Quantification of the Adhesion Strength of Candida albicans to Tooth Enamel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strain and Cultivation
2.2. Enamel Samples
2.3. Human Subjects
2.4. Saliva Treatment of Yeast and Enamel
2.5. Oral Exposure
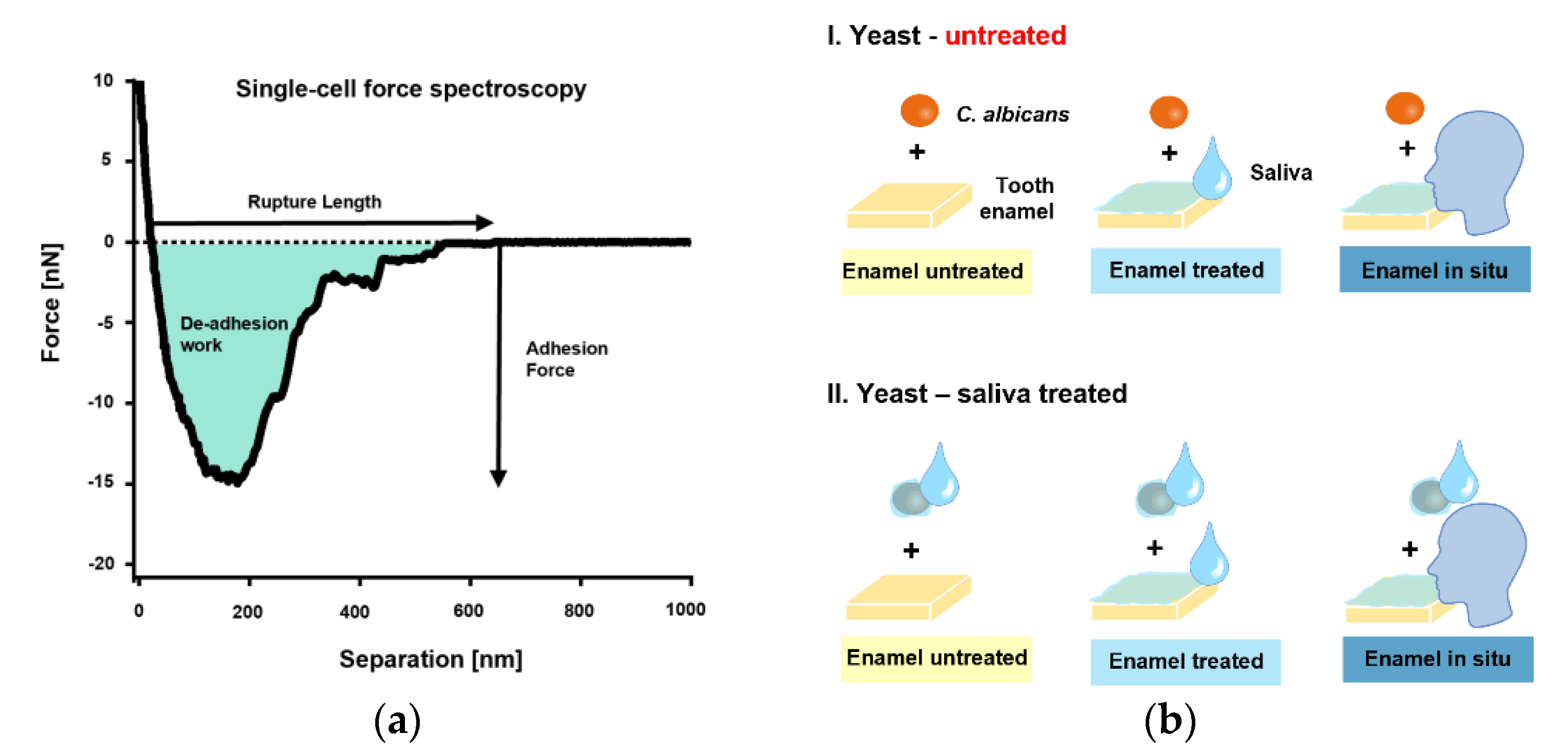
2.6. Single-Cell Force Spectroscopy
2.7. Scanning Electron Microscopy (SEM)
2.8. Statistical Analyses
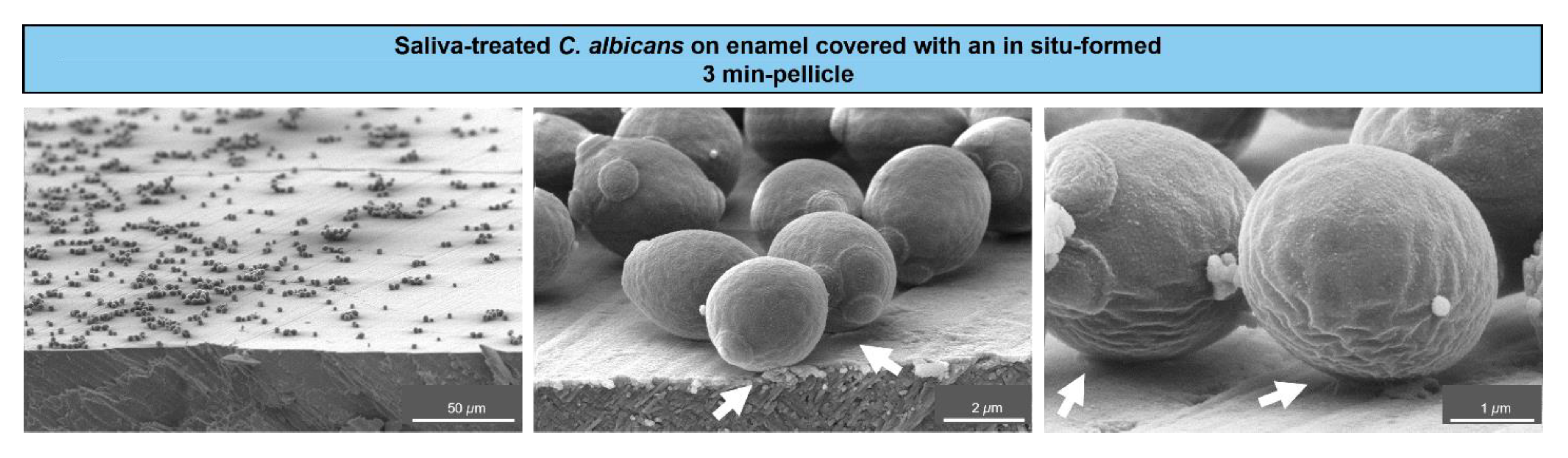
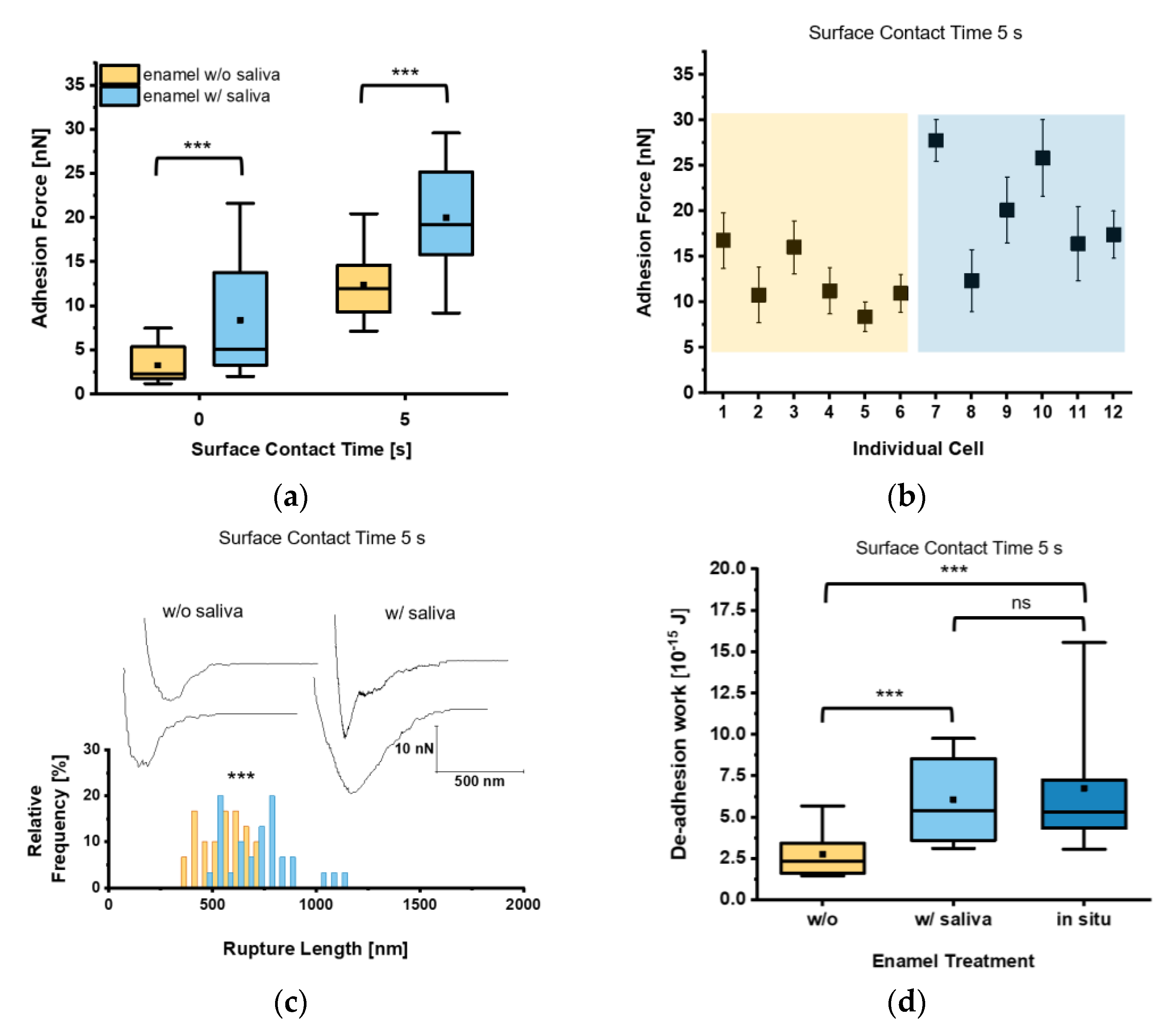
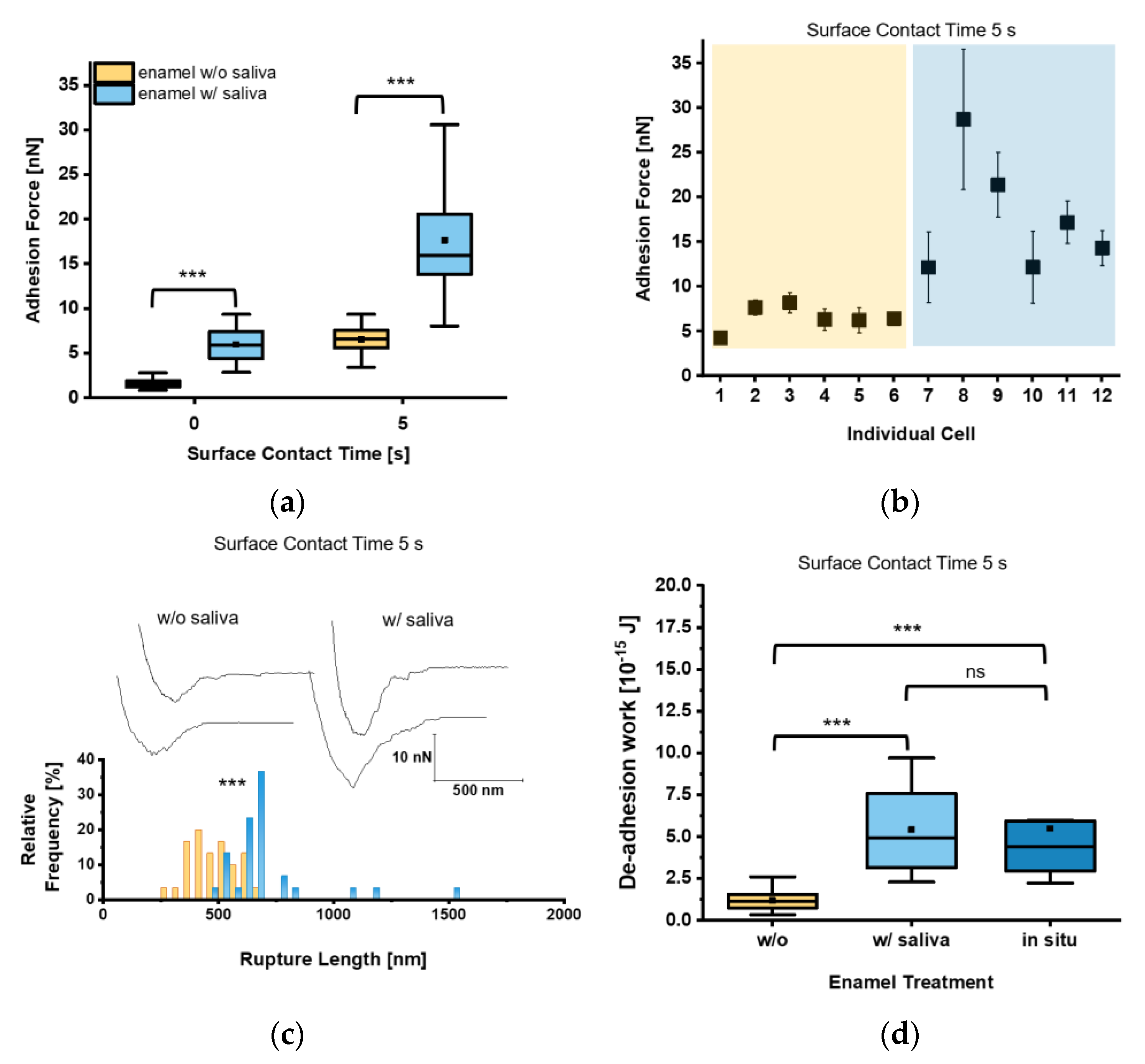
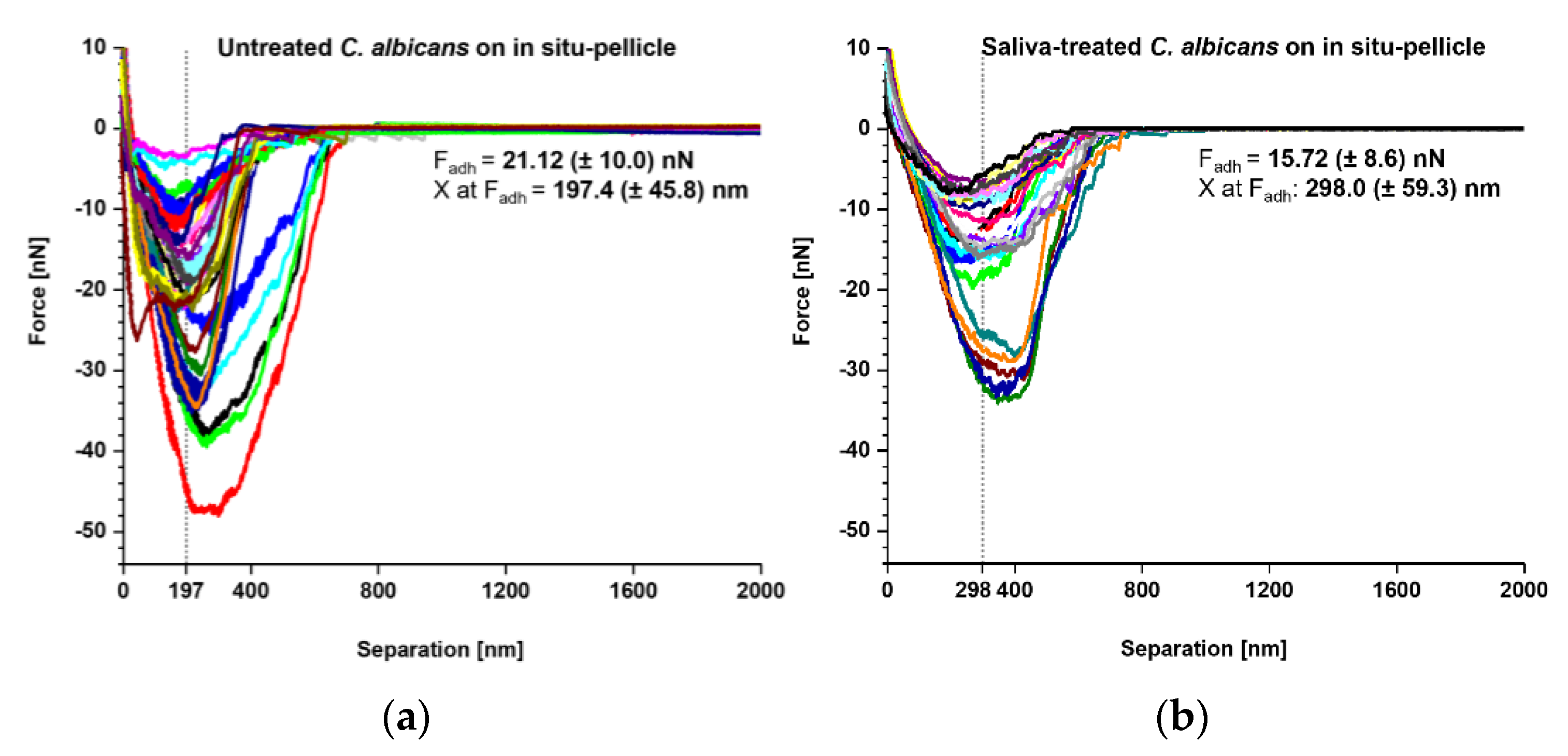
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bar-Or, Y. The Effect of Adhesion on Survival and Growth of Microorganisms. Experientia 1990, 46, 823–826. [Google Scholar] [CrossRef]
- ten Cate, J.M.; Klis, F.M.; Pereira-Cenci, T.; Crielaard, W.; de Groot, P.W.J. Molecular and Cellular Mechanisms That Lead to Candida Biofilm Formation. J. Dent. Res. 2009, 88, 105–115. [Google Scholar] [CrossRef] [PubMed]
- de Barros, P.P.; Rossoni, R.D.; de Souza, C.M.; Scorzoni, L.; Fenley, J.D.C.; Junqueira, J.C. Candida Biofilms: An Update on Developmental Mechanisms and Therapeutic Challenges. Mycopathologia 2020, 185, 415–424. [Google Scholar] [CrossRef]
- Gulati, M.; Nobile, C.J. Candida albicans Biofilms: Development, Regulation, and Molecular Mechanisms. Microbes Infect. 2016, 18, 310–321. [Google Scholar] [CrossRef] [Green Version]
- Jean, J.; Goldberg, S.; Khare, R.; Bailey, L.C.; Forrest, C.B.; Hajishengallis, E.; Koo, H. Retrospective Analysis of Candida-Related Conditions in Infancy and Early Childhood Caries. Pediatr. Dent. 2018, 40, 131–135. [Google Scholar]
