Abstract
Beehives are populated by bacterial species with a protective role against honey bee pathogens thanks to the production of bioactive metabolites. These compounds are largely unexploited despite their high potential interest for pest management. This study evaluated the capability of bacterial species associated with honey bees to produce 2-heptanone, a volatile organic compound with anesthetic properties of the parasitic mite Varroa destructor. The production of this compound was quantified by SPME-GC-MS in a culture filtrate of nine bacterial strains isolated from the surface of honey bees, and the biosynthetic potential was evaluated in bacterial species associated with apiaries by searching for protein homologs putatively involved in its biosynthesis by using biocomputational tools. The findings pointed out that 2-heptanone was produced by Acetobacteraceae bacterium, Bacillus thuringiensis and Apilactobacillus kunkeei isolates in concentrations between 1.5 and 2.6 ng/mL and that its production was strain-specific. Putative methylketone synthase homologs were found in Bacillus, Gilliamella, Acetobacteraceae, Bartonella and Lactobacillaceae, and the protein sequence results were distributed in nine Sequence Similarity Network (SSN) clusters. These preliminary results support the hypothesis that 2-heptanone may act as a mediator of microbial relationships in hives and provide contributions to assess the role and biosynthetic potential of 2-heptanone in apiaries.
1. Introduction
Honey bees (Apis mellifera L., Insecta: Hymenoptera) are managed insects of economic and environmental importance for their role in pollination and for hive products. Over the past few decades in Europe and North America, their health has been threatened by multiple issues, including diseases, parasites, pesticides and environmental and socioeconomic factors [1]. Pests and pathogens, among which the mite Varroa destructor is one of the main causes of colony loss [2], have been controlled in the past years with the use of synthetic chemicals, but nowadays, the search for alternative strategies is of extreme interest to reduce the side effects and the development of resistance [3]. Natural products synthetized by living organisms are valuable resources that can be sustainable alternatives to chemicals [4]. Hundreds of signaling chemicals used by plants, insects and microorganisms for transmitting information between individuals of the same species or of different species (semiochemicals) have been discovered, but their use in pest management is still largely unexploited, despite the high potential interest [5,6]. In the hive environment, chemical signals from multiple biological interactions are countless [6,7,8]. Hundreds of VOCs have been identified in hive products, but studies have focused mainly on their involvement in the aroma of honey and on their use to obtain information on the botanical and geographical origins of honey [9]. Few studies have investigated the functional role of specific volatile compounds in plant–pollinator–predator–microorganism interactions. In this study, we focused on a semiochemical of honey bees, 2-heptanone (heptan-2-one or methyl n-amyl ketone). This methylketone secreted by the mandibular glands of worker honey bees has a debated role; it is thought to signal aversive situations by inducing defensive responses [10], to act as a chemical marker on visited flowers [11] or even as an adaptative modulator of learning and memory [12]. It has been recently discovered that honey bees use their mandibles to bite and remove Varroa mites from the bodies of their nest mates and that the released 2-heptanone acts as a local anesthetic, causing paralysis and death of this parasite [13]. The use of 2-heptanone as a mite repellent has been proposed, taking into consideration that this compound would not be toxic towards bees or contaminate hive products, since it already exists in hives [14,15].
Methylketones can be synthetized by bacteria, fungi, plants, insects and mammals and have been found in numerous natural environments, having various biological roles [16]. In plants, for example, these compounds are highly effective for protection against pests [17]. 2-heptanone produced by lactic acid bacteria (i.e., Lactobacillus casei and Lactobacillus paracasei) is known to contribute to aroma development in many dairy products [18]. Furthermore, this volatile compound was found to be synthetized by species of Bacillus during host–pathogen interactions [16] and by Bacillus amyloliquefaciens with antifungal properties against Fusarium oxysporum [19]. Even though this volatile compound has various bioactive roles of potential practical interest, its biosynthetic pathway has been scarcely investigated. In 2005, two methylketone synthetases from wild tomatoes (Lycopersicon hirsutum) were reported to use intermediates of the fatty acids biosynthetic pathway to synthesize methylketones [17]. In bacteria, a methylketone synthase involved in the production of 2-heptanone was identified in Bacillus nematocida [16].
