; Sekine, M.; Furuchi, T.; Homma, H. Comparative characterization of three d-aspartate oxidases and one d-amino acid oxidase from Caenorhabditis elegans. Chem. Biodivers. 2010, 7, 1424–1434. [Google Scholar] [CrossRef]
- Saitoh, Y.; Katane, M.; Kawata, T.; Maeda, K.; Sekine, M.; Furuchi, T.; Kobuna, H.; Sakamoto, T.; Inoue, T.; Arai, H.; et al. Spatiotemporal localization of d-amino acid oxidase and d-aspartate oxidases during development in Caenorhabditis elegans. Mol. Cell Biol. 2012, 32, 1967–1983. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, Y.; Katane, M.; Miyamoto, T.; Sekine, M.; Sakamoto, T.; Imai, H.; Homma, H. Secreted d-aspartate oxidase functions in C. elegans reproduction and development. FEBS J. 2019, 286, 124–138. [Google Scholar] [CrossRef]
- D’Aniello, A.; D’Onofrio, G.; Pischetola, M.; D’Aniello, G.; Vetere, A.; Petrucelli, L.; Fisher, G.H. Biological role of d-amino acid oxidase and d-aspartate oxidase. Effects of d-amino acids. J. Biol. Chem. 1993, 268, 26941–26949. [Google Scholar] [CrossRef]
- Huang, A.S.; Beigneux, A.; Weil, Z.M.; Kim, P.M.; Molliver, M.E.; Blackshaw, S.; Nelson, R.J.; Young, S.G.; Snyder, S.H. d-aspartate regulates melanocortin formation and function: Behavioral alterations in d-aspartate oxidase-deficient mice. J. Neurosci. 2006, 26, 2814–2819. [Google Scholar] [CrossRef] [PubMed]
- Errico, F.; Napolitano, F.; Nistico, R.; Usiello, A. New insights on the role of free d-aspartate in the mammalian brain. Amino Acids 2012, 43, 1861–1871. [Google Scholar] [CrossRef]
- Krashia, P.; Ledonne, A.; Nobili, A.; Cordella, A.; Errico, F.; Usiello, A.; D’Amelio, M.; Mercuri, N.B.; Guatteo, E.; Carunchio, I. Persistent elevation of d-Aspartate enhances NMDA receptor-mediated responses in mouse substantia nigra pars compacta dopamine neurons. Neuropharmacology 2016, 103, 69–78. [Google Scholar] [CrossRef]
- Nuzzo, T.; Sacchi, S.; Errico, F.; Keller, S.; Palumbo, O.; Florio, E.; Punzo, D.; Napolitano, F.; Copetti, M.; Carella, M.; et al. Decreased free d-aspartate levels are linked to enhanced d-aspartate oxidase activity in the dorsolateral prefrontal cortex of schizophrenia patients. NPJ Schizophr. 2017, 3, 16. [Google Scholar] [CrossRef]
- Errico, F.; Nuzzo, T.; Carella, M.; Bertolino, A.; Usiello, A. The Emerging Role of Altered d-Aspartate Metabolism in Schizophrenia: New Insights From Preclinical Models and Human Studies. Front. Psychiatry 2018, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Wakayama, M.; Nakashima, S.; Sakai, K.; Moriguchi, M. Isolation, Enzyme-Production and Characterization of d-Aspartate Oxidase from Fusarium sacchari var. elongatum Y-105. J. Ferment. Bioeng. 1994, 78, 377–379. [Google Scholar] [CrossRef]
- Fukunaga, S.; Yuno, S.; Takahashi, M.; Taguchi, S.; Kera, Y.; Odani, S.; Yamada, R.H. Purification and properties of d-glutamate oxidase from Candida boidinii 2201. J. Ferment. Bioeng. 1998, 85, 579–583. [Google Scholar] [CrossRef]
- Yamada, R.; Nagasaki, H.; Nagata, Y.; Wakabayashi, Y.; Iwashima, A. Administration of d-aspartate increases d-aspartate oxidase activity in mouse liver. Biochim. Biophys. Acta 1989, 990, 325–328. [Google Scholar] [CrossRef]
- Pronk, J.T.; Yde Steensma, H.; Van Dijken, J.P. Pyruvate metabolism in Saccharomyces cerevisiae. Yeast 1996, 12, 1607–1633. [Google Scholar] [CrossRef]
- Jitrapakdee, S.; St Maurice, M.; Rayment, I.; Cleland, W.W.; Wallace, J.C.; Attwood, P.V. Structure, mechanism and regulation of pyruvate carboxylase. Biochem. J. 2008, 413, 369–387. [Google Scholar] [CrossRef]
