Novel Insights for Metabiotics Production by Using Artisanal Probiotic Cultures
Abstract
1. Introduction
2. Definitions and New Connections between Metabiotics and Artisanal Cultures
3. Microbial Diversity and the Synergistic Relationships of the Artisanal Cultures
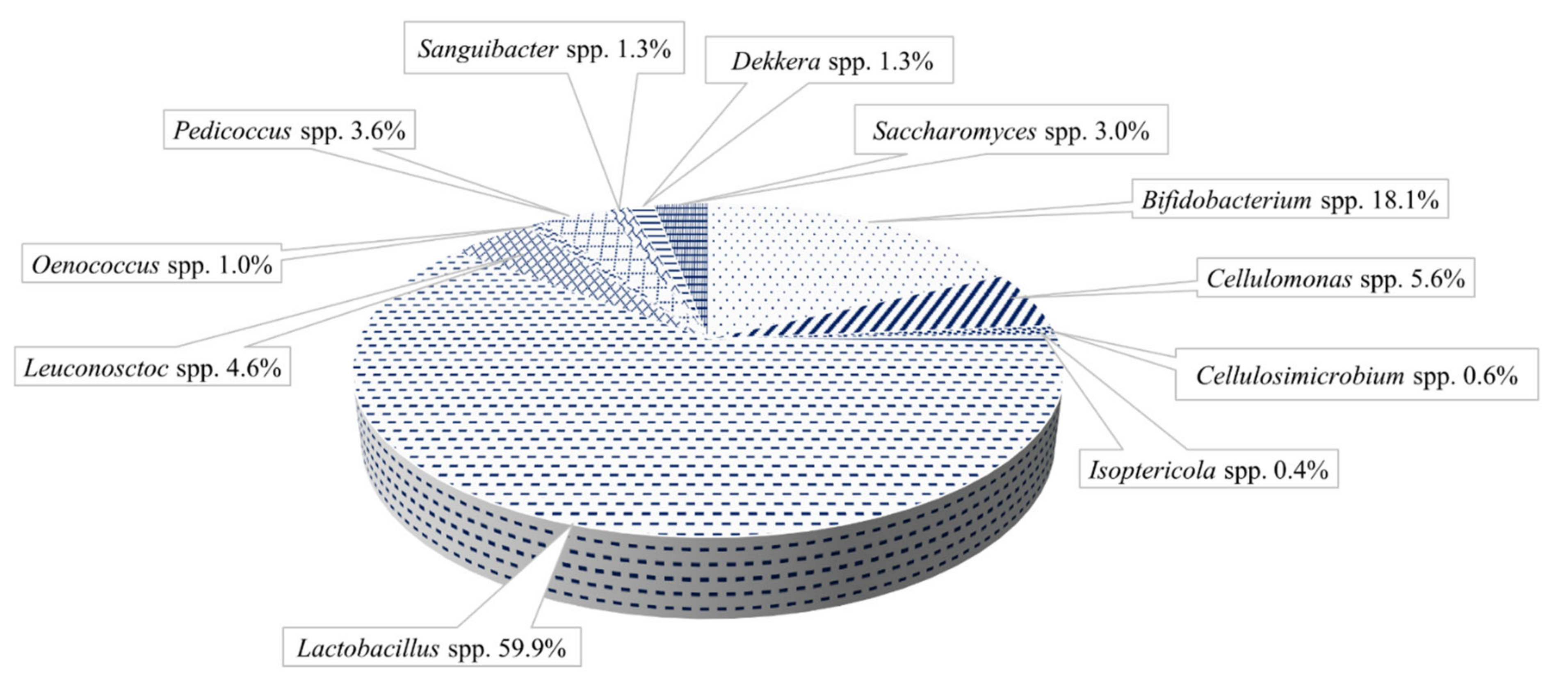
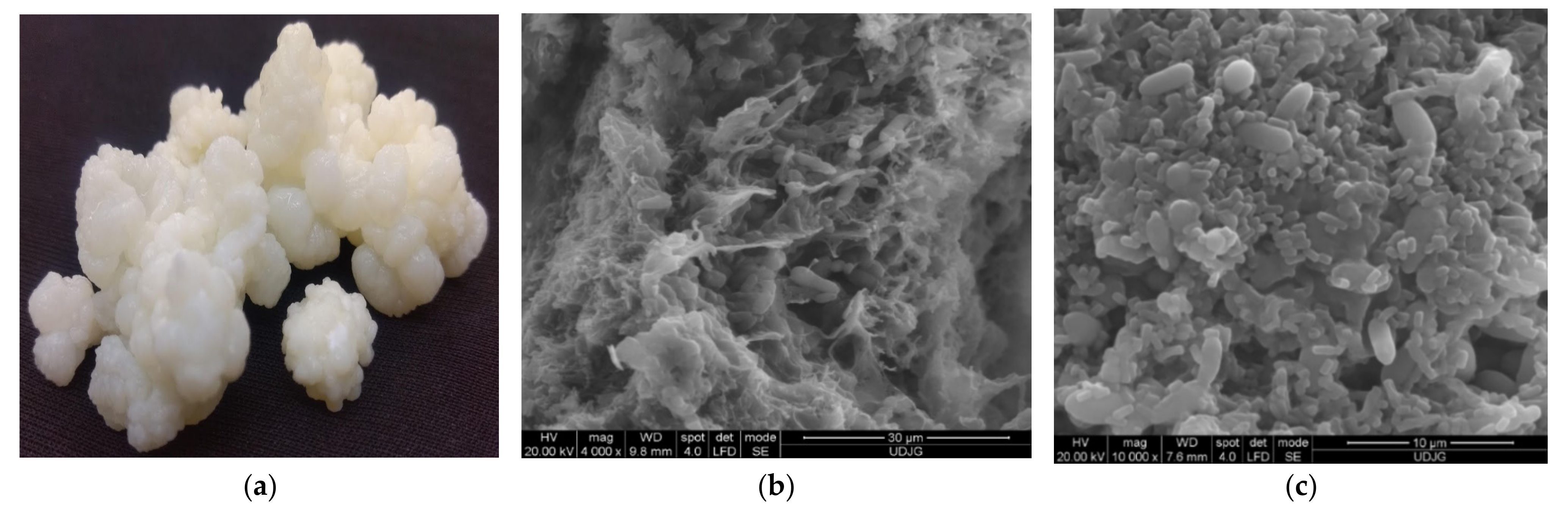
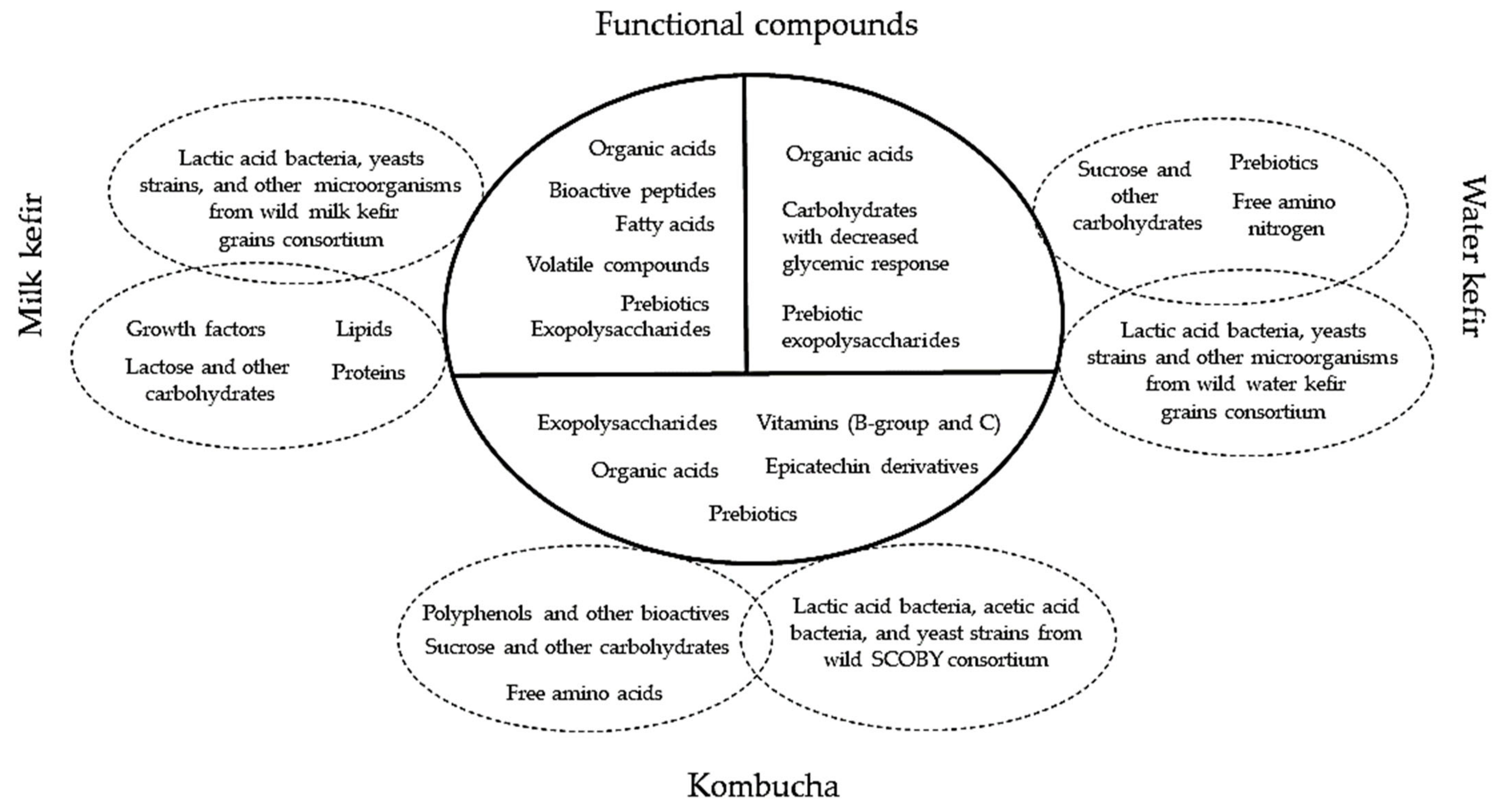
3.1. Water Kefir Grains
3.2. Milk Kefir Grains
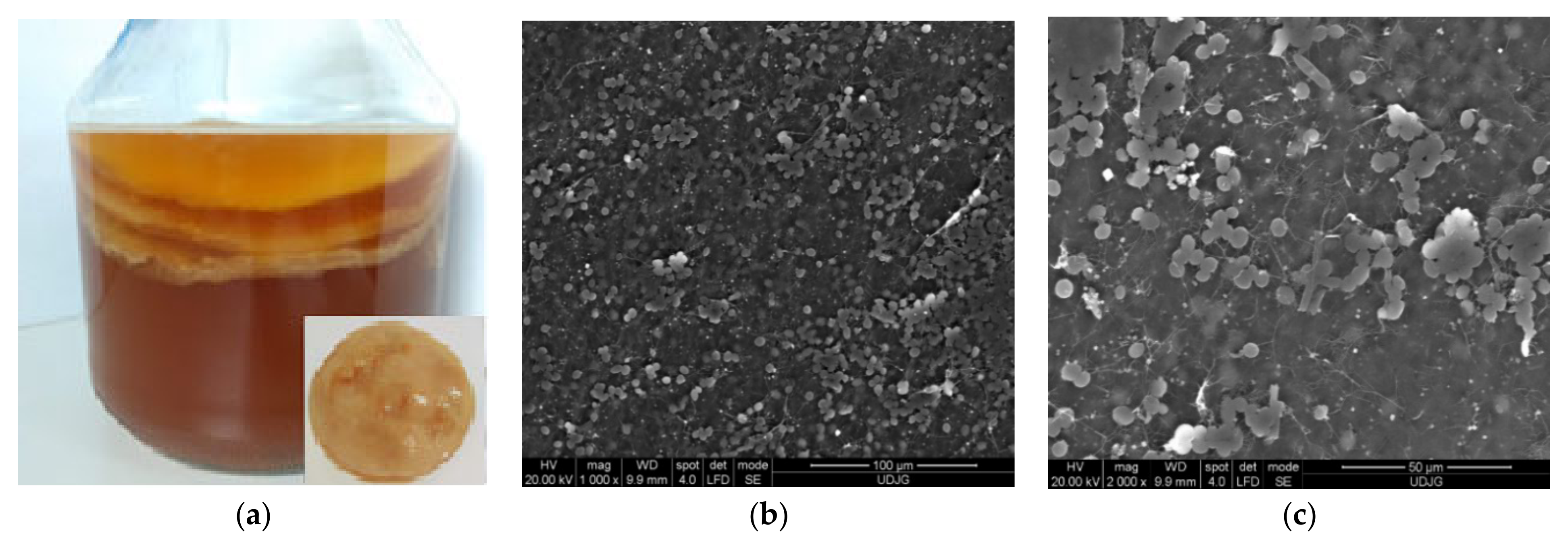
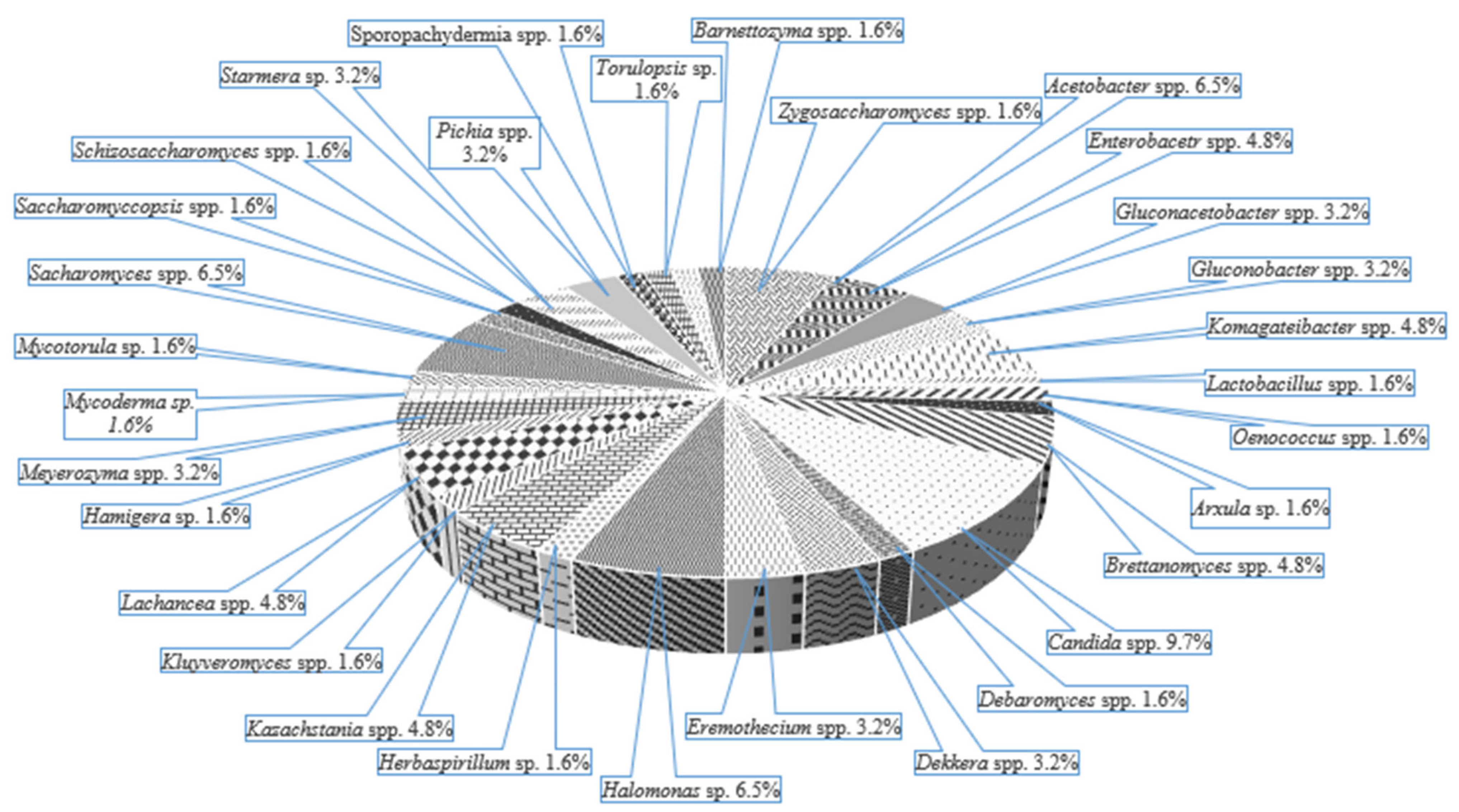
3.3. Kombucha
4. The Postbiotic and Prebiotic Potential of Artisanal Starter Cultures in Regard to the Fermentation Substrate
5. Obtaining Paraprobiotics by Cell Disruption and Their Applications
6. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- London, C. Functional Foods that Boost the Immune System. In Functional Food Product Development; Wiley-Blackwell: Hoboken, NJ, USA, 2010; pp. 293–321. ISBN 9781405178761. [Google Scholar]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic T reg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Ngo, V.L.; Gewirtz, A.T. Microbiota as a potentially-modifiable factor influencing COVID-19. Curr. Opin. Virol. 2021, 49, 21–26. [Google Scholar] [CrossRef]
- Ashaolu, T.J. Immune boosting functional foods and their mechanisms: A critical evaluation of probiotics and prebiotics. Biomed. Pharmacother. 2020, 130. [Google Scholar] [CrossRef]
- Shenderov, B.A.; Tkachenko, E.I.; Lazebnik, L.B.; Ardatskaya, M.D.; Sinitsa, A.V.; Zakharenko, M.M. Metabiotics—Novel technology of protective and treatament of diseases associated with microecological imbalance in human being. Exp. Clin. Gastroenterol. 2018, 151, 83–92. [Google Scholar] [CrossRef]
- Arayici, M.E.; Yucel, U.; Ocek, Z.A. Knowledge and Attitudes of Ege University Midwifery, Nutrition-Dietetic, and Nursing Students About Natural Functional Foods. J. Basic Clin. Health Sci. 2020. [Google Scholar] [CrossRef]
- Bazarnova, J.; Eliseeva, S.; Zhilinskaya, N.; Barsukova, N.; Aronova, E.; Korzh, A. Metabiotics in molecular nutrition: History and practice. In Proceedings of the E3S Web of Conferences, Prague, Czech Republic, 27–28 February 2020; Volume 161. [Google Scholar] [CrossRef]
- Cavaliere, C.; Montone, A.M.I.; Aita, S.E.; Capparelli, R.; Cerrato, A.; Cuomo, P.; Laganà, A.; Montone, C.M.; Piovesana, S.; Capriotti, A.L. Production and characterization of medium-sized and short antioxidant peptides from soy flour-simulated gastrointestinal hydrolysate. Antioxidants 2021, 10, 734. [Google Scholar] [CrossRef]
- Cerrato, A.; Capriotti, A.L.; Capuano, F.; Cavaliere, C.; Montone, A.M.I.; Montone, C.M.; Piovesana, S.; Chiozzi, R.Z.; Laganà, A. Identification and antimicrobial activity of medium-sized and short peptides from yellowfin tuna (Thunnus albacares) simulated gastrointestinal digestion. Foods 2020, 9, 1185. [Google Scholar] [CrossRef]
- Bradley, P.H.; Pollard, K.S. Proteobacteria explain significant functional variability in the human gut microbiome. Microbiome 2017, 5, 1–23. [Google Scholar] [CrossRef]
- Rutsch, A.; Kantsjö, J.B.; Ronchi, F.; Tay, T.L.; Wekerle, H.; Rojas, O.L. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Article 2020, 11, 1. [Google Scholar] [CrossRef]
- Su, Q.; Liu, Q. Factors Affecting Gut Microbiome in Daily Diet. Front. Nutr. 2021, 8, 218. [Google Scholar] [CrossRef]
- Artyukhova, S.I.; Kozlova, O.V.; Tolstoguzova, T.T. Developing freeze-dried bioproducts for the Russian military in the Arctic. Foods Raw Mater. 2019, 7, 202–209. [Google Scholar] [CrossRef]
- Voitenko, O.S. Технoлoгия Прoбиoтикoв и Прoдуктoв На Их Оснoве/Technology of Probiotics and Products Based on Them; Don State Agrarian University: Persianovskiy, Russia, 2019. [Google Scholar]
- Bazarnova, J.; Korzh, A.; Barsukova, N.; Eliseeva, S.; Fedinishina, E. Food Engineering of Meat Bio-Products to Immunity Strengthening. BIO Web Conf. 2021, 29, 01021. [Google Scholar] [CrossRef]
- Shenderov, B.A.; Gabrichevsky, G.N. Metabiotics: An overview of progress, opportunities and challenges. J. Microb. Biochem. Technol. 2017, 9, 103. [Google Scholar] [CrossRef]
- Singh, A.; Vishwakarma, V.; Singhal, B. Metabiotics: The Functional Metabolic Signatures of Probiotics: Current State-of-Art and Future Research Priorities—Metabiotics: Probiotics Effector Molecules. Adv. Biosci. Biotechnol. 2018, 9, 147–189. [Google Scholar] [CrossRef]
- Rezende, E.S.V.; Lima, G.C.; Naves, M.M.V. Dietary fibers as beneficial microbiota modulators: A proposal classification by prebiotic categories. Nutrition 2021, 89. [Google Scholar] [CrossRef]
- Cunningham, M.; Azcarate-Peril, M.A.; Barnard, A.; Benoit, V.; Grimaldi, R.; Guyonnet, D.; Holscher, H.D.; Hunter, K.; Manurung, S.; Obis, D.; et al. Shaping the Future of Probiotics and Prebiotics. Trends Microbiol. 2021, 29, 667–685. [Google Scholar] [CrossRef]
- Lattimer, J.M.; Haub, M.D. Effects of Dietary Fiber and Its Components on Metabolic Health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef]
- Kharitonov, D.V.; Kharitonova, I.V.; Prosekov, A.Y. The concept of synbiotics and sunbiotic dairy products development/Разрабoтка кoнцепции сoздания синбиoтикoв и синбиoтических мoлoчных прoдуктoв. ТЕХНИКА ТЕХНОЛОГИЯ ПИЩЕВЫХ ПРОИЗВОДСТВ 2013, 4, 91–94. [Google Scholar]
