Phenotypic and Molecular Characterization of Commensal, Community-Acquired and Nosocomial Klebsiella spp.
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Molecular Identification of Isolates
2.3. Phylogenetic Analysis
2.4. Genotyping of Isolates
2.5. Antimicrobial Susceptibility Testing
2.6. Virulence Determinants
2.7. Hypermucoviscosity, Biofilms, Siderophores and Bacteriocin Activity Assays
3. Results
3.1. Molecular Identification of Klebsiella Species
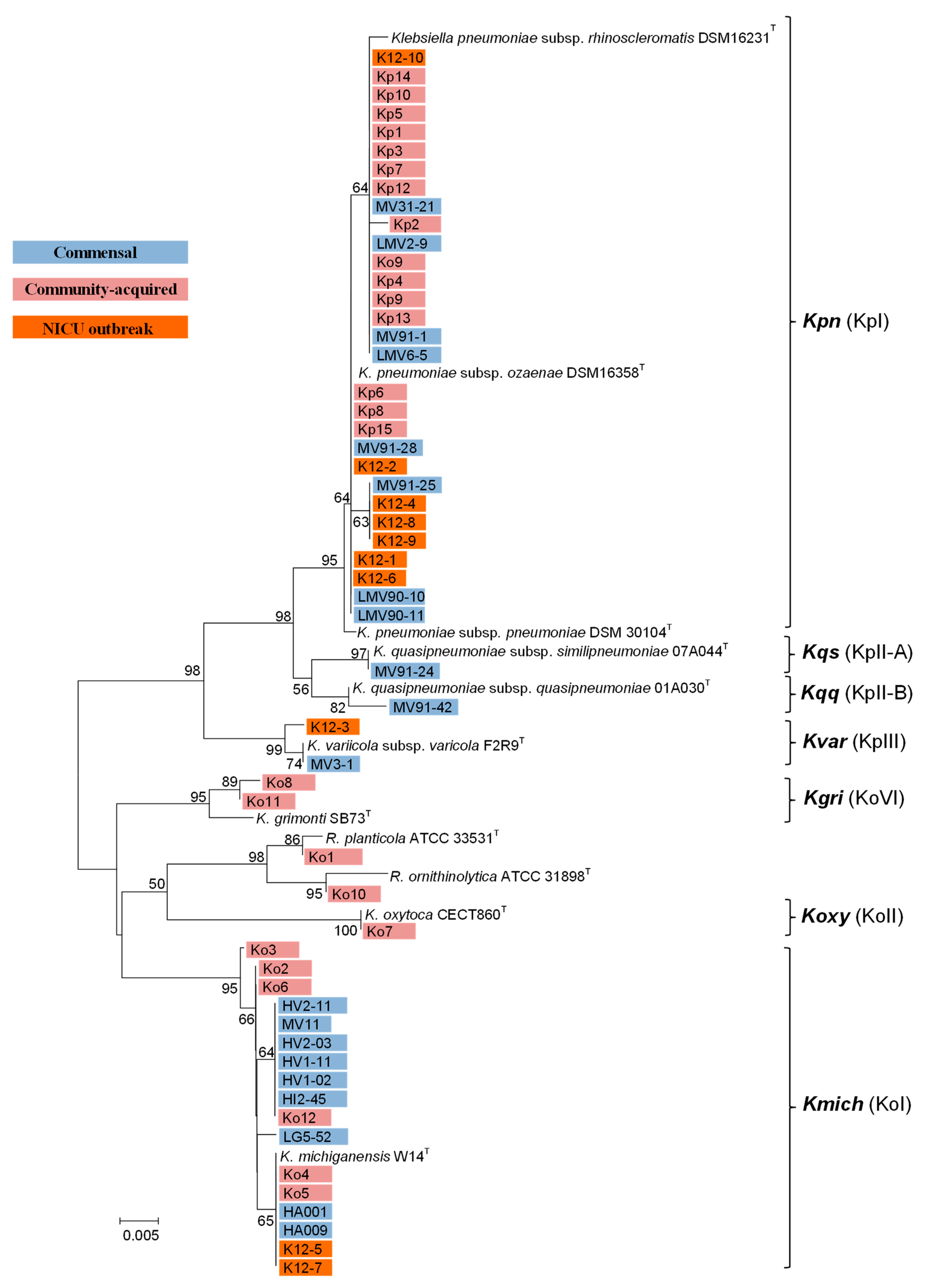
3.2. Phylogenetic Analysis Based on rpoB
3.3. Genetic Diversity
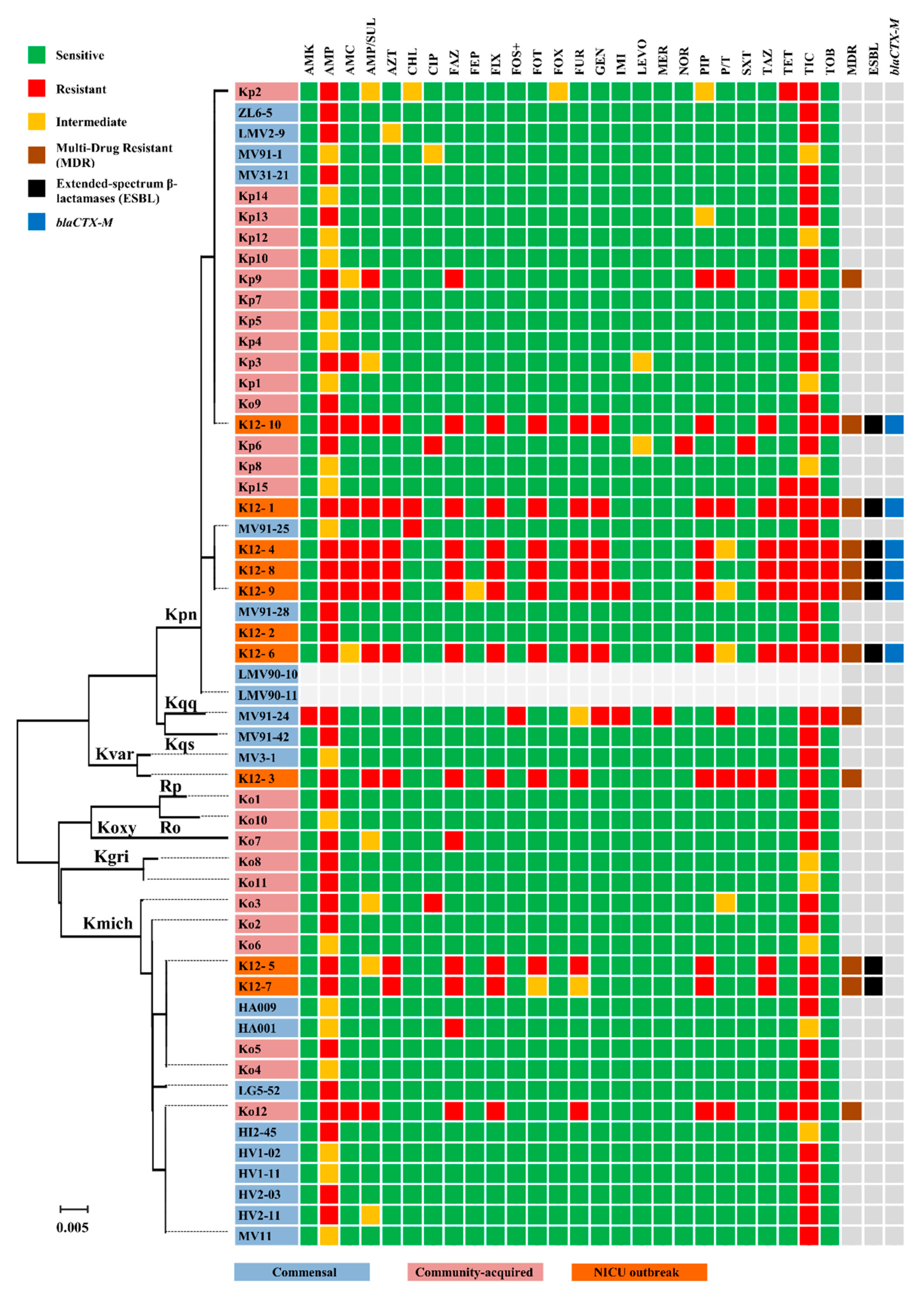
3.4. Antimicrobial Susceptibility
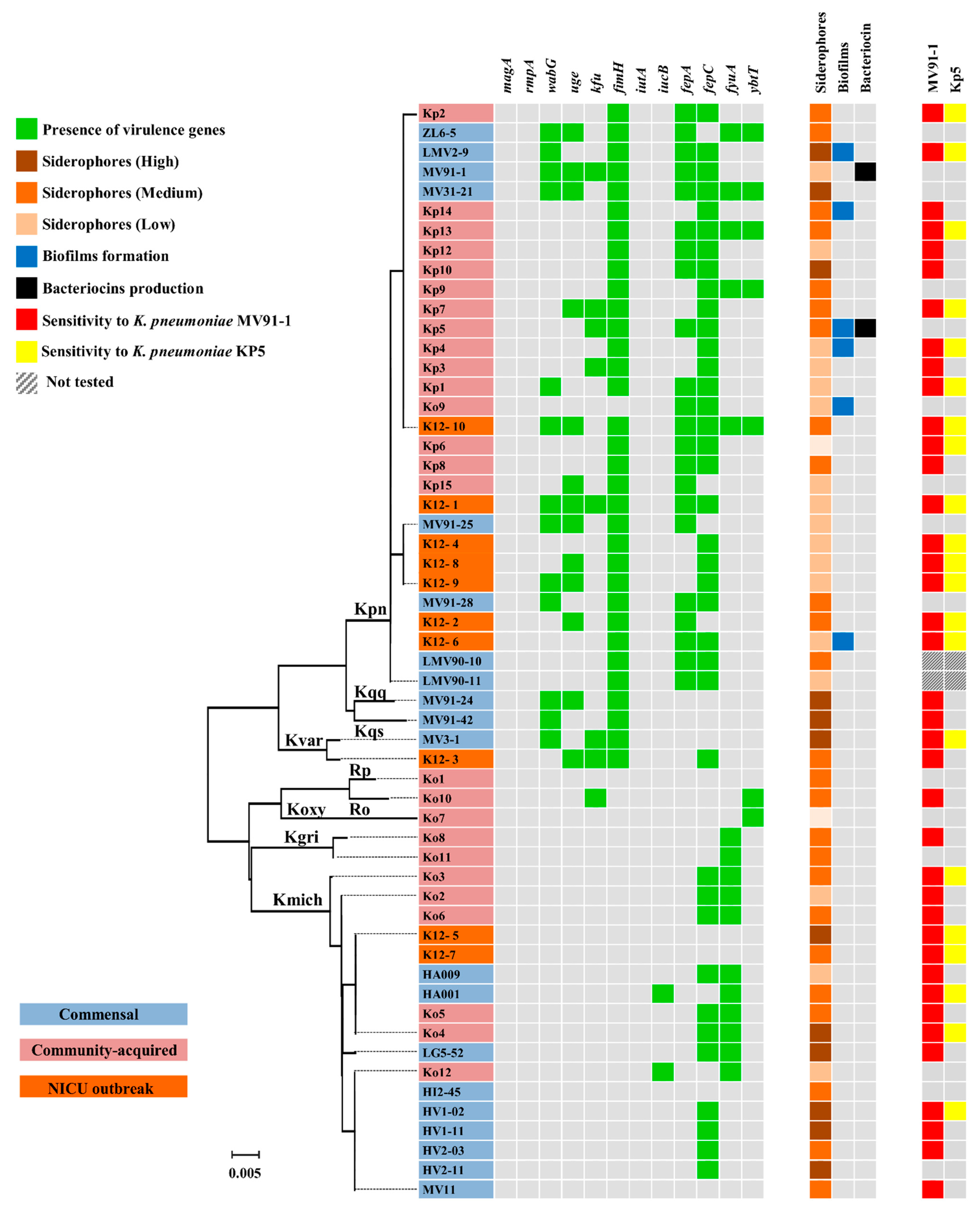
3.5. Virulence Determinants, Hypermucoviscosity, Biofilms and Siderophores
3.6. Antimicrobial Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Podschun, R.; Ullmann, U. Klebsiella spp. as nosocomial pathogens: Epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 1998, 11, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Bengoechea, J.A.; Sa Pessoa, J. Klebsiella pneumoniae infection biology: Living to counteract host defences. FEMS Microbiol. Rev. 2019, 43, 123–144. [Google Scholar] [CrossRef]
- Blin, C.; Passet, V.; Touchon, M.; Rocha, E.P.C.; Brisse, S. Metabolic diversity of the emerging pathogenic lineages of Klebsiella pneumoniae. Environ. Microbiol. 2017, 19, 1881–1898. [Google Scholar] [CrossRef]
- Brisse, S.; Verhoef, J. Phylogenetic diversity of Klebsiella pneumoniae and Klebsiella oxytoca clinical isolates revealed by randomly amplified polymorphic DNA, gyrA and parC genes sequencing and automated ribotyping. Int. J. Syst. Evol. Microbiol. 2001, 51, 915–924. [Google Scholar] [CrossRef]
- Rodrigues, C.; Passet, V.; Rakotondrasoa, A.; Diallo, T.A.; Criscuolo, A.; Brisse, S. Description of Klebsiella africanensis sp. nov., Klebsiella variicola subsp. tropicalensis subsp. nov. and Klebsiella variicola subsp. variicola subsp. nov. Res. Microbiol. 2019, 170, 165–170. [Google Scholar] [CrossRef]
- Merla, C.; Rodrigues, C.; Passet, V.; Corbella, M.; Thorpe, H.A.; Kallonen, T.V.S.; Zong, Z.; Marone, P.; Bandi, C.; Sassera, D.; et al. Description of Klebsiella spallanzanii sp. nov. and of Klebsiella pasteurii sp. nov. Front. Microbiol. 2019, 10, 2360. [Google Scholar] [CrossRef]
- Long, S.W.; Linson, S.E.; Ojeda Saavedra, M.; Cantu, C.; Davis, J.J.; Brettin, T.; Olsen, R.J. Whole-genome sequencing of human clinical Klebsiella pneumoniae isolates reveals misidentification and misunderstandings of Klebsiella pneumoniae, Klebsiella variicola, and Klebsiella quasipneumoniae. mSphere 2017, 2, e00290-17. [Google Scholar] [CrossRef]