- Xiao, J.; Huang, X.; Alkhers, N.; Alzamil, H.; Alzoubi, S.; Wu, T.T.; Castillo, D.A.; Campbell, F.; Davis, J.; Herzog, K.; et al. Candida albicans and Early Childhood Caries: A Systematic Review and Meta-Analysis. Caries Res. 2018, 52, 102–112. [Google Scholar] [CrossRef] [Green Version]
- O’Connell, L.M.; Santos, R.; Springer, G.; Burne, R.A.; Nascimento, M.M.; Richards, V.P. Site-Specific Profiling of the Dental Mycobiome Reveals Strong Taxonomic Shifts during Progression of Early-Childhood Caries. Appl. Environ. Microbiol. 2020, 86, e02825-19. [Google Scholar] [CrossRef]
- Schulz-Weidner, N.; Ansari, F.; Hossain, H.; Chakraborty, T.; Domann, E.; Wetzel, W.-E. Vergleichende PCR-Typisierung von Candida albicans aus der Mundhöhle und dem Magen-Darm-Trakt. Oralprophylaxe Kinderzahnheilkd 2005, 27, 139–143. [Google Scholar]
- Fakhruddin, K.S.; Perera Samaranayake, L.; Egusa, H.; Chi Ngo, H.; Panduwawala, C.; Venkatachalam, T.; Kumarappan, A.; Pesee, S. Candida Biome of Severe Early Childhood Caries (S-ECC) and Its Cariogenic Virulence Traits. J. Oral Microbiol. 2020, 12, 1724484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, J.; Moon, Y.; Li, L.; Rustchenko, E.; Wakabayashi, H.; Zhao, X.; Feng, C.; Gill, S.R.; McLaren, S.; Malmstrom, H.; et al. Candida albicans Carriage in Children with Severe Early Childhood Caries (S-ECC) and Maternal Relatedness. PLoS ONE 2016, 11, e0164242. [Google Scholar] [CrossRef] [Green Version]
- de Carvalho, F.G.; Silva, D.S.; Hebling, J.; Spolidorio, L.C.; Spolidorio, D.M.P. Presence of Mutans Streptococci and Candida Spp. in Dental Plaque/Dentine of Carious Teeth and Early Childhood Caries. Arch. Oral Biol. 2006, 51, 1024–1028. [Google Scholar] [CrossRef]