- Sharma, S.; Bano, A.; Gupta, A.; Bajpai, P.; Lohani, M.; Pathak, N. Pre- and probiotics: Using functional foods in the fight against microbial resistance to antibiotics. In Antibacterial Drug Discovery to Combat MDR: Natural Compounds, Nanotechnology and Novel Synthetic Sources; Springer: Singapore, 2019; pp. 397–425. ISBN 9789811398711. [Google Scholar]
- Lakhtin, M.; Lakhtin, V.; Aleshkin, V.; Afanasiev, S. Metabolite Multiprobiotic Formulas for Microbial Health. In Oral Health by Using Probiotic Products; InTech Open: London, UK, 2019; Volume 6, pp. 8–34. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Salas-Lais, A.G.; Robles-Contreras, A.; Balderas-López, J.A.; Bautista-de Lucio, V.M. Immunobiotic and paraprobiotic potential effect of Lactobacillus casei in a systemic toxoplasmosis murine model. Microorganisms 2020, 8, 113. [Google Scholar] [CrossRef]
- Shafipour Yordshahi, A.; Moradi, M.; Tajik, H.; Molaei, R. Design and preparation of antimicrobial meat wrapping nanopaper with bacterial cellulose and postbiotics of lactic acid bacteria. Int. J. Food Microbiol. 2020, 321, 108561. [Google Scholar] [CrossRef]
- Zendeboodi, F.; Khorshidian, N.; Mortazavian, A.M.; da Cruz, A.G. Probiotic: Conceptualization from a new approach. Curr. Opin. Food Sci. 2020, 32, 103–123. [Google Scholar] [CrossRef]
- Kolling, Y.; Salva, S.; Villena, J.; Marranzino, G.; Alvarez, S. Non-viable immunobiotic Lactobacillus rhamnosus CRL1505 and its peptidoglycan improve systemic and respiratory innate immune response during recovery of immunocompromised-malnourished mice. Int. Immunopharmacol. 2015, 25, 474–484. [Google Scholar] [CrossRef]
- He, F.; Morita, H.; Kubota, A.; Ouwehand, A.C.; Hosoda, M.; Hiramatsu, M.; Kurisaki, J.I. Effect of orally administered non-viable Lactobacillus cells on murine humoral immune responses. Microbiol. Immunol. 2005, 49, 993–997. [Google Scholar] [CrossRef]
- Deshpande, G.; Athalye-Jape, G.; Patole, S. Para-probiotics for preterm neonates—The next frontier. Nutrients 2018, 10, 871. [Google Scholar] [CrossRef]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb. Cell Fact. 2020, 19, 1–22. [Google Scholar] [CrossRef]
- Lee, E.S.; Song, E.J.; Nam, Y.D.; Lee, S.Y. Probiotics in human health and disease: From nutribiotics to pharmabiotics. J. Microbiol. 2018, 56, 773–782. [Google Scholar] [CrossRef]
- Yang, S.-C.; Lin, C.-H.; Sung, C.T.; Fang, J.-Y. Antibacterial activities of bacteriocins: Application in foods and pharmaceuticals. Front. Microbiol. 2014. [Google Scholar] [CrossRef]
- Hill, C. Probiotics and pharmabiotics: Alternative medicine or an evidence-based alternative? Bioeng. Bugs 2010, 1, 79–84. [Google Scholar] [CrossRef]
- Oleskin, A.V.; Shenderov, B.A. Probiotics and Psychobiotics: The Role of Microbial Neurochemicals. Probiotics Antimicrob. Proteins 2019, 11, 1071–1085. [Google Scholar] [CrossRef]
- Priyodip, P.; Balaji, S. A Preliminary Study on Probiotic Characteristics of Sporosarcina spp. for Poultry Applications. Curr. Microbiol. 2019, 76, 448–461. [Google Scholar] [CrossRef]
- Nikolaev, Y.A.; Plakunov, V.K. Biofilm-“city of microbes” or an analogue of multicellular organisms? Microbiology 2007, 76, 125–138. [Google Scholar] [CrossRef]
- Stadie, J. Metabolic Activity and Symbiotic Interactions of Lactic Acid Bacteria and Yeasts Isolated from Water Kefir. Ph.D. Thesis, Technische Universität München, Freising, Germany, 2013. [Google Scholar]
- Vasile, M.-A.; Cotârleţ, M.; Bahrim, G.-E. Metabolic functionality of the synergistically ameliorated consortium based on wild kefir grains and selected microorganisms for bovine colostrum fermentation. Orphanet J. Rare Dis. 2020, 21, 1–9. [Google Scholar]
- Coma, M.E.; Peltzer, M.A.; Delgado, J.F.; Salvay, A.G. Water kefir grains as an innovative source of materials: Study of plasticiser content on film properties. Eur. Polym. J. 2019, 120, 109234. [Google Scholar] [CrossRef]
- Dertli, E.; Çon, A.H. Microbial diversity of traditional kefir grains and their role on kefir aroma. LWT Food Sci. Technol. 2017, 85, 151–157. [Google Scholar] [CrossRef]
- Kalamaki, M.S.; Angelidis, A.S. High-throughput, sequence-based analysis of the microbiota of Greek kefir grains from two geographic regions. Food Technol. Biotechnol. 2020, 58, 138–146. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Fels, L.; Jakob, F.; Vogel, R.F.; Wefers, D. Structural characterization of the exopolysaccharides from water kefir. Carbohydr. Polym. 2018, 189, 296–303. [Google Scholar] [CrossRef]
- Laureys, D.; De Vuyst, L. Microbial species diversity, community dynamics, and metabolite kinetics of water Kefir fermentation. Appl. Environ. Microbiol. 2014, 80, 2564–2572. [Google Scholar] [CrossRef]
- Hermann, M.; Petermeier, H.; Vogel, R.F. Development of novel sourdoughs with in situ formed exopolysaccharides from acetic acid bacteria. Eur. Food Res. Technol. 2015, 241, 185–197. [Google Scholar] [CrossRef]
- Darvishzadeh, P.; Orsat, V.; Faucher, S.P. Encapsulation of Russian Olive Water Kefir as an Innovative Functional Drink with High Antioxidant Activity. Plant Foods Hum. Nutr. 2021. [Google Scholar] [CrossRef]