- Chen, Y.; Brook, T.C.; Soe, C.Z.; O’Neill, I.; Alcon-Giner, C.; Leelastwattanagul, O.; Phillips, S.; Caim, S.; Clarke, P.; Hall, L.J.; et al. Preterm infants harbour diverse Klebsiella populations, including atypical species that encode and produce an array of antimicrobial resistance- and virulence-associated factors. Microb. Genom. 2020, 6, e000377. [Google Scholar] [CrossRef] [PubMed]
- Conlan, S.; Kong, H.H.; Segre, J.A. Species-level analysis of DNA sequence data from the NIH Human Microbiome Project. PLoS ONE 2012, 7, e47075. [Google Scholar] [CrossRef] [PubMed]
- Amaretti, A.; Righini, L.; Candeliere, F.; Musmeci, E.; Bonvicini, F.; Gentilomi, G.A.; Rossi, M.; Raimondi, S. Antibiotic resistance, virulence factors, phenotyping, and genotyping of non-Escherichia coli Enterobacterales from the gut microbiota of healthy subjects. Int. J. Mol. Sci. 2020, 21, 1847. [Google Scholar] [CrossRef]
- Chung, D.R.; Lee, H.; Park, M.H.; Jung, S.I.; Chang, H.H.; Kim, Y.S.; Son, J.S.; Moon, C.; Kwon, K.T.; Ryu, S.Y.; et al. Fecal carriage of serotype K1 Klebsiella pneumoniae ST23 strains closely related to liver abscess isolates in Koreans living in Korea. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Gorrie, C.L.; Mirceta, M.; Wick, R.R.; Edwards, D.J.; Thomson, N.R.; Strugnell, R.A.; Pratt, N.F.; Garlick, J.S.; Watson, K.M.; Pilcher, D.V.; et al. Gastrointestinal carriage is a major reservoir of Klebsiella pneumoniae infection in intensive care patients. Clin. Infect. Dis. 2017, 65, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.M.; Cao, J.; Brisse, S.; Passet, V.; Wu, W.; Zhao, L.; Malani, P.N.; Rao, K.; Bachman, M.A. Molecular epidemiology of colonizing and infecting isolates of Klebsiella pneumoniae. mSphere 2016, 1, e00261-16. [Google Scholar] [CrossRef]
- Clark, R.; Powers, R.; White, R.; Bloom, B.; Sanchez, P.; Benjamin, D.K., Jr. Nosocomial infection in the NICU: A medical complication or unavoidable problem? J. Perinatol. 2004, 24, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.; Langa, S.; Martín, V.; Maldonado, A.; Jiménez, E.; Martín, R.; Rodríguez, J.M. The human milk microbiota: Origin and potential roles in health and disease. Pharmacol. Res. 2013, 69, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Cruz, M.; Alba, C.; Aparicio, M.; Checa, M.Á.; Fernández, L.; Rodríguez, J.M. Effect of sample collection (manual expression vs. pumping) and skimming on the microbial profile of human milk using culture techniques and metataxonomic analysis. Microorganisms 2020, 8, 1278. [Google Scholar] [CrossRef]