- Lozano Moraga, C.P.; Rodríguez Martínez, G.A.; Lefimil Puente, C.A.; Morales Bozo, I.C.; Urzúa Orellana, B.R. Prevalence of Candida albicans and Carriage of Candida non-albicans in the Saliva of Preschool Children, According to Their Caries Status. Acta Odontol. Scand. 2017, 75, 30–35. [Google Scholar] [CrossRef]
- Trautmann, S.; Künzel, N.; Fecher-Trost, C.; Barghash, A.; Schalkowsky, P.; Dudek, J.; Delius, J.; Helms, V.; Hannig, M. Deep Proteomic Insights into the Individual Short-Term Pellicle Formation on Enamel—An In Situ Pilot Study. Prot. Clin. Appl. 2020, 14, 1900090. [Google Scholar] [CrossRef] [PubMed]
- Samaranayake, L.P.; MacFarlane, T.W. Factors Affecting the In-Vitro Adherence of the Fungal Oral Pathogen Candida albicans to Epithelial Cells of Human Origin. Arch. Oral Biol. 1982, 27, 869–873. [Google Scholar] [CrossRef]
- Lendenmann, U.; Grogan, J.; Oppenheim, F.G. Saliva and Dental Pellicle-A Review. Adv. Dent. Res. 2000, 14, 22–28. [Google Scholar] [CrossRef]
- Samaranayake, L.P.; McCourtie, J.; MacFarlane, T.W. Factors Affecting the In-Vitro Adherence of Candida albicans to Acrylic Surfaces. Arch. Oral Biol. 1980, 25, 611–615. [Google Scholar] [CrossRef]
- Bürgers, R.; Hahnel, S.; Reichert, T.E.; Rosentritt, M.; Behr, M.; Gerlach, T.; Handel, G.; Gosau, M. Adhesion of Candida albicans to Various Dental Implant Surfaces and the Influence of Salivary Pellicle Proteins. Acta Biomater. 2010, 6, 2307–2313. [Google Scholar] [CrossRef] [PubMed]
- Oncul, B.; Karakis, D.; Dogruman Al, F. The Effect of Two Artificial Salivas on the Adhesion of Candida albicans to Heat-Polymerized Acrylic Resin. J. Adv. Prosthodont. 2015, 7, 93. [Google Scholar] [CrossRef]