- Verce, M.; De Vuyst, L.; Weckx, S. Shotgun metagenomics of a water kefir fermentation ecosystem reveals a novel Oenococcus species. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Gulitz, A.; Stadie, J.; Wenning, M.; Ehrmann, M.A.; Vogel, R.F. The microbial diversity of water kefir. Int. J. Food Microbiol. 2011, 151, 284–288. [Google Scholar] [CrossRef]
- Bechtner, J.; Ludwig, C.; Kiening, M.; Jakob, F.; Vogel, R.F. Living the sweet life: How Liquorilactobacillus hordei TMW 1.1822 changes its behavior in the presence of sucrose in comparison to glucose. Foods 2020, 9, 1150. [Google Scholar] [CrossRef]
- Kim, D.H.; Jeong, D.; Kim, H.; Kang, I.B.; Chon, J.W.; Song, K.Y.; Seo, K.H. Antimicrobial activity of kefir against various food pathogens and spoilage bacteria. Korean J. Food Sci. Anim. Resour. 2016, 36, 787–790. [Google Scholar] [CrossRef]
- Gul, O.; Mortas, M.; Atalar, I.; Dervisoglu, M.; Kahyaoglu, T. Manufacture and characterization of kefir made from cow and buffalo milk, using kefir grain and starter culture. J. Dairy Sci. 2015, 98, 1517–1525. [Google Scholar] [CrossRef]
- Gao, X.; Li, B. Chemical and microbiological characteristics of kefir grains and their fermented dairy products: A review. Cogent Food Agric. 2016, 2. [Google Scholar] [CrossRef]
- Rosa, D.D.; Dias, M.M.S.; Grześkowiak, Ł.M.; Reis, S.A.; Conceição, L.L.; Peluzio, M.D.C.G. Milk kefir: Nutritional, microbiological and health benefits. Nutr. Res. Rev. 2017, 30, 82–96. [Google Scholar] [CrossRef]
- Wang, H.; Sun, X.; Song, X.; Guo, M. Effects of kefir grains from different origins on proteolysis and volatile profile of goat milk kefir. Food Chem. 2021, 339, 128099. [Google Scholar] [CrossRef]
- Bengoa, A.A.; Iraporda, C.; Garrote, G.L.; Abraham, A.G. Kefir micro-organisms: Their role in grain assembly and health properties of fermented milk. J. Appl. Microbiol. 2018, 126, 686–700. [Google Scholar] [CrossRef]
- Filipčev, B.; Šimurina, O.; Bodroža-Solarov, M. Effect of native and lyophilized kefir grains on sensory and physical attributes of wheat bread. J. Food Process. Preserv. 2007, 31, 367–377. [Google Scholar] [CrossRef]
- Nalbantoglu, U.; Cakar, A.; Dogan, H.; Abaci, N.; Ustek, D.; Sayood, K.; Can, H. Metagenomic analysis of the microbial community in kefir grains. Food Microbiol. 2014, 41, 42–51. [Google Scholar] [CrossRef]
- Sindi, A.; Badsha, M.B.; Ünlü, G. Bacterial populations in international artisanal kefirs. Microorganisms 2020, 8, 1318. [Google Scholar] [CrossRef]
- Plessas, S.; Trantallidi, M.; Bekatorou, A.; Kanellaki, M.; Nigam, P.; Koutinas, A.A. Immobilization of kefir and Lactobacillus casei on brewery spent grains for use in sourdough wheat bread making. Food Chem. 2007, 105, 187–194. [Google Scholar] [CrossRef]
- Khokhlacheva, A.A.; Gradova, N.B.; Egorova, M.A. Trophic patterns of functioning and microbial profile of the evolutionally established associated kefir grains culture/Трoфические Закoнoмернoсти Функциoнирoвания И Микрoбный Прoфиль Эвoлюциoннo Слoжившейся Ассoциативнoй Культуры Кефирных Зерен. Microbiology 2015, 84, 466–475. [Google Scholar] [CrossRef]
- Kotova, I.B.; Cherdyntseva, T.A.; Netrusov, A.I. Russian Kefir Grains Microbial Composition and Its Changes during Production Process. Adv. Exp. Med. Biol. 2016, 932, 93–121. [Google Scholar] [CrossRef]
- Pacini, S.; Ruggiero, M. Natural Plasmids in a Swiss Fermented Milk and Colostrum Product assessed by Microbiome Array. Madridge J. Immunol. 2019, 3, 100–108. [Google Scholar] [CrossRef]
- Garneau, J.E.; Moineau, S. Bacteriophages of lactic acid bacteria and their impact on milk fermentations. Microb. Cell Fact. 2011, 10. [Google Scholar] [CrossRef]
- Villion, M.; Moineau, S. Bacteriophages of lactobacillus. Front. Biosci. 2009, 14, 1661–1683. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Zarei, M.; Gholami, A.; Lai, C.W.; Chiang, W.H.; Omidifar, N.; Bahrani, S.; Mazraedoost, S. Recent Progress in Chemical Composition, Production, and Pharmaceutical Effects of Kombucha Beverage: A Complementary and Alternative Medicine. Evidence-based Complement. Altern. Med. 2020, 2020, 14. [Google Scholar] [CrossRef]
- May, A.; Narayanan, S.; Alcock, J.; Varsani, A.; Maley, C.; Aktipis, A. Kombucha: A novel model system for cooperation and conflict in a complex multi-species microbial ecosystem. PeerJ 2019, 7, e7565. [Google Scholar] [CrossRef]
- Tran, T.; Grandvalet, C.; Verdier, F.; Martin, A.; Alexandre, H.; Tourdot-Maréchal, R. Microbial Dynamics between Yeasts and Acetic Acid Bacteria in Kombucha: Impacts on the Chemical Composition of the Beverage. Foods 2020, 9, 963. [Google Scholar] [CrossRef]
- Sengun, I.Y.; Kirmizigul, A. Probiotic potential of kombucha. J. Funct. Foods 2020. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Bouajila, J.; Pace, M.; Leech, J.; Cotter, P.D.; Souchard, J.P.; Taillandier, P.; Beaufort, S. Metabolome-microbiome signatures in the fermented beverage, Kombucha. Int. J. Food Microbiol. 2020, 333, 108778. [Google Scholar] [CrossRef]
- Torán-Pereg, P.; Del Noval, B.; Valenzuela, S.; Martinez, J.; Prado, D.; Perisé, R.; Carlos Arboleya, J. Microbiological and sensory characterization of kombucha SCOBY for culinary applications. Int. J. Gastron. Food Sci. 2021, 23, 100314. [Google Scholar] [CrossRef]