The mediation of extracellular bacterial metabolites in beneficial interactions with honey bees has been reported [20,21], but little is known on VOCs produced by bacterial populations inhabiting hives. The microbiota associated with honey bees has lately been the subject of an increasing number of studies, and the most frequently reported taxa include Lactobacillaceae, Bacillaceae, Acetobacteraceae, Bifidobacteriaceae, Gilliamella and Fructobacillus [22,23,24,25,26,27,28].
Hence, the aims of this study were to (i) establish and quantify the production of 2-heptanone in the culture filtrate by bacterial species isolated from honey bees and (ii) investigate the potential of honey bee-associated bacteria to produce this natural compound with a protective role against hive pathogens by using biocomputational tools.
2. Materials and Methods
2.1. Bacterial Cultures
Nine bacterial strains isolated from the surfaces of healthy honey bees in a previous study [27] were selected based on their abundance in the microbiota of bees for testing their capability to produce 2-heptanone in a liquid culture. The selected isolates from the CREA-AA bacterial collection were the following: two strains of Acetobacteraceae bacterium (BO_L(L)1 and IM_G(L)3); three strains of Bacillus thuringiensis (BI_G1, PD_L1 and RN_G(L)2); one of Bifidobacterium asteroides (LE_V(L)2) and three Apilactobacillus kunkeei (BO_G1, LE_L(L)2 and LG_V1). They were stored in FGYP broth [29] with 20% (v/v) glycerol at −80 °C, and prior to use, bacterial colonies were regenerated in the same medium containing 10 g of D-fructose, 10 g of D-glucose, 10 g of yeast extract, 5 g of polypeptone, 2 g of sodium acetate, 0.5 g of Tween 80, 0.2 g of MgSO4∗7H2O, 0.01 g of MnSO4∗4H2O, 0.01 g of FeSO4∗7H2O and 0.01 g of NaCl per liter, pH 6.8. This medium was the same selective medium used for isolating bacterial strains from honey bees; the use of fructose and glucose as growth substrates supported the optimal growth, considering that these isolates originated from a sugar-rich environment such as an apiary [27]. Liquid cultures for the analysis of 2-heptanone were obtained by growing bacterial isolates in FGYP broth (50 mL) at 30 °C under aerobic conditions and shaking at 125 rpm for three days to permit the fermentation of carbohydrates and accumulation of byproducts. After incubation, the concentration of the cells in the stationary phase was around 107 cell/mL (OD 0.8). The cell-free supernatants (CFS) were recovered from the fermentation broth by centrifuging (14,000× g, 10 min), filter-sterilizing (using cellulose acetate syringe filters, 0.22-µm pore size, GVS Life Sciences, Bologna, Italy) and keeping them at −80 °C until analysis.
2.2. GC-MS Analysis of 2-Heptanone
The analysis was performed following the method of Reference [16], with modifications. Each sample was composed of 10 mL of CFS in a 20-mL glass vial, with 2 g of NaCl added and closed with an aluminum–silicone/PTFE septum. The extraction was carried out using a DVB/CAR/PDMS solid-phase micro extraction (SPME) fiber (Supelco, Milan, Italy) exposed to the sample headspace at 60 °C for 30 min. Volatile compound desorption was obtained by exposing the fiber in the GC injector at 200 °C for 5 min. GC-MS analyses were carried out with an Agilent 6890 N GC connected to an Agilent 5973 mass spectrometer (Agilent Technologies, Cernusco sul Naviglio, Italy) and equipped with a DB-1 column (60 m × 0.25 mm I.D., film thickness 0.25 µm) in splitless mode using He as the carrier gas (flow 1 mL/min). The column temperature program was: 40 °C for 5 min, 3 °C/min to 180 °C and 8 °C/min to 220 °C for 5 min. The injector and detector temperatures were 200 and 230 °C, respectively, interconnecting the line temperature at 200 °C. The MS settings were as follows: filament voltage, 70 eV; scan range, 39–450 amu and scan speed, 1.4 scan/s. Uninoculated growth medium was used as the control. 2-Heptanone was identified by comparing its mass spectra with that stored in the Wiley 7n library and analyzing the authentic standard. Quantification was performed by the interpolation of a calibration curve made with known concentrations of 2-heptanone (Sigma-Aldrich, Italy), and the values were expressed as ng/mL; the detection limit was below 0.2 ng/mL.