- Ozimek, P.; van Dijk, R.; Latchev, K.; Gancedo, C.; Wang, D.Y.; van der Klei, I.J.; Veenhuis, M. Pyruvate carboxylase is an essential protein in the assembly of yeast peroxisomal oligomeric alcohol oxidase. Mol. Biol. Cell 2003, 14, 786–797. [Google Scholar] [CrossRef] [PubMed]
- Ozimek, P.Z.; Klompmaker, S.H.; Visser, N.; Veenhuis, M.; van der Klei, I.J. The transcarboxylase domain of pyruvate carboxylase is essential for assembly of the peroxisomal flavoenzyme alcohol oxidase. FEMS Yeast Res. 2007, 7, 1082–1092. [Google Scholar] [CrossRef] [PubMed]
- Huberts, D.H.; Venselaar, H.; Vriend, G.; Veenhuis, M.; van der Klei, I.J. The moonlighting function of pyruvate carboxylase resides in the non-catalytic end of the TIM barrel. Biochim. Biophys. Acta 2010, 1803, 1038–1042. [Google Scholar] [CrossRef] [PubMed]
- Van der Klei, I.J.; Harder, W.; Veenhuis, M. Methanol metabolism in a peroxisome-deficient mutant of Hansenula polymorpha: A physiological study. Arch. Microbiol. 1991, 156, 15–23. [Google Scholar] [CrossRef]
- Klompmaker, S.H.; Kilic, A.; Baerends, R.J.; Veenhuis, M.; van der Klei, I.J. Activation of a peroxisomal Pichia pastoris d-amino acid oxidase, which uses d-alanine as a preferred substrate, depends on pyruvate carboxylase. FEMS Yeast Res. 2010, 10, 708–716. [Google Scholar] [CrossRef]
- Takahashi, S.; Matsunaga, R.; Kera, Y.; Yamada, R.H. Isolation of the Cryptococcus humicolus URA3 gene encoding orotidine-5’-phosphate decarboxylase and its use as a selective marker for transformation. J. Biosci. Bioeng. 2003, 96, 23–31. [Google Scholar] [CrossRef]
- Wallis, J.W.; Chrebet, G.; Brodsky, G.; Rolfe, M.; Rothstein, R. A hyper-recombination mutation in S. cerevisiae identifies a novel eukaryotic topoisomerase. Cell 1989, 58, 409–419. [Google Scholar] [CrossRef]
- Imanishi, D.; Abe, K.; Kera, Y.; Takahashi, S. Draft Genome Sequence of the Yeast Vanrija humicola (Formerly Cryptococcus humicola) Strain UJ1, a Producer of d-Aspartate Oxidase. Genome Announc. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Takahashi, T.; Shimoi, H.; Ito, K. Identification of genes required for growth under ethanol stress using transposon mutagenesis in Saccharomyces cerevisiae. Mol. Genet. Genom. 2001, 265, 1112–1119. [Google Scholar] [CrossRef]
- Payne, J.; Morris, J.G. Pyruvate carboxylase in Rhodopseudomonas spheroides. J. Gen. Microbiol. 1969, 59, 97–101. [Google Scholar] [CrossRef]
- Molla, G.; Piubelli, L.; Volonte, F.; Pilone, M.S. Enzymatic detection of d-amino acids. Methods Mol. Biol. 2012, 794, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Kajitani, K.; Ishikawa, T.; Shibata, K.; Kouya, T.; Kera, Y.; Takahashi, S. Development of an enzymatic screening method for d-aspartate-producing lactic acid bacteria. Enzyme Microb. Technol. 2021, 149, 109835. [Google Scholar] [CrossRef]
- Zhu, A.; Romero, R.; Petty, H.R. A sensitive fluorimetric assay for pyruvate. Anal. Biochem. 2010, 396, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Attwood, P.V. The structure and the mechanism of action of pyruvate carboxylase. Int. J. Biochem. Cell Biol. 1995, 27, 231–249. [Google Scholar] [CrossRef]
- Yang, S.T.; Zhang, K.; Zhang, B.; Huang, H. Fumaric Acid. In Comprehensive Biotechnology; Elsevier: Amsterdam, The Netherlands, 2011; pp. 163–177. [Google Scholar]
- Imanishi, D.; Kera, Y.; Takahashi, S. Identification of an Acidic Amino Acid Permease Involved in d-Aspartate Uptake in the Yeast Cryptococcus humicola. Microorganisms 2021, 9, 192. [Google Scholar] [CrossRef]