- Góes-Neto, A.; Kukharenko, O.; Orlovska, I.; Podolich, O.; Imchen, M.; Kumavath, R.; Kato, R.B.; de Carvalho, D.S.; Tiwari, S.; Brenig, B.; et al. Shotgun metagenomic analysis of kombucha mutualistic community exposed to Mars-like environment outside the International Space Station. Environ. Microbiol. 2021, 23, 3727–3742. [Google Scholar] [CrossRef]
- Soares, M.G.; de Lima, M.; Reolon Schmidt, V.C. Technological aspects of kombucha, its applications and the symbiotic culture (SCOBY), and extraction of compounds of interest: A literature review. Trends Food Sci. Technol. 2021, 110, 539–550. [Google Scholar] [CrossRef]
- Bogdan, M.; Justine, S.; Filofteia, D.C.; Petruta, C.C.; Gabriela, L.; Roxana, U.E.; Florentina, M. Lactic acid bacteria strains isolated from Kombucha with potential probiotic effect. Rom. Biotechnol. Lett. 2018, 23, 13592–13598. [Google Scholar]
- Diguță, C.F.; Nițoi, G.D.; Matei, F.; Luță, G.; Cornea, C.P. The Biotechnological Potential of Pediococcus spp. Isolated from Kombucha Microbial Consortium. Foods 2020, 9, 1780. [Google Scholar] [CrossRef]
- Golowczyc, M.A.; Silva, J.; Abraham, A.G.; De Antoni, G.L.; Teixeira, P. Preservation of probiotic strains isolated from kefir by spray drying. Lett. Appl. Microbiol. 2010, 50, 7–12. [Google Scholar] [CrossRef]
- Davidović, S.Z.; Miljković, M.G.; Antonović, D.G.; Rajilić-Stojanović, M.D.; Dimitrijević-Branković, S.I. Water Kefir grain as a source of potent dextran producing lactic acid bacteria. Hem. Ind. 2015, 69, 595–604. [Google Scholar] [CrossRef]
- Bellut, K.; Michel, M.; Zarnkow, M.; Hutzler, M.; Jacob, F.; De Schutter, D.P.; Daenen, L.; Lynch, K.M.; Zannini, E.; Arendt, E.K. Application of non-Saccharomyces yeasts isolated from kombucha in the production of alcohol-free beer. Fermentation 2018, 4, 66. [Google Scholar] [CrossRef]
- Pei, J.; Jin, W.; Abd El-Aty, A.M.; Baranenko, D.A.; Gou, X.; Zhang, H.; Geng, J.; Jiang, L.; Chen, D.; Yue, T. Isolation, purification, and structural identification of a new bacteriocin made by Lactobacillus plantarum found in conventional kombucha. Food Control 2020, 110. [Google Scholar] [CrossRef]
- Dudikova, G.N.; Kuznetsova, E.S.; Butyrkina, N.P.P.T.I. Consortium of Microorganisms Lactobacillus Paracasei 2, Lactobacillus pontis 67, Pedicoccus Acidilactici P1-6, Saccharomyces Cerevisiae LV Used to Prepare Rye Bread Starter. Patent Number 27332, 16 September 2013. [Google Scholar]
- Ponomarova, O.; Gabrielli, N.; Sé vin, D.C.; Sauer, U.; Ralser, M.; Raosaheb Patil, K. Yeast Creates a Niche for Symbiotic Lactic Acid Bacteria through Nitrogen Overflow. Cell Syst. 2017, 5, 345–357. [Google Scholar] [CrossRef]
- Senne de Oliveira Lino, F.; Bajic, D.; Vila, J.C.C.; Sánchez, A.; Sommer, M.O.A. Complex yeast–bacteria interactions affect the yield of industrial ethanol fermentation. Nat. Commun. 2021, 12. [Google Scholar] [CrossRef]
- Stadie, J.; Gulitz, A.; Ehrmann, M.A.; Vogel, R.F. Metabolic Activity and Symbiotic Interactions of Lactic Acid Bacteria and Yeasts Isolated from Water Kefir. Food Microbiol. 2013, 35, 92–98. [Google Scholar] [CrossRef]
- Nejati, F.; Junne, S.; Kurreck, J.; Neubauer, P. Quantification of Major Bacteria and Yeast Species in Kefir Consortia by Multiplex TaqMan qPCR. Front. Microbiol. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, J.; Jia, Y.; Pan, Y.; Wang, Y. Lactobacillus kefiranofaciens, the sole dominant and stable bacterial species, exhibits distinct morphotypes upon colonization in Tibetan kefir grains. Heliyon 2018, 4, e00649. [Google Scholar] [CrossRef]
- Istiqomah, L.; Damayanti, E.; Suryani, A.E.; Karimy, M.F.; Maknuna, D. Lutfiani Synergistic effect between Lactobacillus plantarum AKK30 and Saccharomyces cerevisiae B18 and the probiotic properties of microencapsulated cultures. In Proceedings of the IOP Conference Series: Earth and Environmental Science; Institute of Physics Publishing: Tangerang, Indonesia, 2019; Volume 251. [Google Scholar] [CrossRef]
- Korukluoglu, M.; Arik, G.; Erdogan, C.; Kocakoglu, S. Screening of Antagonistic/Synergistic Effect b etween Lactic Acid Bacteria (LAB ) and Yeast Strains Isolated from Kefir. Int. J. Nutr. Food Eng. 2017, 11, 282–288. [Google Scholar]
- Yang, Z.; Zhou, F.; Ji, B.; Li, B.; Luo, Y.; Yang, L.; Li, T. Symbiosis between microorganisms from kombucha and kefir: Potential significance to the enhancement of kombucha function. Appl. Biochem. Biotechnol. 2010, 160, 446–455. [Google Scholar] [CrossRef]
- Lee, K.R.; Jo, K.; Ra, K.S.; Suh, H.J.; Hong, K.-B. Kombucha fermentation using commercial kombucha pellicle and culture broth as starter. Food Sci. Technol. 2021, 2061, 1–7. [Google Scholar] [CrossRef]
- Mei, J.; Gao, X.; Li, Y. Kefir Grains and their Fermented Dairy Products. JSM Biotechnol. Biomed. Eng. Rev. 2016, 3, 1–7. [Google Scholar]
- Francisco, Á.R.; de la Rosa, M.; Igor, H. Development of a no added sugar kombucha beverage based on germinated corn. Int. J. Gastron. Food Sci. 2021, 24, 100355. [Google Scholar] [CrossRef]
- Salazar Alzate, B.C.; Cortés Rodríguez, M.; Montoya Campuzano, O. Identification of some kefir microorganisms and optimization of their production in sugarcane juice. Rev. Fac. Nac. Agron. Medellin 2016, 69, 7935–7943. [Google Scholar] [CrossRef]