2.3. Sequence Analysis
A protein sequence BLAST was performed against the nonredundant protein database using acyl-CoA thioester hydrolase QBO55937 as the input to search for potential homologs in bacterial species populating apiaries. The search was oriented towards species reported to be associated with honey bees [25,26,27]. The following organisms were included in the search: Acetobacteraceae (taxid:433), Bacillus (taxid:1386), Bartonella (taxid:773), Bifidobacteriaceae (taxid:31953), Bombella (taxid:1654741), Fructobacillus (taxid:559173), Gilliamella (taxid:1193503), Lactobacillus (taxid:1578), Lactobacillus kunkeei (taxid:148814), Leuconostoc (taxid:1243), Parasaccharibacter (taxid:1602345) and Snodgrassella (taxid:1193515). The sequence of a thioesterase-like protein reported to synthesize methylketone using intermediates of the fatty acids biosynthetic pathway from L. hirsutum was also included in the dataset.
The GenBank protein accession IDs of 72 sequences, with a minimum 23.5% identity, 3×10−8 E-values and 52% query coverage, were then submitted to the Enzyme Similarity Tool for generating Sequence Similarity Networks (SSNs) for visualization of the relationships among the protein sequences by grouping together the most related proteins in the clusters [30]. An alignment score threshold of 35 and a minimum length of 100 and maximum length 280 were set up for the analysis, and the networks were visualized in Cytoscape (v3.80). The obtained SSN was then used as the input for generating the Genome Neighborhood Diagrams (GNDs).
The evolutionary distances of the amino acid sequences were computed in MEGA7 [31] using the Poisson correction model with 1000 bootstrap replications [32].
3. Results and Discussion
3.1. 2-Heptanone Production in Bacterial Cultures
This study aimed at the identification and quantification, in bacterial culture filtrates, of 2-heptanone, whose protective role against honey bee pathogens has been previously reported [13]. The GC-MS analysis of CFS from the selected bacterial strains revealed a complex mixture of volatile compounds; among them, 2-heptanone was found at the retention time of 19.10 min (Figure 1). The gas chromatographic profile comprised many other compounds potentially interesting for their bioactivity; among which were 2,5-dimethylpyrazine at a retention time of 20.46 min, phenyl methanol (retention time of 26.90 min) and phenyl ethanol (retention time of 31.13 min). Interestingly, ant-associated bacteria have been reported to produce volatile pyrazines, including 2,5-dimethylpyrazine, previously identified as ant trail and alarm pheromones [33]. These compounds will be the object of further investigations on bioactive volatiles produced by hive-associated bacteria. Most of the other identified peaks are referred to as volatile fatty acids produced by microbial fermentation.
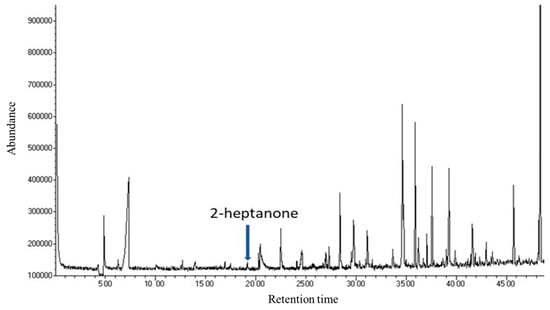
Figure 1.
GC-MS chromatogram of the Acetobacteraceae bacterium strain IM_G(L)3 culture filtrate.
The mass spectra of 2-heptanone from the extract of bacterial culture filtrates corresponded to that from the synthetic standard and to the spectrum in the Wiley 7n library, thus confirming the specificity of the results (Figure 2).

Figure 2.
Comparison of the mass spectra of 2-heptanone from the SPME extract of the Acetobacteraceae bacterium strain IM_G(L)3 culture filtrate (a) and from the synthetic standard (b) to the spectrum stored in the Wiley 7n library (c). m/z = mass/charge.
2-heptanone was found to be produced by both tested A. bacterium strains, by one B. thuringiensis and by one A. kunkeei in concentrations ranging from 1.5 to 2.6 ng/mL (Table 1). Traces were found in the culture filtrate of B. asteroides and of the other two A. kunkeei strains. The production of this compound therefore seemed to be strain-specific in the case of B. thuringiensis and A. kunkeei, since it was found only in one out of three isolates in both cases.

Table 1.
Concentration of 2-heptanone in the bacterial culture filtrates (average of 2 replicates ± standard deviation). Tr = traces and n.d. = not detected.