- O’Sullivan, J.M.; Jenkinson, H.F.; Cannon, R.D. Adhesion of Candida albicans to Oral Streptococci Is Promoted by Selective Adsorption of Salivary Proteins to the Streptococcal Cell Surface. Microbiology 2000, 146, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Ponton, J.; Elguezabal, N.; Maza, J.L.; Dorronsoro, S. Whole Saliva Has a Dual Role on the Adherence of Candida albicans to Polymethylmetacrylate. TODENTJ 2008, 2, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Nikawa, H.; Hamada, T.; Yamashiro, H.; Murata, H.; Subiwahjudi, A. The Effect of Saliva or Serum on Streptococcus Mutans and Candida albicans Colonization of Hydroxylapatite Beads. J. Dent. 1998, 26, 31–37. [Google Scholar] [CrossRef]
- Hahnel, S.; Ettl, T.; Gosau, M.; Rosentritt, M.; Handel, G.; Bürgers, R. Influence of Saliva Substitute Films on the Initial Adhesion of Candida albicans to Dental Substrata Prior to and after Artificial Ageing. Arch. Oral Biol. 2010, 55, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Cannon, R.D.; Nand, A.K.; Jenkinson, H.F. Adherence of Candida albicans to Human Salivary Components Adsorbed to Hydroxylapatite. Microbiology 1995, 141 Pt 1, 213–219. [Google Scholar] [CrossRef] [Green Version]
- Thanh Nguyen, H.; Zhang, R.; Inokawa, N.; Oura, T.; Chen, X.; Iwatani, S.; Niimi, K.; Niimi, M.; Holmes, A.R.; Cannon, R.D.; et al. Candida albicans Bgl2p, Ecm33p, and Als1p Proteins Are Involved in Adhesion to Saliva-Coated Hydroxyapatite. J. Oral Microbiol. 2021, 13, 1879497. [Google Scholar] [CrossRef]
- Potthoff, E.; Guillaume-Gentil, O.; Ossola, D.; Polesel-Maris, J.; LeibundGut-Landmann, S.; Zambelli, T.; Vorholt, J.A. Rapid and Serial Quantification of Adhesion Forces of Yeast and Mammalian Cells. PLoS ONE 2012, 7, e52712. [Google Scholar] [CrossRef] [Green Version]
- Hofherr, L.; Müller-Renno, C.; Ziegler, C. FluidFM as a Tool to Study Adhesion Forces of Bacteria—Optimization of Parameters and Comparison to Conventional Bacterial Probe Scanning Force Spectroscopy. PLoS ONE 2020, 15, e0227395. [Google Scholar] [CrossRef]