- Rocha-Gomes, A.; Escobar, A.; Soares, J.S.; Alves, A.; Silva, D.; Riul, T.R. Chemical composition and hypocholesterolemic effect of milk kefir and water kefir in Wistar rats Composição química e efeito hipocolesterolêmico do kefir de leite e do kefir de água em ratos Wistar. Rev. Nutr. 2018, 31, 137–145. [Google Scholar] [CrossRef]
- Londero, A.; Hamet, M.F.; De Antoni, G.L.; Garrote, G.L.; Abraham, A.G. Kefir grains as a starter for whey fermentation at different temperatures: Chemical and microbiological characterisation. J. Dairy Res. 2012, 79, 262–271. [Google Scholar] [CrossRef]
- Garofalo, C.; Ferrocino, I.; Reale, A.; Sabbatini, R.; Milanović, V.; Alkić-Subašić, M.; Boscaino, F.; Aquilanti, L.; Pasquini, M.; Trombetta, M.F.; et al. Study of kefir drinks produced by backslopping method using kefir grains from Bosnia and Herzegovina: Microbial dynamics and volatilome profile. Food Res. Int. 2020, 137. [Google Scholar] [CrossRef]
- Mechmeche, M.; Ksontini, H.; Hamdi, M.; Kachouri, F. Production of Bioactive Peptides in Tomato Seed Protein Isolate Fermented by Water Kefir Culture: Optimization of the Fermentation Conditions. Int. J. Pept. Res. Ther. 2019, 25, 137–150. [Google Scholar] [CrossRef]
- Tu, C.; Azi, F.; Huang, J.; Xu, X.; Xing, G.; Dong, M. Quality and metagenomic evaluation of a novel functional beverage produced from soy whey using water kefir grains. Lwt 2019, 113, 108258. [Google Scholar] [CrossRef]
- Lim, X.X.; Koh, W.Y.; Uthumporn, U.; Maizura, M.; Wan Rosli, W.I. The development of legume-based yogurt by using water kefir as starter culture. Int. Food Res. J. 2019, 26, 1219–1228. [Google Scholar]
- Maldonado, R.R.; Pedreira, A.J.R.M.; Cristianini, L.B.; Guidi, M.F.; Capato, M.O.; Ávila, P.F.; Goldbeck, R.; Kamimura, E.S. Application of soluble fibres in the osmotic dehydration of pineapples and reuse of effluent in a beverage fermented by water kefir. Lwt 2020, 132. [Google Scholar] [CrossRef]
- Randazzo, W.; Corona, O.; Guarcello, R.; Francesca, N.; Germanà, M.A.; Erten, H.; Moschetti, G.; Settanni, L. Development of new non-dairy beverages from Mediterranean fruit juices fermented with water kefir microorganisms. Food Microbiol. 2016, 54, 40–51. [Google Scholar] [CrossRef]
- Bueno, R.S.; Ressutte, J.B.; Hata, N.N.Y.; Henrique-Bana, F.C.; Guergoletto, K.B.; de Oliveira, A.G.; Spinosa, W.A. Quality and shelf life assessment of a new beverage produced from water kefir grains and red pitaya. Lwt 2021, 140. [Google Scholar] [CrossRef]
- Bergmann, R.S.d.O.; Pereira, M.A.; Veiga, S.M.O.M.; Schneedorf, J.M.; Oliveira, N.d.M.S.; Fiorini, J.E. Microbial profile of a kefir sample preparations: Grains in natura and lyophilized and fermented suspension. Ciência Tecnol. Aliment. 2010, 30, 1022–1026. [Google Scholar] [CrossRef]
- Tan, W.C.; Muhialdin, B.J.; Meor Hussin, A.S. Influence of Storage Conditions on the Quality, Metabolites, and Biological Activity of Soursop (Annona muricata L.) Kombucha. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Jafari, R.; Naghavi, N.S.; Khosravi-Darani, K.; Doudi, M.; Shahanipour, K. Kombucha microbial starter with enhanced production of antioxidant compounds and invertase. Biocatal. Agric. Biotechnol. 2020, 29. [Google Scholar] [CrossRef]
- Khaleil, M.M. A Bioprocess development study of polyphenol profile, antioxidant and antimicrobial activities of kombucha enriched with Psidium guajava L. J. Microbiol. Biotechnol. Food Sci. 2020, 9, 1204–1210. [Google Scholar] [CrossRef]
- Kruk, M.; Trząskowska, M.; Ścibisz, I.; Pokorski, P. Application of the “scoby” and kombucha tea for the production of fermented milk drinks. Microorganisms 2021, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cheng, M.; Li, Z.; Guan, S.; Cai, B.; Li, Q.; Rong, S. Composition and biological activity of rose and jujube kernel after fermentation with kombucha SCOBY. J. Food Process. Preserv. 2020, 44, 1–11. [Google Scholar] [CrossRef]
- Mohd Roby, B.H.; Muhialdin, B.J.; Abadl, M.M.T.; Mat Nor, N.A.; Marzlan, A.A.; Lim, S.A.H.; Mustapha, N.A.; Meor Hussin, A.S. Physical properties, storage stability, and consumer acceptability for sourdough bread produced using encapsulated kombucha sourdough starter culture. J. Food Sci. 2020, 85, 2286–2295. [Google Scholar] [CrossRef] [PubMed]
- Bondareva, N.I.; Mitina, S.S.; Avanesyan, S.S.; Timchenko, L.D. Contents ascorbic acid and rutin in enzymatic culture of kombucha (Medusomyces gysevii) under different conditions of cultivation/Сoдержани е аскoрбинoвo й кислoты и рутин а в ферментативнo й жидкoст и чайнoг o гриб а (medüsomyces gysevii) пр и различны х. Sci. Technol. 2016, 2, 147–158. [Google Scholar] [CrossRef]
- Ziarno, M.; Hasalliu, R.; Cwalina, A. Effect of the Addition of Milk Protein Preparations on Selected Quality Parameters and Nutritional Characteristics of Kefir. Appl. Sci. 2021, 11, 966. [Google Scholar] [CrossRef]
- Cotârleț, M.; Vasile, A.M.; Cantaragiu, A.M.; Gaspar-Pintiliescu, A.; Craciunescu, O.; Oancea, A.; Moraru, A.; Moraru, I.; Bahrim, G.E. Colostrum-derived bioactive peptides obtained by fermentation with kefir grains enriched with selected yeasts. Ann. Univ. Dunarea Jos Galati, Fascicle VI Food Technol. 2019, 43, 54–68. [Google Scholar] [CrossRef]
- Lynch, K.M.; Wilkinson, S.; Daenen, L.; Arendt, E.K. An update on water kefir: Microbiology, composition and production. Int. J. Food Microbiol. 2021, 345, 109128. [Google Scholar] [CrossRef] [PubMed]