- Martín, V.; Maldonado, A.; Rodríguez-Baños, M.; del Campo, R.; Rodríguez, J.M.; Jiménez, E. Sharing of bacterial strains between breast milk and infant faeces. J. Hum. Lact. 2012, 28, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Kaufmann, M.E. Pulsed-field gel electrophoresis. In Molecular Bacteriology: Protocols and Clinical Applications; Woodford, N., Johnson, A.P., Eds.; Humana Press: Totowa, NJ, USA, 1998; pp. 33–50. [Google Scholar]
- Jarlier, V.; Nicolas, M.-H.; Fournier, G.; Philippon, A. Extended broad-spectrum β-lactamases conferring transferable resistance to newer β-lactam agents in Enterobacteriaceae: Hospital prevalence and susceptibility pattern. Rev. Infect. Dis. 1988, 10, 867–878. [Google Scholar] [CrossRef]
- Woodford, N.; Fagan, E.J.; Ellington, M.J. Multiplex PCR for rapid detection of genes encoding CTX-M extended-spectrum (beta)-lactamases. J. Antimicrob. Chemother. 2006, 57, 154–155. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Kelkar, S.; Wu, W.; Chen, M.; Quinn, J.I. Clinical isolates of Enterobacteriaceae producing extended-spectrum beta-lactamases: Prevalence of CTX-M-3 at a hospital in China. Antimicrob. Agents Chemother. 2003, 47, 790–793. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Fang, C.T.; Chuang, Y.P.; Shun, C.T.; Chang, S.C.; Wang, J.T. A novel virulence gene in Klebsiella pneumoniae strains causing primary liver abscess and septic metastatic complications. J. Exp. Med. 2004, 199, 697–705. [Google Scholar] [CrossRef]
- Yu, W.L.; Ko, W.C.; Cheng, K.C.; Lee, H.C.; Ke, D.S.; Lee, C.C.; Fung, C.P.; Chuang, Y.C. Association between rmpA and magA genes and clinical syndromes caused by Klebsiella pneumoniae in Taiwan. Clin. Infect. Dis. 2006, 42, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Shon, A.S.; Bajwa, R.P.; Russo, T.A. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: A new and dangerous breed. Virulence 2013, 4, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, M. Adhesion and biofilm development of acetate-, propionate-, and butyrate-degrading microorganisms on glass surfaces. Can. J. Microbiol. 1977, 23, 1–6. [Google Scholar] [CrossRef]
- O’Toole, G.A.; Kolter, R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: A genetic analysis. Mol. Microbiol. 1998, 28, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Tagg, J.R.; Dajani, A.S.; Wannamaker, L.W. Bacteriocins of Gram-positive bacteria. Bacteriol. Rev. 1976, 40, 722–756. [Google Scholar] [CrossRef]
- Harris, L.J.; Daescheyl, M.A.; Stiles, M.E.; Klaenhammer, T.R. Antimicrobial activity of lactic acid bacteria against Listeria monocytogenes. J. Food Prot. 1999, 52, 384–887. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report 2019; ECDC: Stockholm, Sweden, 2020.