Aliphatic ketones are historically known as insect alarm pheromones. Previous quantitative data, measured on insects, gave higher concentrations of these ketones than the present ones. In extracts of heads of Atta texana and Atta cephalotes ants, 2-heptanone was determined at around 160 ppb and in trace amounts, respectively [34]. In hives, the role of 2-heptanone appears to depend on its concentration; it is thought to be attractive in low concentrations and repulsive in higher concentrations, but contrasting results have been reported so far. It was originally hypothesized that this compound, secreted from the glands in honey bee mandibles, acted as an alarm pheromone stimulating defensive reactions against a potential threat [35,36]. Nevertheless, Papachristoforou and colleagues (2012) [13] demonstrated that it triggered no defensive responses when applied at colony entrances in doses of 0.001, 10 and 1000 µL, whilst it acted as a local anesthetic of Varroa mites when 0.061 µL of pure compound were applied topically on mites. In hive applications of up to 500 µL of pure 2-heptanone, it resulted that at no time did all bees fully or permanently exit the observation hive [14]. The low concentrations of 2-heptanone produced by the bacterial strains investigated in this study (<3 ng/mL), together with the high volatility of this compound, imply no side effects on the bees. Furthermore, in hives, its evaporation due to high temperature and humidity would be so quick that an investigation was specifically performed to maintain the compound longer to use it as a mite repellent [15]. The results of this study rather suggest that 2-heptanone may have a role in microbial chemical communication, and further studies are needed for the exploitation of 2-heptanone-producing bacteria for the control of honey bee pests and pathogens.
In this study, 2-heptanone production by A. bacterium was reported for the first time. This result is particularly interesting, since this species has been found to be associated with honey bees in different environments and suggested to have a beneficial role towards these insects [26]. Within Lactobacillaceae, 2-heptanone has been previously found to be produced by a honey bee bacterial symbiont Apilactobacillus apinorum, which showed antimicrobial properties against clinical isolates of pathogenic wound bacteria [37]; this is the first report of 2-heptanone production by A. kunkeei.
3.2. Biosynthetic Genes
The investigation of methylketone synthase proteins showed that putative homologs are encoded in the genomes of the bacterial taxa populating apiaries, such as Acetobacteraceae, Bacillus, Bartonella, Bombella, Gilliamella and Parasaccharibacter and that no significant similarities were found by restricting the search to A. kunkeei, Bifidobacterium, Fructobacillus, Leuconostoc and Snodgrassella. The presence of 2-heptanone in the culture filtrates of A. kunkeei and of B. asteroides in traces suggests that these species may use a different biosynthetic pathway to synthetize this compound yet to be described. After length filtering, 68 sequences were submitted to SSN analysis and resulted distributed in nine SSN clusters and one singleton (Figure 3 and Table 2). The only protein that did not cluster with the others was that of the wild tomato L. hirsutum, suggesting that putative methylketone synthase homologs evolved within the bacterial phylum, and a coevolutionary relation between honey bees and its associated microbiota may be hypothesized.
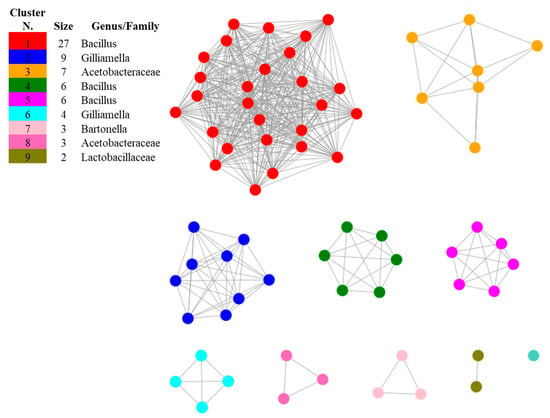
Figure 3.
Sequence Similarity Network (SSN) showing the relationships among the protein sequences of putative homologs of methylketone synthase in bacterial species inhabiting apiaries. The most related proteins are grouped together in clusters. The singleton was constituted by the sequence of Lycopersicon hirsutum included in the dataset as the reference of a methylketone synthase described in plants.

Table 2.
List of potential methylketone homologs and their distribution in clusters, as calculated by the Sequence Similarity Network (SSN).