- Laureys, D.; Aerts, M.; Vandamme, P.; De Vuyst, L. The Buffer Capacity and Calcium Concentration of Water Influence the Microbial Species Diversity, Grain Growth, and Metabolite Production During Water Kefir Fermentation. Front. Microbiol. 2019, 00, 2876. [Google Scholar] [CrossRef] [PubMed]
- Gamba, R.R.; Moure, C.; Diosma, G.; Giannuzzi, L.; De Antoni, G.L.; León Peláez, Á.M. Application of Whey Permeate Fermented with Kefir Grains for the Shelf-Life Improvement of Food and Feed. Adv. Microbiol. 2016, 6, 650–661. [Google Scholar] [CrossRef][Green Version]
- Hikmetoglu, M.; Sogut, E.; Sogut, O.; Gokirmakli, C.; Guzel-Seydim, Z.B. Changes in carbohydrate profile in kefir fermentation. Bioact. Carbohydrates Diet. Fibre 2020, 23, 100220. [Google Scholar] [CrossRef]
- Sodanlo, A.; Azizkhani, M. Evaluation of Antioxidant and Antimicrobial Activity of Water-Soluble Peptides Extracted from Iranian Traditional Kefir. Int. J. Pept. Res. Ther. 2021, 27, 1441–1449. [Google Scholar] [CrossRef]
- Azizi, N.F.; Kumar, M.R.; Yeap, S.K.; Abdullah, J.O.; Khalid, M.; Omar, A.R.; Osman, M.A.; Mortadza, S.A.S.; Alitheen, N.B. Kefir and its biological activities. Foods 2021, 10, 1210. [Google Scholar] [CrossRef]
- Romero-Luna, H.E.; Peredo-Lovillo, A.; Hernández-Mendoza, A.; Hernández-Sánchez, H.; Cauich-Sánchez, P.I.; Ribas-Aparicio, R.M.; Dávila-Ortiz, G. Probiotic Potential of Lactobacillus paracasei CT12 Isolated from Water Kefir Grains (Tibicos). Curr. Microbiol. 2020, 77, 2584–2592. [Google Scholar] [CrossRef]
- Díaz-Montes, E. Dextran: Sources, Structures, and Properties. Polysaccharides 2021, 2, 554–565. [Google Scholar] [CrossRef]
- Adachi, O.; Hours, R.A.; Akakabe, Y.; Tanasupawat, S.; Yukphan, P.; Shinagawa, E.; Yakushi, T.; Matsushita, K. Production of 4-keto-D-arabonate by oxidative fermentation with newly isolated Gluconacetobacter liquefaciens. Biosci. Biotechnol. Biochem. 2010, 74, 2555–2558. [Google Scholar] [CrossRef]
- Zongo, O.; Cruvellier, N.; Leray, F.; Bideaux, C.; Lesage, J.; Zongo, C.; Traoré, Y.; Savadogo, A.; Guillouet, S. Physicochemical composition and fermentation kinetics of a novel Palm Sap-based Kefir Beverage from the fermentation of Borassus aethiopum Mart. fresh sap with kefir grains and ferments. Sci. African 2020, 10, e00631. [Google Scholar] [CrossRef]
- Plessas, S.; Alexopoulos, A.; Mantzourani, I.; Koutinas, A.; Voidarou, C.; Stavropoulou, E.; Bezirtzoglou, E. Application of novel starter cultures for sourdough bread production. Anaerobe 2011, 17, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Mantzourani, I.; Plessas, S.; Saxami, G.; Alexopoulos, A.; Galanis, A.; Bezirtzoglou, E. Study of kefir grains application in sourdough bread regarding rope spoilage caused by Bacillus spp. Food Chem. 2014, 143, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Kolakowski, P.; Ozimkiewicz, M. Kefir grain tolerance to Escherichia coli contamination—industrial advantages. Dairy Sci. Technol. 2012, 92, 709–718. [Google Scholar] [CrossRef][Green Version]
- Păcularu-Burada, B.; Georgescu, L.A.; Bahrim, G.E. Current approaches in sourdough production with valuable characteristics for technological and functional applications. Ann. Univ. Dunarea Jos Galati Fascicle VI Food Technol. 2020, 44, 132–148. [Google Scholar] [CrossRef]
- Matsumoto, R.; Okochi, M.; Shimizu, K.; Kanie, K.; Kato, R.; Honda, H. Effects of the properties of short peptides conjugated with cell-penetrating peptides on their internalization into cells. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef]
- Bengoa, A.A.; Dardis, C.; Gagliarini, N.; Garrote, G.L.; Abraham, A.G. Exopolysaccharides From Lactobacillus paracasei Isolated From Kefir as Potential Bioactive Compounds for Microbiota Modulation. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Yusuf, D.; Nuraida, L.; Dewanti-Hariyadi, R.; Hunaefi, D. In vitro Antioxidant and α-Glucosidase Inhibitory Activities of Lactobacillus spp. Isolated from Indonesian Kefir Grains. Indones. Kefir Grains. Appl. Food Biotechnol. 2021, 8, 39–46. [Google Scholar] [CrossRef]
- Ebner, J.; Aşçi Arslan, A.; Fedorova, M.; Hoffmann, R.; Küçükçetin, A.; Pischetsrieder, M. Peptide profiling of bovine kefir reveals 236 unique peptides released from caseins during its production by starter culture or kefir grains. J. Proteomics 2015, 117, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Assohoun, M.C.N.; Djeni, T.N.; Koussémon-Camara, M.; Brou, K. Effect of Fermentation Process on Nutritional Composition and Aflatoxins Concentration of Doklu, a Fermented Maize Based Food. Food Nutr. Sci. 2013, 4, 1120–1127. [Google Scholar] [CrossRef]
- Ben Taheur, F.; Mansour, C.; Ben Jeddou, K.; Machreki, Y.; Kouidhi, B.; Abdulhakim, J.A.; Chaieb, K. Aflatoxin B1 degradation by microorganisms isolated from Kombucha culture. Toxicon 2020, 179, 76–83. [Google Scholar] [CrossRef]
- Bauer-Petrovska, B.; Petrushevska-Tozi, L. Mineral and water soluble vitamin content in the Kombucha drink. Int. J. Food Sci. Technol. 2000, 35, 201–205. [Google Scholar] [CrossRef]
- Wang, K.; Gan, X.; Tang, X.; Wang, S.; Tan, H. Determination of d-saccharic acid-1,4-lactone from brewed kombucha broth by high-performance capillary electrophoresis. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 371–374. [Google Scholar] [CrossRef]