- Boye, K.; Hansen, D.S. Sequencing of 16S rDNA of Klebsiella: Taxonomic relations within the genus and to other Enterobacteriaceae. Int. J. Med. Microbiol. 2003, 292, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Drancourt, M.; Bollet, C.; Carta, A.; Rousselier, P. Phylogenetic analyses of Klebsiella species delineate Klebsiella and Raoultella gen. nov., with description of Raoultella ornithinolytica comb. nov., Raoultella terrigena comb. nov. and Raoultella planticola comb. nov. Int. J. Syst. Evol. Microbiol. 2001, 51, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Garza-Ramos, U.; Barrios-Camacho, H.; Moreno-Domínguez, S.; Toribio-Jiménez, J.; Jardón-Pineda, D.; Cuevas-Peña, J.; Sánchez-Pérez, A.; Duran-Bedolla, J.; Olguín-Rodriguez, J.; Román-Román, A. Phenotypic and molecular characterization of Klebsiella spp. isolates causing community-acquired infections. New Microbes New Infect. 2018, 23, 17–27. [Google Scholar] [CrossRef]
- Hajjar, R.; Ambaraghassi, G.; Sebajang, H.; Schwenter, F.; Su, S.H. Raoultella ornithinolytica: Emergence and resistance. Infect. Drug Resist. 2020, 13, 1091–1104. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, H.; Pervin, N.; Israr Ul Haq, M.; Kamal, K.R.; Marwat, A.; Khan, M. A rare case of Raoultella planticola urinary tract infection in a patient with immunoglobulin a nephropathy. J. Investig. Med. High Impact Case Rep. 2018, 6, 2324709618780422. [Google Scholar]
- Chapman, P.; Forde, B.M.; Roberts, L.W.; Bergh, H.; Vesey, D.; Jennison, A.V.; Moss, S.; Paterson, D.L.; Beatson, S.A.; Harris, P.N.A. Genomic Investigation reveals contaminated detergent as the source of an extended-spectrum-β-lactamase-producing Klebsiella michiganensis outbreak in a neonatal unit. J. Clin. Microbiol. 2020, 58, e01980-19. [Google Scholar] [CrossRef]
- Rodríguez-Medina, N.; Barrios-Camacho, H.; Duran-Bedolla, J.; Garza-Ramos, U. Klebsiella variicola: An emerging pathogen in humans. Emerg. Microbes Infect. 2019, 8, 973–988. [Google Scholar] [CrossRef]
- Perdigão, J.; Caneiras, C.; Elias, R.; Modesto, A.; Spadar, A.; Phelan, J.; Campino, S.; Clark, T.G.; Costa, E.; Saavedra, M.J.; et al. Genomic epidemiology of carbapenemase producing Klebsiella pneumoniae strains at a northern Portuguese hospital enables the detection of a misidentified Klebsiella variicola KPC-3 producing strain. Microorganisms 2020, 8, 1986. [Google Scholar] [CrossRef]
- Nordmann, P.; Cuzon, G.; Naas, T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect. Dis. 2009, 9, 228–236. [Google Scholar] [CrossRef]
- Fiett, J.; Palucha, A.; Miaczynska, B.; Stankiewicz, M.; Przondo-Mordarska, H.; Hryniewicz, W.; Gniadkowski, M. A novel complex mutant beta-lactamase, TEM-68, identified in a Klebsiella pneumoniae isolate from an outbreak of extended-spectrum beta-lactamase-producing Klebsiellae. Antimicrob. Agents Chemother. 2000, 44, 1499–1505. [Google Scholar] [CrossRef][Green Version]