- Jung, P.; Mischo, C.E.; Gunaratnam, G.; Spengler, C.; Becker, S.L.; Hube, B.; Jacobs, K.; Bischoff, M. Candida albicans Adhesion to Central Venous Catheters: Impact of Blood Plasma-Driven Germ Tube Formation and Pathogen-Derived Adhesins. Virulence 2020, 11, 1453–1465. [Google Scholar] [CrossRef] [PubMed]
- Gunaratnam, G.; Spengler, C.; Trautmann, S.; Jung, P.; Mischo, J.; Wieland, B.; Metz, C.; Becker, S.L.; Hannig, M.; Jacobs, K.; et al. Human Blood Plasma Factors Affect the Adhesion Kinetics of Staphylococcus Aureus to Central Venous Catheters. Sci. Rep. 2020, 10, 20992. [Google Scholar] [CrossRef]
- Potthoff, E.; Ossola, D.; Zambelli, T.; Vorholt, J.A. Bacterial Adhesion Force Quantification by Fluidic Force Microscopy. Nanoscale 2015, 7, 4070–4079. [Google Scholar] [CrossRef]
- Spengler, C.; Thewes, N.; Nolle, F.; Faidt, T.; Umanskaya, N.; Hannig, M.; Bischoff, M.; Jacobs, K. Enhanced Adhesion of Streptococcus Mutans to Hydroxyapatite after Exposure to Saliva: Enhanced Adhesion of Streptococcus Mutans to Hydroxyapatite after Exposure to Saliva. J. Mol. Recognit. 2017, 30, e2615. [Google Scholar] [CrossRef]
- Mischo, J.; Faidt, T.; McMillan, R.B.; Dudek, J.; Gunaratnam, G.; Bayenat, P.; Holtsch, A.; Spengler, C.; Müller, F.; Hähl, H.; et al. Hydroxyapatite Pellets as Versatile Model Surfaces for Systematic Studies on Enamel. bioRxiv 2021. [Google Scholar] [CrossRef]
- Trautmann, S.; Barghash, A.; Fecher-Trost, C.; Schalkowsky, P.; Hannig, C.; Kirsch, J.; Rupf, S.; Keller, A.; Helms, V.; Hannig, M. Proteomic Analysis of the Initial Oral Pellicle in Caries-Active and Caries-Free Individuals. Prot. Clin. Appl. 2019, 13, 1800143. [Google Scholar] [CrossRef] [PubMed]