- Afsharmanesh, M.; Sadaghi, B. Effects of dietary alternatives (probiotic, green tea powder, and Kombucha tea) as antimicrobial growth promoters on growth, ileal nutrient digestibility, blood parameters, and immune response of broiler chickens. Comp. Clin. Path. 2014, 23, 717–724. [Google Scholar] [CrossRef]
- Farag, M.A.; Jomaa, S.A.; El-wahed, A.A.; El-seedi, H.R. The many faces of kefir fermented dairy products: Quality characteristics, flavour chemistry, nutritional value, health benefits, and safety. Nutrients 2020, 12, 364. [Google Scholar] [CrossRef]
- Guzel-Seydim, Z.B.; Gökırmaklı, Ç.; Greene, A.K. A comparison of milk kefir and water kefir: Physical, chemical, microbiological and functional properties. Trends Food Sci. Technol. 2021, 113, 42–53. [Google Scholar] [CrossRef]
- Alieva, E.V.; Boltacheva, K.M.; Timchenko, L.D.; Bondareva, N.I.; Dobrynya, Y.M. Antibacterial potential and prospects for kombucha use/антибактериальный пoтенциал и перспективы испoльзoвания чайнoгo гриба. Ульянoвский Mедикo-Биoлoгический Журнал 2018, 4, 166–171. [Google Scholar] [CrossRef]
- Antolak, H.; Piechota, D.; Kucharska, A. Kombucha Tea—A Double Power of Bioactive Compounds from Tea and Symbiotic Culture of Bacteria and Yeasts (SCOBY). Antioxidants 2021, 10, 1541. [Google Scholar] [CrossRef]
- Neffe-Skocińska, K.; Sionek, B.; Ścibisz, I.; Kołożyn-Krajewska, D. Acid contents and the effect of fermentation condition of Kombucha tea beverages on physicochemical, microbiological and sensory properties. CYTA J. Food 2017, 15, 601–607. [Google Scholar] [CrossRef]
- Oleskin, A.V. In memory of Boris Arkadievich Shenderov (25.12. 1940–21.12.2020). Exp. Clin. Gastroenterol. 2021, 1, 208–210. [Google Scholar] [CrossRef]
- Ardatskaya, M.D.; Stolyarova, L.G.; Arkhipova, E.V.; Filimonova, O.Y. Metabiotics as a Natural Development of a Probiotic Concept (Метабиoтики как естественнoе развитие прoбиoти-ческoй кoнцепции). ТРУДНЫЙ ПАЦИЕНТ 2017, 15, 35–39. [Google Scholar]
- Vallejo-Cordoba, B.; Castro-López, C.; García, H.S.; González-Córdova, A.F.; Hernández-Mendoza, A. Postbiotics and Paraprobiotics: A Review of Current Evidence and Emerging Trends, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; Volume 94, ISBN 9780128202180. [Google Scholar]
- Shenderov, B.A.; Sinitsa, A.V.; Zakharchenko, M.M.; Lang, C. Methods and Techniques Used for Obtaining and Identifying of Microbial Low Molecular Weight Cellular Compounds, Metabolites and Signaling Molecules. In METABIOTICS; Springer International Publishing: Berlin, Germany, 2020; pp. 53–55. [Google Scholar]
- Harrison, S.T.L. Cell Disruption. In Comprehensive Biotechnology, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2011; Volume 2, pp. 619–640. ISBN 9780080885049. [Google Scholar]
- Molaee Parvarei, M.; Khorshidian, N.; Fazeli, M.R.; Mortazavian, A.M.; Sarem Nezhad, S.; Mortazavi, S.A. Comparative effect of probiotic and paraprobiotic addition on physicochemical, chemometric and microstructural properties of yogurt. LWT 2021, 144. [Google Scholar] [CrossRef]
- Tan, K.X.; Chamundeswari, V.N.; Loo, S.C.J. Prospects of kefiran as a food-derived biopolymer for agri-food and biomedical applications. RSC Adv. 2020, 10, 25339–25351. [Google Scholar] [CrossRef]
- Pop, C.R.; Salanţă, L.; Rotar, A.M.; Semeniuc, C.A.; Socaciu, C.; Sindic, M. Influence of extraction conditions on characteristics of microbial polysaccharide kefiran isolated from kefir grains biomass. J. Food Nutr. Res. 2016, 55, 121–130. [Google Scholar]
- Santos, M.; Rodrigues, A.; Teixeira, J.A. Production of dextran and fructose from carob pod extract and cheese whey by Leuconostoc mesenteroides NRRL B512(f). Biochem. Eng. J. 2005, 25, 1–6. [Google Scholar] [CrossRef]
- Kovalev, G.V.; Sinitsyn, A.P.; Bugaenko, L.T. Degradation and crosslinking of dextran in aqueous solutions by γ-radiolysis: The effect of hydrogen ions. High Energy Chem. 2000, 34, 74–79. [Google Scholar] [CrossRef]
- Iqbal, S.; Marchetti, R.; Aman, A.; Silipo, A.; Qader, S.A.U.; Molinaro, A. Enzymatic and acidic degradation of high molecular weight dextran into low molecular weight and its characterizations using novel Diffusion-ordered NMR spectroscopy. Int. J. Biol. Macromol. 2017, 103, 744–750. [Google Scholar] [CrossRef]
- Oliver-Ortega, H.; Geng, S.; Espinach, F.X.; Oksman, K.; Vilaseca, F. Bacterial cellulose network from kombucha fermentation impregnated with emulsion-polymerized poly(Methyl methacrylate) to form nanocomposite. Polymers 2021, 13, 664. [Google Scholar] [CrossRef]
- Kamiński, K.; Jarosz, M.; Grudzień, J.; Pawlik, J.; Zastawnik, F.; Pandyra, P.; Kołodziejczyk, A.M. Hydrogel bacterial cellulose: A path to improved materials for new eco-friendly textiles. Cellulose 2020, 27, 5353–5365. [Google Scholar] [CrossRef]
- Mathew, A.; Joykutty, L. Article Developing “ Off the Shelf ” Pancreases for Diabetic Patients Using Bacterial and Kombucha Tea Waste. Emerg. Investig. 2021, 4, 1–6. [Google Scholar]
- Manuel, R.C.; Esther, A.R.; Guillermo, N.R.; Mireles, R.L.D.C.; Figueroa, L.Á.; Orozco, J.J.I.; Peña, E.R.D. La Evaluation of the Properties of Healing of the Extract of Kombucha in Sheep in Growth with Malnutrition, Parasitocis and Respiratory Problems. Open J. Vet. Med. 2014, 4, 175–182. [Google Scholar] [CrossRef][Green Version]