- Bingen, E.H.; Desjardins, P.; Arlet, G.; Bourgeois, F.; Mariani-Kurkdjian, P.; Lambert-Zechovsky, N.Y.; Denamur, E.; Philippon, A.; Elion, J. Molecular epidemiology of plasmid spread among extended broad-spectrum beta-lactamase-producing Klebsiella pneumoniae isolates in a pediatric hospital. J. Clin. Microbiol. 1993, 31, 179–184. [Google Scholar] [CrossRef] [PubMed]
- San Millan, A. Evolution of plasmid-mediated antibiotic resistance in the clinical context. Trends Microbiol. 2018, 26, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Hennequin, C.; Robin, F. Correlation between antimicrobial resistance and virulence in Klebsiella pneumoniae. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 333–341. [Google Scholar] [CrossRef]
- Beceiro, A.; Tomás, M.; Bou, G. Antimicrobial resistance and virulence: A successful or deleterious association in the bacterial world? Clin. Microbiol. Rev. 2013, 26, 185–230. [Google Scholar] [CrossRef] [PubMed]
- Brisse, S.; Fevre, C.; Passet, V.; Issenhuth-Jeanjean, S.; Tournebize, R.; Diancourt, L.; Grimont, P. Virulent clones of Klebsiella pneumoniae: Identification and evolutionary scenario based on genomic and phenotypic characterization. PLoS ONE 2009, 4, e4982. [Google Scholar] [CrossRef]
- Izquierdo, L.; Coderch, N.; Piqué, N.; Bedini, E.; Corsaro, M.M.; Merino, S.; Fresno, S.; Tomas, J.M.; Regué, M. The Klebsiella pneumoniae wabG gene: Role in biosynthesis of the core lipopolysaccharide and virulence. J. Bacteriol. 2003, 185, 7213–7221. [Google Scholar] [CrossRef]
- Regué, M.; Hita, B.; Piqué, N.; Izquierdo, L.; Merino, S.; Fresno, S.; Benedí, V.J.; Tomás, J.M. A gene, uge, is essential for Klebsiella pneumoniae virulence. Infect. Immun. 2004, 72, 54–61. [Google Scholar] [CrossRef]
- Fursova, N.K.; Astashkin, E.I.; Ershova, O.N.; Aleksandrova, I.A.; Savin, I.A.; Novikova, T.S.; Fedyukina, G.N.; Kislichkina, A.A.; Fursov, M.V.; Kuzina, E.S.; et al. Multidrug-resistant Klebsiella pneumoniae causing severe infections in the neuro-ICU. Antibiotics 2021, 10, 979. [Google Scholar] [CrossRef]
- Ma, L.C.; Fang, C.T.; Lee, C.Z.; Shun, C.T.; Wang, J.T. Genomic heterogeneity in Klebsiella pneumoniae strains is associated with primary pyogenic liver abscess and metastatic infection. J. Infect. Dis. 2005, 192, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Dogan, O.; Vatansever, C.; Atac, N.; Albayrak, O.; Karahuseyinoglu, S.; Sahin, O.E.; Kilicoglu, B.K.; Demiray, A.; Ergonul, O.; Gönen, M.; et al. Virulence determinants of colistin-resistant K. pneumoniae high-risk clones. Biology (Basel) 2021, 10, 436. [Google Scholar] [CrossRef] [PubMed]
