Virulence Potential and Treatment Options of Multidrug-Resistant (MDR) Acinetobacter baumannii
Abstract
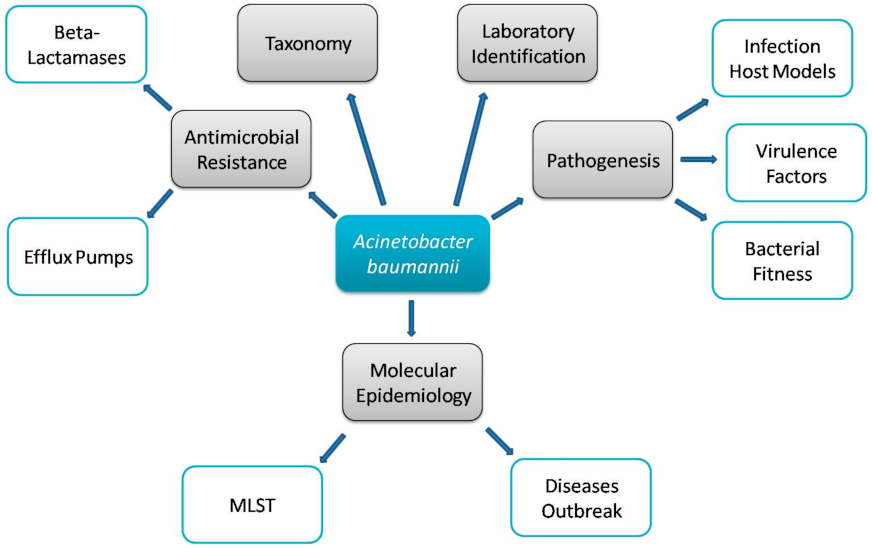
1. Introduction
2. Multidrug Resistance Mechanisms
- Enzymatic mechanisms including; deferent β-lactamases.
- Non-enzymatic mechanisms involving efflux pumps and membrane permeability.
- Change in the sequence of penicillin-binding proteins (PBPs).
2.1. Enzymatic Mechanisms (Beta-Lactamases)
2.2. Non-Enzymatic Mechanisms (Efflux Pumps)
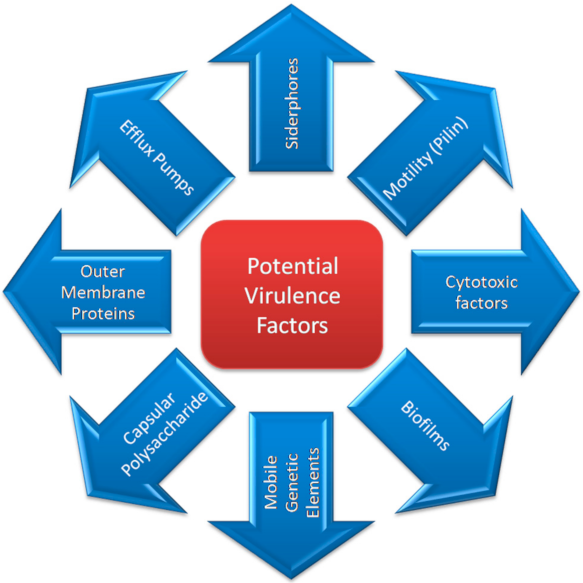
3. Virulence Factors
3.1. Outer Membrane Proteins (OMPs) and Outer Membrane Vesicles (OMVs)
3.2. Biofilm
3.3. Penicillin-Binding Proteins (PBPs)
3.4. Siderophores/Iron
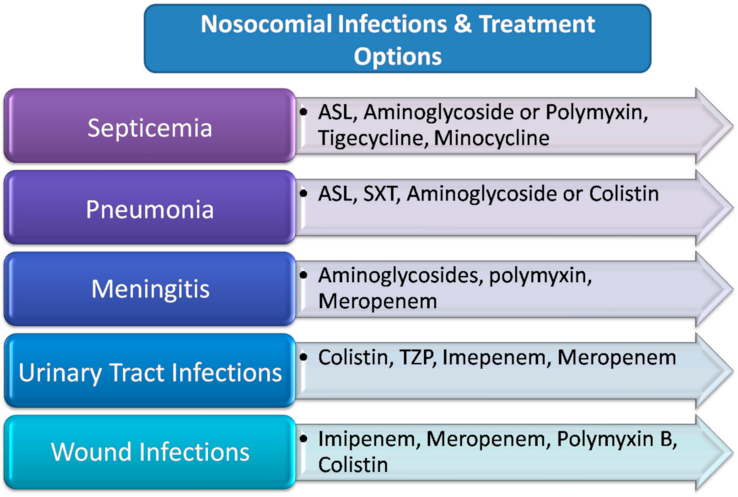
4. Antimicrobial Therapy
4.1. Monotherapy
4.2. Synergy and Combination Therapy
4.3. Dose and Drug of Choice
4.4. Polymyxins
4.5. Sulbactam/β-Lactamase Inhibitors
4.6. Tigecycline
4.7. Aminoglycosides
4.8. Tetracyclines
5. Prevention and Control
6. Study of Virulence Using Animal Models
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, M.F.; Lin, Y.Y.; Yeh, H.W.; Lan, C.Y. Role of the BaeSR two-component system in the regulation of Acinetobacter baumannii adeAB genes and its correlation with tigecycline susceptibility. BMC Microbiol. 2014, 14, 119. [Google Scholar] [CrossRef]
- Chan, J.Z.; Halachev, M.R.; Loman, N.J.; Constantinidou, C.; Pallen, M.J. Defining bacterial species in the genomic era: Insights from the genus Acinetobacter. BMC Microbiol. 2012, 12, 302. [Google Scholar] [CrossRef] [PubMed]
- Perez, S.; Innes, G.K.; Walters, M.S.; Mehr, J.; Arias, J.; Greeley, R.; Chew, D. Increase in Hospital-Acquired Carbapenem-Resistant Acinetobacter baumannii Infection and Colonization in an Acute Care Hospital During a Surge in COVID-19 Admissions—New Jersey, February–July 2020. MMWR Morb. Mortal Wkly. Rep. 2020, 69, 1827–1831. [Google Scholar] [CrossRef]
- Shu, H.; Li, L.; Wang, Y.; Guo, Y.; Wang, C.; Yang, C.; Gu, L.; Cao, B. Prediction of the Risk of Hospital Deaths in Patients with Hospital-Acquired Pneumonia Caused by Multidrug-Resistant Acinetobacter baumannii Infection: A Multi-Center Study. Infect. Drug Resist. 2020, 13, 4147–4154. [Google Scholar] [CrossRef]
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter baumannii: Emergence of a successful pathogen. Clin. Microbiol. Rev. 2008, 21, 538–582. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Shao, N.; Zheng, J. Integrated Co-functional Network Analysis on the Resistance and Virulence Features in Acinetobacter baumannii. Front. Microbiol. 2020, 11, 598380. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, B.; Bazgir, Z.N.; Goli, H.R.; Iranpour, F.; Mohammadi, F.; Babaei, R. Prevalence of multi-drug resistant (MDR) and extensively drug-resistant (XDR) phenotypes of Pseudomonas aeruginosa and Acinetobacter baumannii isolated in clinical samples from Northeast of Iran. BMC Res. Notes 2020, 13, 380. [Google Scholar] [CrossRef] [PubMed]