- Sen, B.H.; Safavi, K.E.; Spångberg, L.S. Colonization of Candida albicans on Cleaned Human Dental Hard Tissues. Arch. Oral Biol. 1997, 42, 513–520. [Google Scholar] [CrossRef]
- Gregoire, S.; Xiao, J.; Silva, B.B.; Gonzalez, I.; Agidi, P.S.; Klein, M.I.; Ambatipudi, K.S.; Rosalen, P.L.; Bauserman, R.; Waugh, R.E.; et al. Role of Glucosyltransferase B in Interactions of Candida albicans with Streptococcus Mutans and with an Experimental Pellicle on Hydroxyapatite Surfaces. Appl. Environ. Microbiol. 2011, 77, 6357–6367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metwalli, K.H.; Khan, S.A.; Krom, B.P.; Jabra-Rizk, M.A. Streptococcus Mutans, Candida albicans, and the Human Mouth: A Sticky Situation. PLoS Pathog. 2013, 9, e1003616. [Google Scholar] [CrossRef] [Green Version]
- Dauben, T.J.; Dewald, C.; Firkowska-Boden, I.; Helbing, C.; Peisker, H.; Roth, M.; Bossert, J.; Jandt, K.D. Quantifying the Relationship between Surfaces’ Nano-Contact Point Density and Adhesion Force of Candida albicans. Colloids Surf. B Biointerfaces 2020, 194, 111177. [Google Scholar] [CrossRef] [PubMed]
- Jeng, H.-W.; Holmes, A.R.; Cannon, R.D. Characterization of Two Candida albicans Surface Mannoprotein Adhesins That Bind Immobilized Saliva Components. Med. Mycol. 2005, 43, 209–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thewes, N.; Thewes, A.; Loskill, P.; Peisker, H.; Bischoff, M.; Herrmann, M.; Santen, L.; Jacobs, K. Stochastic Binding of Staphylococcus Aureus to Hydrophobic Surfaces. Soft Matter 2015, 11, 8913–8919. [Google Scholar] [CrossRef]
- Alsteens, D.; Dupres, V.; Klotz, S.A.; Gaur, N.K.; Lipke, P.N.; Dufrêne, Y.F. Unfolding Individual Als5p Adhesion Proteins on Live Cells. ACS Nano 2009, 3, 1677–1682. [Google Scholar] [CrossRef] [Green Version]