- Kramer, J.; Özkaya, Ö.; Kümmerli, R. Bacterial siderophores in community and host interactions. Nat. Rev. Microbiol. 2020, 18, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocins: Developing innate immunity for food. Nat. Rev. Microbiol. 2005, 3, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Denkovskienė, E.; Paškevičius, Š.; Misiūnas, A.; Stočkūnaitė, B.; Starkevič, U.; Vitkauskienė, A.; Hahn-Löbmann, S.; Schulz, S.; Giritch, A.; Gleba, Y.; et al. Broad and efficient control of Klebsiella pathogens by peptidoglycan-degrading and pore-forming bacteriocins klebicins. Sci. Rep. 2019, 9, 15422. [Google Scholar] [CrossRef]
- Martin, R.M.; Bachman, M.A. Colonization, Infection, and the Accessory Genome of Klebsiella pneumoniae. Front. Cell. Infect. Microbiol. 2018, 8, 4. [Google Scholar] [CrossRef]
- Ghenea, A.E.; Cioboată, R.; Drocaş, A.I.; Țieranu, E.N.; Vasile, C.M.; Moroşanu, A.; Țieranu, C.G.; Salan, A.-I.; Popescu, M.; Turculeanu, A.; et al. Prevalence and Antimicrobial Resistance of Klebsiella Strains Isolated from a County Hospital in Romania. Antibiotics 2021, 10, 868. [Google Scholar] [CrossRef]
- Dorman, M.J.; Short, F.L. Genome watch: Klebsiella pneumoniae: When a colonizer turns bad. Nat. Rev. Microbiol. 2017, 15, 384. [Google Scholar] [CrossRef]
- Arroyo, R.; Martín, V.; Maldonado, A.; Jiménez, E.; Fernández, L.; Rodríguez, J.M. Treatment of infectious mastitis during lactation: Antibiotics versus oral administration of Lactobacilli isolated from breast milk. Clin. Infect. Dis. 2010, 50, 1551–1558. [Google Scholar] [CrossRef]
- Maldonado, A.; Ruiz-Barba, J.L.; Jiménez-Díaz, R. Purification and genetic characterization of plantaricin NC8, a novel coculture-inducible two-peptide bacteriocin from Lactobacillus plantarum NC8. Appl. Environ. Microbiol. 2003, 69, 383–389. [Google Scholar] [CrossRef]
- Bengtsson, T.; Selegård, R.; Musa, A.; Hultenby, K.; Utterström, J.; Sivlér, P.; Skog, M.; Nayeri, F.; Hellmark, B.; Söderquist, B.; et al. Plantaricin NC8 αβ exerts potent antimicrobial activity against Staphylococcus spp. and enhances the effects of antibiotics. Sci. Rep. 2020, 10, 3580. [Google Scholar] [CrossRef] [PubMed]

| Strains | Biochemical ID | rpoB (% Identity) | Origin | Source * |
|---|---|---|---|---|
| Commensal | ||||
| HA001 | K. oxytoca | K. michiganensis (100) | Faeces | UCM |
| HA009 | K. oxytoca | K. michiganensis (100) | Faeces | UCM |
| HI2-45 | K. oxytoca | K. michiganensis (100) | Faeces | UCM |
| HV1-02 | K. oxytoca | K. michiganensis (99.52) | Faeces | UCM |