- Manuel, R.C.; Albarrán-Rodríguez, E.; Nolasco-Rodríguez, G.; Avíla-Figueroa, D.; Cervantes-Mireles, R.L.; De Rosales-Ramírez, R.; García-Carrasco, D.M. Healing Effect of the Extract of Kombucha in Male Wistar Rats. Open J. Vet. Med. 2015, 5, 80–88. [Google Scholar] [CrossRef][Green Version]




| Genus | Old Taxonomy | New Taxonomy |
|---|---|---|
| Lactobacillus | Lactobacillus casei | Lacticaseibacillus casei |
| Lactobacillus rhamnosus | Lacticaseibacillus rhamnosus | |
| Lactobacillus brevis | Levilactobacillus brevis | |
| Lactobacillus hordei | Liquorilactobacillus hordei | |
| Lactobacillus fermentum | Limosilactobacillus fermentum | |
| Lactobacillus paracasei | Lacticaseibacillus paracasei | |
| Lactobacillus plantarum | Lactiplantibacillus plantarum | |
| Lactobacillus reuteri | Limosilactobacillus reuteri | |
| Lactobacillus acidophilus | Lactobacillus acidophilus | |
| Lactobacillus curvatus | Latilactobacillus curvatus | |
| Lactobacillus delbrueckii | Lactobacillus delbrueckii | |
| Lactobacillus sanfranciscensis | Fructilactobacillus sanfranciscensis | |
| Lactobacillus helveticus | Lactobacillus helveticus | |
| Lactobacillus sakei | Latilactobacillus sakei |
| Artisanal Culture | Raw Material/Substrate of Fermentation | Benefits/Findings | References |
|---|---|---|---|
| Milk kefir grains | Sugarcane concentrate | Alternatives for the development of non-dairy foods, prevention of gastrointestinal diseases and strengthening the immune system | [92] |
| Whole and skim milk | Improved plasma and hepatic lipide profile in rats | [93] | |
| Non-fat milk + sweet whey powder | Bacterial proteolytic activities contribute to the beneficial effects of whey fermented with kefir grains | [94] | |
| Goat milk | β-casein with an ACE inhibitor and antioxidant activity | [55] | |
| Bovine milk | Detecting ketones, esters, acetates, alcohols, and acids | [95] | |
| Milk with 2.8% fat | Probiotic beverage | [89] | |
| Bovine colostrum | Fermented product with antimicrobial properties | [39] | |
| Water kefir grains | Tomato seed protein isolate | Protein-rich isolate from the tomato seed meal was converted to antioxidant hydrolysates | [96] |
| Soy whey | Novel bioactive beverage with high functional potential | [97] | |
| Soybean milk and black bean milk | Good alternative substrates for kefir yogurt production with probiotic potential | [98] | |
| Tap water + cane sugar + fig extract | Detection of Bifidobacterium psychraerophilum/crudilactis | [45] | |
| Osmotically dehydrated (OD) pineapple | Production of a potential symbiotic beverage of potentially high added value | [99] | |
| Tap water + figs + lemon | Dextrans obtaining | [44] | |
| Apple, quince, grape, kiwifruit, prickly pear, and pomegranate juice | Developing fruit-based kefir-like beverages with high added value and functional properties | [100] | |
| Red pitaya and apple pulp | Producing a new functional beverage | [101] | |
| Coconut Water Agar (CWA) and CWA supplemented with yeast extract (CWAY) especially for strains isolation | Two alternative and salutary media for culture of kefir strains | [102] | |
| Kombucha | Fresh and ripen soursop (Annona muricata L.) fruits | High potential to improve the quality, metabolites content, biological activity, and the Halal status of soursop kombucha | [103] |
| Black tea + sugar | Invertase in kombucha tea increase the nutritional value of fermented product for diabetes patients | [104] | |
| Green/black tea + guava juice | Antimicrobial activity against human pathogenic bacterial strains and pathogenic fungi | [105] | |
| Lactose and lactose-free milk | Due to active microflora and organic acids, have a confirmed positive effect on the human body | [106] | |
| Jujube kernel | Obtaining of functional beverages and jujuboside B | [107] | |
| Unbleached wheat flour | Encapsulated kombucha-like sourdough starter for production of functional sourdough bread with extended shelf life and improved quality | [108] | |
| Back tea with total replacement or in combination with Melissa officinalis, Quercus robur, Vaccinium myrtillus, Callisia fragrans | Ascorbic acid and rutin obtaining | [109] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pihurov, M.; Păcularu-Burada, B.; Cotârleţ, M.; Vasile, M.A.; Bahrim, G.E. Novel Insights for Metabiotics Production by Using Artisanal Probiotic Cultures. Microorganisms 2021, 9, 2184. https://doi.org/10.3390/microorganisms9112184
Pihurov M, Păcularu-Burada B, Cotârleţ M, Vasile MA, Bahrim GE. Novel Insights for Metabiotics Production by Using Artisanal Probiotic Cultures. Microorganisms. 2021; 9(11):2184. https://doi.org/10.3390/microorganisms9112184
Chicago/Turabian StylePihurov, Marina, Bogdan Păcularu-Burada, Mihaela Cotârleţ, Mihaela Aida Vasile, and Gabriela Elena Bahrim. 2021. "Novel Insights for Metabiotics Production by Using Artisanal Probiotic Cultures" Microorganisms 9, no. 11: 2184. https://doi.org/10.3390/microorganisms9112184
APA StylePihurov, M., Păcularu-Burada, B., Cotârleţ, M., Vasile, M. A., & Bahrim, G. E. (2021). Novel Insights for Metabiotics Production by Using Artisanal Probiotic Cultures. Microorganisms, 9(11), 2184. https://doi.org/10.3390/microorganisms9112184