The first biggest cluster was composed by proteins from different species of Bacillus, including B. nematocida (Table 2), indicating that the proteins that are mostly related with putative methylketone synthase used as starting point in this study are found within this genus. The second cluster composed exclusively by Gilliamella apicola indicates that this species, widely distributed in the honey bee environment [23,24], is a potential producer of 2-heptanone. The compositions of the other minor clusters suggests that putative methylketone synthase homologs may be found in Acetobacteraceae, as well as in Bartonella apis, Parasaccharibacter apium and Bombella apis, well-known symbionts of honey bees [23,25,38,39], and, among Lactobacillaceae, in Pediococcus acidilactici, recently shown to have a protective effect towards honey bees [40].
Most of the bacterial thioesterases described in Table 2 have yet to be functionally characterized. The role of these enzymes has been studied more extensively in plants, where acyl thioesterases are reported to produce fatty acids that play roles in plant–insect and plant–microbial interactions [41]. A similar role of these enzymes in bacteria–bacteria and bacteria–insect interactions may be hypothesized and be the object of further studies.
The overall mean distance of the sequences listed in Table 2 was 1.33 ± 0.07, and the pairwise distance of the representative sequences from each cluster highlighted that the most diverse were those of B. apis from cluster 7 and B. cereus from cluster 4 (Figure 4).

Figure 4.
Estimates of the evolutionary divergences between a selection of representative protein sequences from each cluster. UniProt ID, cluster number and bacterial species are reported for each sequence. The number of amino acid substitutions per site from between sequences are shown. The values highlighted in blue indicate the most similar sequences, while those in red the most diverse.
GNDs representing genomic regions around the genes encoded for the sequences from the submitted SSN highlighted the presence of two main protein families in the analyzed sequences: the 4HBT Thioesterase superfamily (PF03061), shared by clusters 1 (Bacillus), 3 (Acetobacteraceae), 6 (Gilliamella), 7 (Bartonella) and 8 (Acetobacteraceae) (Figure 5), and the 4HBT_2 Thioesterase-like superfamily (PF13279), shared by clusters 2 (Gilliamella), 4 (Bacillus) and 5 (Bacillus). The Acyl-ACP thioesterase (PF01643) protein family was found in Lactobacillaceae from cluster 9, suggesting that this family may have evolved separately. The fact that those thioesterase superfamilies were shared by different taxonomic groups, together with the high similarities of these sequences, reinforces the possibility of finding methylketone synthase homologs in the selected bacterial taxa. From a practical point of view, the presence of a conserved protein family putatively involved in the biosynthesis of methylketones in different bacterial taxa suggests the possibility of using this sequence fragment as a target when aiming at assessing the biosynthetic potential of 2-heptanone in apiaries.

Figure 5.
Genome Neighborhood Diagrams (GNDs) showing the 4HBT Thioesterase superfamily (PF03061) (red) in the genomes of the representative bacterial species from clusters 1, 3, 6, 7 and 8.
In a previous study, putative acyl-CoA thioesterase biosynthetic genes were sequenced from eight B. thuringiensis strains isolated from honey bees [42], among which were the three isolates tested in this study for 2-heptanone production. Therefore, the occurrence of this metabolite in the culture filtrate of only one out of three bacterial isolates suggests that the targeted acyl-CoA thioesterase genes may not always function as methylketone synthase and that the biosynthesis of 2-heptanone may be strain-specific and dependent from environmental factors. Certainly, studies oriented towards fermentation process optimization are needed for practical implications of 2-heptanone-producing strains in pest management in apiaries.
Volatile compounds affecting the behavior of Varroa mites have been proposed as control strategies, but these semiochemical-based methods have received no validation in the field [8]. The use of 2-heptanone to control Varroa has been evaluated by the application of the pure compound in hives at mite-attracting and at miticidal concentrations [14], but issues have been encountered to ensure the persistence of this volatile compound at the desired levels. The possibility of using 2-heptanone-producing bacteria in naturally colonizing apiaries has so far never been evaluated.
The production of 2-heptanone by two A. bacterium strains together with a high number of putative methylketone synthase homologs found within this species, as well as in Acetobacteraceae, suggests that active producers of this compound may be found within this family. Since the beneficial role of these bacteria towards honey bees has been frequently reported [26,43] but never associated with a particular mechanism, it may be hypothesized that this compound has a role in the interactions between Acetobacteraceae and honey bees. These results are in line with previous reports on the mediation of bee alarm pheromones in intra- and interspecies communications [7] and support the idea that microbially produced volatiles have an effect on honey bee physiology and behavior [44].