| HV1-11 | K. oxytoca | K. michiganensis (99.52) | Faeces | UCM |
| HV2-03 | K. oxytoca | K. michiganensis (99.52) | Faeces | UCM |
| HV2-11 | K. oxytoca | K. michiganensis (99.52) | Faeces | UCM |
| LG5-52 | K. oxytoca | K. michiganensis (99.52) | Milk | UCM |
| MV11 | K. oxytoca | K. michiganensis (99.52) | Meconium | UCM |
| LMV2-9 | K. pneumoniae | K. pneumoniae (99.76) | Milk | UCM |
| LMV6-5 | K. pneumoniae | K. pneumoniae (99.76) | Milk | UCM |
| LMV90-10 | K. pneumoniae | K. pneumoniae (100) | Milk | UCM |
| LMV90-11 | K. pneumoniae | K. pneumoniae (100) | Milk | UCM |
| MV3-1 | K. pneumoniae | K. variicola (100) | Faeces | UCM |
| MV31-21 | K. pneumoniae | K. pneumoniae (99.76) | Faeces | UCM |
| MV91-1 | K. pneumoniae | K. pneumoniae (99.76) | Faeces | UCM |
| MV91-24 | K. pneumoniae | K. quasipneumoniae subsp. similipneumoniae (100) | Faeces | UCM |
| MV91-25 | K. pneumoniae | K. pneumoniae (99.76) | Faeces | UCM |
| MV91-28 | K. pneumoniae | K. pneumoniae (100) | Faeces | UCM |
| MV91-42 | K. pneumoniae | K. quasipneumoniae subsp. quasipneumoniae (99.52) | Faeces | UCM |
| Community-acquired | ||||
| Ko1 | K. pneumoniae | R. planticola (100) | Blood culture | RYC |
| Ko2 | K. oxytoca | K. michiganensis (99.76) | Blood culture | RYC |
| Ko3 | K. oxytoca | K. michiganensis (99.52) | Blood culture | RYC |
| Ko4 | K. oxytoca | K. michiganensis (100) | Blood culture | RYC |
| Ko5 | K. oxytoca | K. michiganensis (100) | Blood culture | RYC |
| Ko6 | K. oxytoca | K. michiganensis (99.76) | Blood culture | RYC |
| Ko7 | K. oxytoca | K. oxytoca (100) | Blood culture | RYC |
| Ko8 | K. oxytoca | K. grimontii (98.80) | Blood culture | RYC |
| Ko9 | K. oxytoca | K. pneumoniae (99.76) | Blood culture | RYC |
| Ko10 | K. oxytoca | R. ornithinolytica (100) | Blood culture | RYC |
| Ko11 | K. oxytoca | K. grimontii (99.04) | Blood culture | RYC |
| Ko12 | K. oxytoca | K. michiganensis (99.52) | Blood culture | RYC |
| Kp1 | K. pneumoniae | K. pneumoniae (99.76) | Blood culture | RYC |
| Kp2 | K. pneumoniae | K. pneumoniae (99.52) | Blood culture | RYC |
| Kp3 | K. pneumoniae | K. pneumoniae (99.76) | Blood culture | RYC |
| Kp4 | K. pneumoniae | K. pneumoniae (99.76) | Blood culture | RYC |
| Kp5 | K. pneumoniae | K. pneumoniae (99.76) | Blood culture | RYC |
| Kp6 | K. pneumoniae | K. pneumoniae (100) | Blood culture | RYC |
| Kp7 | K. pneumoniae | K. pneumoniae (99.76) | Blood culture | RYC |
| Kp8 | K. pneumoniae | K. pneumoniae (100) | Blood culture | RYC |
| Kp9 | K. pneumoniae | K. pneumoniae (99.76) | Blood culture | RYC |
| Kp10 | K. pneumoniae | K. pneumoniae (99.76) | Blood culture | RYC |
| Kp12 | K. pneumoniae | K. pneumoniae (99.76) | Blood culture | RYC |