- Skinner, V.M.; Armitt, S. Mutants of Aspergillus nidulans lacking pyruvate carboxylase. FEBS Lett. 1972, 20, 16–18. [Google Scholar] [CrossRef]
- Stucka, R.; Dequin, S.; Salmon, J.M.; Gancedo, C. DNA sequences in chromosomes II and VII code for pyruvate carboxylase isoenzymes in Saccharomyces cerevisiae: Analysis of pyruvate carboxylase-deficient strains. Mol. Gen. Genet. 1991, 229, 307–315. [Google Scholar] [CrossRef]
- Menendez, J.; Delgado, J.; Gancedo, C. Isolation of the Pichia pastoris PYC1 gene encoding pyruvate carboxylase and identification of a suppressor of the pyc phenotype. Yeast 1998, 14, 647–654. [Google Scholar] [CrossRef]
- Wood, H.G. Mechanism of formation of oxaloacetate and phosphoenol pyruvate from pyruvate. J. Vitaminol. (Kyoto) 1968, 14, 59–67. [Google Scholar] [CrossRef]
- Brewster, N.K.; Val, D.L.; Walker, M.E.; Wallace, J.C. Regulation of pyruvate carboxylase isozyme (PYC1, PYC2) gene expression in Saccharomyces cerevisiae during fermentative and nonfermentative growth. Arch. Biochem. Biophys. 1994, 311, 62–71. [Google Scholar] [CrossRef]
- Menendez, J.; Gancedo, C. Regulatory regions in the promoters of the Saccharomyces cerevisiae PYC1 and PYC2 genes encoding isoenzymes of pyruvate carboxylase. FEMS Microbiol. Lett. 1998, 164, 345–352. [Google Scholar] [CrossRef][Green Version]
- Huet, C.; Menendez, J.; Gancedo, C.; Francois, J.M. Regulation of pyc1 encoding pyruvate carboxylase isozyme I by nitrogen sources in Saccharomyces cerevisiae. Eur. J. Biochem. 2000, 267, 6817–6823. [Google Scholar] [CrossRef] [PubMed]
- Cazzulo, J.J.; Stoppani, A.O. Effects of adenosine phosphates and nicotinamide nucleotides on pyruvate carboxylase from baker’s yeast. Biochem. J. 1969, 112, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Jitrapakdee, S.; Adina-Zada, A.; Besant, P.G.; Surinya, K.H.; Cleland, W.W.; Wallace, J.C.; Attwood, P.V. Differential regulation of the yeast isozymes of pyruvate carboxylase and the locus of action of acetyl CoA. Int. J. Biochem. Cell Biol. 2007, 39, 1211–1223. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakagawa, T.; Inagaki, A.; Ito, T.; Fujimura, S.; Miyaji, T.; Yurimoto, H.; Kato, N.; Sakai, Y.; Tomizuka, N. Regulation of two distinct alcohol oxidase promoters in the methylotrophic yeast Pichia methanolica. Yeast 2006, 23, 15–22. [Google Scholar] [CrossRef]
- Fujimura, S.; Nakagawa, T.; Ito, T.; Matsufuji, Y.; Miyaji, T.; Tomizuka, N. Peroxisomal metabolism is regulated by an oxygen-recognition system through organelle crosstalk between the mitochondria and peroxisomes. Yeast 2007, 24, 491–498. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imanishi, D.; Zaitsu, S.; Takahashi, S. Regulation of d-Aspartate Oxidase Gene Expression by Pyruvate Metabolism in the Yeast Cryptococcus humicola. Microorganisms 2021, 9, 2444. https://doi.org/10.3390/microorganisms9122444
Imanishi D, Zaitsu S, Takahashi S. Regulation of d-Aspartate Oxidase Gene Expression by Pyruvate Metabolism in the Yeast Cryptococcus humicola. Microorganisms. 2021; 9(12):2444. https://doi.org/10.3390/microorganisms9122444
Chicago/Turabian StyleImanishi, Daiki, Sota Zaitsu, and Shouji Takahashi. 2021. "Regulation of d-Aspartate Oxidase Gene Expression by Pyruvate Metabolism in the Yeast Cryptococcus humicola" Microorganisms 9, no. 12: 2444. https://doi.org/10.3390/microorganisms9122444
APA StyleImanishi, D., Zaitsu, S., & Takahashi, S. (2021). Regulation of d-Aspartate Oxidase Gene Expression by Pyruvate Metabolism in the Yeast Cryptococcus humicola. Microorganisms, 9(12), 2444. https://doi.org/10.3390/microorganisms9122444