4. Conclusions
The production of 2-heptanone in culture filtrates of A. bacterium, B. thuringiensis and A. kunkeei isolates indicates that this bioactive metabolite is produced by honey bee-associated bacteria. Putative methylketone synthase homologs were found in bacterial taxa known to inhabit apiaries that were considered in this study, such as the Bacillus genus, Acetobacteraceae family and Gilliamella genus. The role of 2-heptanone in protecting honey bees from pathogens and as a chemical signal in microbial communication, as well as the optimization of the strain and fermentation process, and the biosynthetic pathway need further investigation. These preliminary results may find applications in the evaluation of the biosynthetic potential of the protective natural compound in apiaries.
Author Contributions
Conceptualization, M.L.S. and R.L.S.; methodology, M.L.S. and G.B.; investigation, M.L.S., G.B. and R.L.S.; resources, M.L.S. and G.B.; data curation, M.L.S., G.B. and R.L.S.; writing—original draft preparation, M.L.S. and writing—review and editing, G.B. and R.L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This study was performed within the frame of the CREA-AA (Bologna) and CREA-IT (Milano) research facilities by using institutional resources.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vanengelsdorp, D.; Meixner, M.D. A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. J. Invertebr. Pathol. 2010, 103, S80–S95. [Google Scholar] [CrossRef]
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and control of Varroa destructor. J. Invertebr. Pathol. 2010, 103, S96–S119. [Google Scholar] [CrossRef]
- Lodesani, M.; Costa, C. Limits of chemotherapy in beekeeping: Development of resistance and the problem of residues. Bee World 2005, 86, 102–109. [Google Scholar] [CrossRef]
- Tauber, J.P.; Collins, W.R.; Schwarz, R.S.; Chen, Y.; Grubbs, K.; Huang, Q.; Lopez, D.; Peterson, R.; Evans, J.D. Natural product medicines for honey bees: Perspective and protocols. Insects 2019, 10, 356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witzgall, P.; Kirsch, P.; Cork, A. Sex pheromones and their impact on pest management. J. Chem. Ecol. 2010, 36, 80–100. [Google Scholar] [CrossRef]
- Beemelmanns, C.; Guo, H.; Rischer, M.; Poulsen, M. Natural products from microbes associated with insects. Beilstein J. Org. Chem. 2016, 12, 314–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Tan, K. Honey bee alarm pheromone mediates communication in plant-pollinator-predator interactions. Insects 2019, 10, 366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plettner, E.; Eliash, N.; Singh, N.K.; Pinnelli, G.R.; Soroker, V. The chemical ecology of host-parasite interaction as a target of Varroa destructor control agents. Apidologie 2017, 48, 78–92. [Google Scholar] [CrossRef] [Green Version]
- Manyi-Loh, C.E.; Ndip, R.N.; Clarke, A.M. Volatile compounds in honey: A review on their involvement in aroma, botanical origin determination and potential biomedical activities. Int. J. Mol. Sci. 2011, 12, 9514–9532. [Google Scholar] [CrossRef] [Green Version]
- Shearer, D.A.; Boch, R. 2-Heptanone in the mandibular gland secretion of the honey-bee. Nature 1965, 206, 530. [Google Scholar] [CrossRef]
- Rieth, J.P.; Wilson, W.T.; Levin, M.D. Repelling Honeybees from Insecticide-Treated Flowers with 2-Heptanone. J. Apic. Res. 1986, 25, 78–84. [Google Scholar] [CrossRef]
- Baracchi, D.; Cabirol, A.; Devaud, J.-M.; Haase, A.; d’Ettorre, P.; Giurfa, M. Pheromone components affect motivation and induce persistent modulation of associative learning and memory in honey bees. Commun. Biol. 2020, 3, 447. [Google Scholar] [CrossRef] [PubMed]
- Papachristoforou, A.; Kagiava, A.; Papaefthimiou, C.; Termentzi, A.; Fokialakis, N.; Skaltsounis, A.-L.; Watkins, M.; Arnold, G.; Theophilidis, G. The Bite of the Honeybee: 2-Heptanone Secreted from Honeybee Mandibles during a Bite Acts as a Local Anaesthetic in Insects and Mammals. PLoS ONE 2012, 7, e47432. [Google Scholar] [CrossRef] [Green Version]