- Gaur, N.K.; Smith, R.L.; Klotz, S.A. Candida Albicans and Saccharomyces Cerevisiae Expressing ALA1/ALS5 Adhere to Accessible Threonine, Serine, or Alanine Patches. Cell Commun. Adhes. 2002, 9, 45–57. [Google Scholar] [CrossRef]
- Finkel, J.S.; Xu, W.; Huang, D.; Hill, E.M.; Desai, J.V.; Woolford, C.A.; Nett, J.E.; Taff, H.; Norice, C.T.; Andes, D.R.; et al. Portrait of Candida albicans Adherence Regulators. PLoS Pathog. 2012, 8, e1002525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Sullivan, J.M.; Cannon, R.D.; Sullivan, P.A.; Jenkinson, H.F. Identification of Salivary Basic Proline-Rich Proteins as Receptors for Candida albicans Adhesion. Microbiology 1997, 143, 341–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, A.R.; Rodrigues, E.; van der Wielen, P.; Lyons, K.M.; Haigh, B.J.; Wheeler, T.T.; Dawes, P.J.D.; Cannon, R.D. Adherence of Candida albicans to Silicone Is Promoted by the Human Salivary Protein SPLUNC2/PSP/BPIFA2. Mol. Oral Microbiol. 2014, 29, 90–98. [Google Scholar] [CrossRef]
- Hannig, C.; Helbig, R.; Hilsenbeck, J.; Werner, C.; Hannig, M. Impact of the Springtail’s Cuticle Nanotopography on Bioadhesion and Biofilm Formation in Vitro and in the Oral Cavity. R. Soc. Open Sci. 2018, 5, 171742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibbons, R.J.; Hay, D.I.; Schlesinger, D.H. Delineation of a Segment of Adsorbed Salivary Acidic Proline-Rich Proteins Which Promotes Adhesion of Streptococcus Gordonii to Apatitic Surfaces. Infect. Immun. 1991, 59, 2948–2954. [Google Scholar] [CrossRef] [Green Version]