| Kp13 | K. pneumoniae | K. pneumoniae (99.76) | Blood culture | RYC |
| Kp14 | K. pneumoniae | K. pneumoniae (99.76) | Blood culture | RYC |
| Kp15 | K. pneumoniae | K. pneumoniae (100) | Blood culture | RYC |
| NICU outbreak | ||||
| K12-1 | K. pneumoniae | K. pneumoniae (100) | Blood culture | HUDO |
| K12-2 | K. pneumoniae | K. pneumoniae (100) | Blood culture | HUDO |
| K12-3 | K. pneumoniae | K. variicola (99.52) | NICU environment | HUDO |
| K12-4 | K. pneumoniae | K. pneumoniae (99.76) | NICU environment | HUDO |
| K12-5 | K. pneumoniae | K. michiganensis (100) | Faeces | HUDO |
| K12-6 | K. pneumoniae | K. pneumoniae (100) | Vascular catheter | HUDO |
| K12-7 | K. oxytoca | K. michiganensis (100) | Faeces | HUDO |
| K12-8 | K. pneumoniae | K. pneumoniae (99.76) | Faeces | HUDO |
| K12-9 | K. pneumoniae | K. pneumoniae (99.76) | Faeces | HUDO |
| K12-10 | K. pneumoniae | K. pneumoniae (99.76) | Faeces | HUDO |
| Reference type strains (T) | ||||
| DSM 30104T | K. pneumoniae subsp. pneumoniae | K. pneumoniae (100) | Unknown | DSMZ |
| CECT 142 | K. pneumoniae subsp. pneumoniae | K. pneumoniae (99.76) | Unknown | CECT |
| CECT 517 | K. pneumoniae subsp. pneumoniae | K. pneumoniae (100) | Urine | CECT |
| DSM 16231T | K. pneumoniae subsp. rhinoscleromatis | K. pneumoniae (100) | Nose rhinoscleroma | DSMZ |
| DSM 16358T | K. pneumoniae subsp. ozaenae | K. pneumoniae (100) | Nose | DSMZ |
| CECT 860T | K. oxytoca | K. oxytoca (100) | Pharyngeal tonsil | CECT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez, M.; Valverde, A.; del Campo, R.; Rodríguez, J.M.; Maldonado-Barragán, A. Phenotypic and Molecular Characterization of Commensal, Community-Acquired and Nosocomial Klebsiella spp. Microorganisms 2021, 9, 2344. https://doi.org/10.3390/microorganisms9112344
Gómez M, Valverde A, del Campo R, Rodríguez JM, Maldonado-Barragán A. Phenotypic and Molecular Characterization of Commensal, Community-Acquired and Nosocomial Klebsiella spp. Microorganisms. 2021; 9(11):2344. https://doi.org/10.3390/microorganisms9112344
Chicago/Turabian StyleGómez, Marta, Arancha Valverde, Rosa del Campo, Juan Miguel Rodríguez, and Antonio Maldonado-Barragán. 2021. "Phenotypic and Molecular Characterization of Commensal, Community-Acquired and Nosocomial Klebsiella spp." Microorganisms 9, no. 11: 2344. https://doi.org/10.3390/microorganisms9112344
APA StyleGómez, M., Valverde, A., del Campo, R., Rodríguez, J. M., & Maldonado-Barragán, A. (2021). Phenotypic and Molecular Characterization of Commensal, Community-Acquired and Nosocomial Klebsiella spp. Microorganisms, 9(11), 2344. https://doi.org/10.3390/microorganisms9112344