- Erickson, E.; Degrandi-Hoffman, G.; Becker, C.; Whitson, R.; Deeby, T. Control of Parasitic Mites of Honey Bees. US Patent 2005/0090560A1, 28 April 2005. [Google Scholar]
- Borries, F.A.; Kudla, A.M.; Kim, S.; Allston, T.D.; Eddingsaas, N.C.; Shey, J.; Orts, W.J.; Klamczynski, A.P.; Glenn, G.M.; Miri, M.J. Ketalization of 2-heptanone to prolong its activity as mite repellant for the protection of honey bees. J. Sci. Food Agric. 2019, 99, 6267–6277. [Google Scholar] [CrossRef]
- Zhu, M.; Xu, X.; Li, Y.; Wang, P.; Niu, S.; Zhang, K.; Huang, X. Biosynthesis of the Nematode Attractant 2-Heptanone and Its Co-evolution Between the Pathogenic Bacterium Bacillus nematocida and Non-pathogenic Bacterium Bacillus subtilis. Front. Microbiol. 2019, 10, 1489. [Google Scholar] [CrossRef]
- Fridman, E.; Wang, J.; Iijima, Y.; Froehlich, J.E.; Gang, D.R.; Ohlrogge, J.; Pichersky, E. Metabolic, genomic, and biochemical analyses of glandular trichomes from the wild tomato species Lycopersicon hirsutum identify a key enzyme in the biosynthesis of methylketones. Plant. Cell 2005, 17, 1252–1267. [Google Scholar] [CrossRef] [Green Version]
- Gallegos, J.; Arce, C.; Jordano, R.; Arce, L.; Medina, L.M. Target identification of volatile metabolites to allow the differentiation of lactic acid bacteria by gas chromatography-ion mobility spectrometry. Food Chem. 2017, 220, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, J.; Li, C.; Ma, Y. Antifungal and plant growth promotion activity of volatile organic compounds produced by Bacillus amyloliquefaciens. Microbiol. Open 2019, 8, e00813. [Google Scholar] [CrossRef] [Green Version]
- Lamei, S.; Stephan, J.G.; Riesbeck, K.; Vasquez, A.; Olofsson, T.; Nilson, B.; de Miranda, J.R.; Forsgren, E. The secretome of honey bee-specific lactic acid bacteria inhibits Paenibacillus larvae growth. J. Apic. Res. 2019, 58, 405–412. [Google Scholar] [CrossRef] [Green Version]
- Butler, È.; Alsterfjord, M.; Olofsson, T.C.; Karlsson, C.; Malmström, J.; Vásquez, A. Proteins of novel lactic acid bacteria from Apis mellifera mellifera: An insight into the production of known extra-cellular proteins during microbial stress. BMC Microbiol. 2013, 13, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rokop, Z.P.; Horton, M.A.; Newton, I.L.G. Interactions between cooccurring lactic acid bacteria in honey bee hives. Appl. Environ. Microbiol. 2015, 81, 7261–7270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribière, C.; Hegarty, C.; Stephenson, H.; Whelan, P.; O’Toole, P.W. Gut and Whole-Body Microbiota of the Honey Bee Separate Thriving and Non-thriving Hives. Microb. Ecol. 2019, 78, 195–205. [Google Scholar] [CrossRef] [PubMed]
- McFrederick, Q.S.; Thomas, J.M.; Neff, J.L.; Vuong, H.Q.; Russell, K.A.; Hale, A.R.; Mueller, U.G. Flowers and Wild Megachilid Bees Share Microbes. Microb. Ecol. 2016, 73, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Corby-Harris, V.; Maes, P.; Anderson, K.E. The Bacterial Communities Associated with Honey Bee (Apis mellifera) Foragers. PLoS ONE 2014, 9, e95056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, K.E.; Sheehan, T.H.; Mott, B.M.; Maes, P.; Snyder, L.; Schwan, M.R.; Walton, A.; Jones, B.M.; Corby-Harris, V. Microbial ecology of the hive and pollination landscape: Bacterial associates from floral nectar, the alimentary tract and stored food of honey bees (Apis mellifera). PLoS ONE 2013, 8, e83125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saccà, M.L.; Lodesani, M. Isolation of bacterial microbiota associated to honey bees and evaluation of potential biocontrol agents of Varroa destructor. Benef. Microbes 2020, 11, 641–654. [Google Scholar] [CrossRef]