- Klotz, S.A.; Smith, R.L. A fibronectin receptor on Candida albicans mediates adherence of the fungus to extracellular matrix. J. Infect. Dis. 1991, 163, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Hannig, M.; Herzog, S.; Willigeroth, S.F.; Zimehl, R. Atomic Force Microscopy Study of Salivary Pellicles Formed on Enamel and Glass in Vivo. Colloid Polym. Sci. 2001, 279, 479–483. [Google Scholar] [CrossRef]
- Spengler, C.; Glatz, B.A.; Maikranz, E.; Bischoff, M.; Klatt, M.A.; Santen, L.; Fery, A.; Jacobs, K. The Adhesion Capability of S. Aureus Cells Is Heterogeneously Distributed over the Cell Envelope. bioRxiv 2021. [Google Scholar] [CrossRef]
- Spengler, C.; Maikranz, E.; Santen, L.; Jacobs, K. Modeling Bacterial Adhesion to Unconditioned Abiotic Surfaces. Front. Mech. Eng. 2021, 7, 661370. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunaratnam, G.; Dudek, J.; Jung, P.; Becker, S.L.; Jacobs, K.; Bischoff, M.; Hannig, M. Quantification of the Adhesion Strength of Candida albicans to Tooth Enamel. Microorganisms 2021, 9, 2213. https://doi.org/10.3390/microorganisms9112213
Gunaratnam G, Dudek J, Jung P, Becker SL, Jacobs K, Bischoff M, Hannig M. Quantification of the Adhesion Strength of Candida albicans to Tooth Enamel. Microorganisms. 2021; 9(11):2213. https://doi.org/10.3390/microorganisms9112213
Chicago/Turabian StyleGunaratnam, Gubesh, Johanna Dudek, Philipp Jung, Sören L. Becker, Karin Jacobs, Markus Bischoff, and Matthias Hannig. 2021. "Quantification of the Adhesion Strength of Candida albicans to Tooth Enamel" Microorganisms 9, no. 11: 2213. https://doi.org/10.3390/microorganisms9112213
APA StyleGunaratnam, G., Dudek, J., Jung, P., Becker, S. L., Jacobs, K., Bischoff, M., & Hannig, M. (2021). Quantification of the Adhesion Strength of Candida albicans to Tooth Enamel. Microorganisms, 9(11), 2213. https://doi.org/10.3390/microorganisms9112213