- Manici, L.M.; Saccà, M.L.; Lodesani, M. Secondary Metabolites Produced by Honey Bee-Associated Bacteria for Apiary Health: Potential Activity of Platynecine. Curr. Microbiol. 2020, 77, 3441–3449. [Google Scholar] [CrossRef]
- Endo, A.; Salminen, S. Honeybees and beehives are rich sources for fructophilic lactic acid bacteria. Syst. Appl. Microbiol. 2013, 36, 444–448. [Google Scholar] [CrossRef]
- Zallot, R.; Oberg, N.; Gerlt, J.A. The EFI Web Resource for Genomic Enzymology Tools: Leveraging Protein, Genome, and Metagenome Databases to Discover Novel Enzymes and Metabolic Pathways. Biochemistry 2019, 58, 4169–4182. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Zuckerkandl, E.; Pauling, L. Evolutionary Divergence and Convergence in Proteins. In Evolving Genes and Proteins; Bryson, V., Vogel, H.J., Eds.; Academic Press: Cambridge, MA, USA, 1965; pp. 97–166. [Google Scholar]
- Silva-Junior, E.A.; Ruzzini, A.C.; Paludo, C.R.; Nascimento, F.S.; Currie, C.R.; Clardy, J.; Pupo, M.T. Pyrazines from bacteria and ants: Convergent chemistry within an ecological niche. Sci. Rep. 2018, 8, 2595. [Google Scholar] [CrossRef] [Green Version]
- Riley, R.G.; Silverstein, R.M.; Moser, J.C. Isolation, identification, synthesis and biological activity of volatile compounds from the heads of Atta ants. J. Insect Physiol. 1974, 20, 1629–1637. [Google Scholar] [CrossRef]
- Boch, R.; Shearer, D.A.; Petrasovits, A. Efficacies of two alarm substances of the honey bee. J. Insect Physiol. 1970, 16, 17–24. [Google Scholar] [CrossRef]
- Breed, M.D.; Guzmán-Novoa, E.; Hunt, G.J. Defensive behavior of honey bees: Organization, Genetics, and Comparisons with Other Bees. Annu. Rev. Entomol. 2003, 49, 271–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olofsson, T.C.; Butler, E.; Markowicz, P.; Lindholm, C.; Larsson, L.; Vasquez, A. Lactic acid bacterial symbionts in honeybees—An unknown key to honey’s antimicrobial and therapeutic activities. Int. Wound J. 2014, 13, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Corby-Harris, V.; Snyder, L.A.; Schwan, M.R.; Maes, P.; McFrederick, Q.S.; Anderson, K.E. Origin and Effect of Alpha 2.2 Acetobacteraceae in Honey Bee Larvae and Description of Parasaccharibacter apium gen. nov., sp. nov. Appl. Environ. Microbiol. 2014, 80, 7460–7472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, J.-H.; Lee, J.-Y.; Hyun, D.-W.; Jung, M.-J.; Bae, J.-W. Bombella apis sp. nov., an acetic acid bacterium isolated from the midgut of a honey bee. Int. J. Syst. Evol. Microbiol. 2017, 67, 2184–2188. [Google Scholar] [CrossRef] [PubMed]
- Peghaire, E.; Moné, A.; Delbac, F.; Debroas, D.; Chaucheyras-Durand, F.; El Alaoui, H. A Pediococcus strain to rescue honeybees by decreasing Nosema ceranae-and pesticide-induced adverse effects. Pestic. Biochem. Physiol. 2020, 163, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Kalinger, R.S.; Pulsifer, I.P.; Hepworth, S.R.; Rowland, O. Fatty Acyl Synthetases and Thioesterases in Plant Lipid Metabolism: Diverse Functions and Biotechnological Applications. Lipids 2020, 55, 25–455. [Google Scholar] [CrossRef]
- Saccà, M.L.; Manici, L.M. Honey bee-associated bacteria as producers of bioactive compounds for protecting hives. A biosynthetic gene-based approach. Microbiol. Res. 2021, 252, 126860. [Google Scholar] [CrossRef]
- Alberoni, D.; Baffoni, L.; Gaggìa, F.; Ryan, P.M.; Murphy, K.; Ross, P.R.; Stanton, C.; Di Gioia, D. Impact of beneficial bacteria supplementation on the gut microbiota, colony development and productivity of Apis mellifera L. Benef. Microbes 2018, 9, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Rering, C.C.; Beck, J.J.; Hall, G.W.; McCartney, M.M.; Vannette, R.L. Nectar-inhabiting microorganisms influence nectar volatile composition and attractiveness to a generalist pollinator. New Phytol. 2018, 220, 750–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).