- Jovcic, B.; Novovic, K.; Dekic, S.; Hrenovic, J. Colistin Resistance in Environmental Isolates of Acinetobacter baumannii. Microb. Drug Resist. 2021, 27, 328–336. [Google Scholar] [CrossRef]
- Zeng, X.; Gu, H.; Cheng, Y.; Jia, K.R.; Liu, D.; Yuan, Y.; Chen, Z.F.; Peng, L.S.; Zou, Q.M.; Shi, Y. A lethal pneumonia model of Acinetobacter baumannii: An investigation in immunocompetent mice. Clin. Microbiol Infect. 2019, 25, 516e1–516e4. [Google Scholar] [CrossRef]
- Zurawski, D.V.; Black, C.C.; Alamneh, Y.A.; Biggemann, L.; Banerjee, J.; Thompson, M.G.; Wise, M.C.; Honnold, C.L.; Kim, R.K.; Paranavitana, C.; et al. A Porcine Wound Model of Acinetobacter baumannii Infection. Adv. Wound Care. 2019, 8, 14–27. [Google Scholar] [CrossRef]
- Palmer, L.D.; Green, E.R.; Sheldon, J.R.; Skaar, E.P. Assessing Acinetobacter baumannii Virulence and Persistence in a Murine Model of Lung Infection. Methods Mol. Biol. 2019, 1946, 289–305. [Google Scholar] [CrossRef]
- Geisinger, E.; Isberg, R.R. Interplay Between Antibiotic Resistance and Virulence During Disease Promoted by Multidrug-Resistant Bacteria. J. Infect. Dis. 2017, 215, S9–S17. [Google Scholar] [CrossRef] [PubMed]
- Tipton, K.A.; Farokhyfar, M.; Rather, P.N. Multiple roles for a novel RND-type efflux system in Acinetobacter baumannii AB5075. Microbiologyopen 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Shin, D.S.; Jang, H.I.; Eom, Y.B. Anti-biofilm and anti-virulence effects of zerumbone against Acinetobacter baumannii. Microbiology 2020, 166, 717–726. [Google Scholar] [CrossRef]
- Harding, C.M.; Hennon, S.W.; Feldman, M.F. Uncovering the mechanisms of Acinetobacter baumannii virulence. Nat.Rev. Microbiol. 2018, 16, 91–102. [Google Scholar] [CrossRef]
- Kumar, S.; Anwer, R.; Yadav, M.; Sehrawat, N.; Singh, M.; Kumar, V. MALDI-TOF MS and Molecular methods for identifying Multidrug resistant clinical isolates of Acinetobacter baumannii. Res. J. Biotechnol. 2021, 16, 47–52. [Google Scholar]
- Sheldon, J.R.; Skaar, E.P. Acinetobacter baumannii can use multiple siderophores for iron acquisition, but only acinetobactin is required for virulence. PLoS Pathog. 2020, 16, e1008995. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, Z.S.; Hu, P.; Cai, L.; Fu, B.Q.; Li, Y.S.; Lu, S.Y.; Liu, N.N.; Ma, X.L.; Chi, D.; et al. Characterization of surface antigen protein 1 (SurA1) from Acinetobacter baumannii and its role in virulence and fitness. Vet. Microbiol. 2016, 186, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, A.; Valliammai, A.; Sivasankar, C.; Suba, M.; Sakthivel, G.; Pandian, S.K. Antibiofilm and antivirulence efficacy of myrtenol enhances the antibiotic susceptibility of Acinetobacter baumannii. Sci. Rep. 2020, 10, 21975. [Google Scholar] [CrossRef]
- Balcazar, J.L.; Subirats, J.; Borrego, C.M. The role of biofilms as environmental reservoirs of antibiotic resistance. Front. Microbiol. 2015, 6, 1216. [Google Scholar] [CrossRef]
- Moosavian, M.; Ahmadi, K.; Shoja, S.; Mardaneh, J.; Shahi, F.; Afzali, M. Antimicrobial resistance patterns and their encoding genes among clinical isolates of Acinetobacter baumannii in Ahvaz, Southwest Iran. MethodsX 2020, 7, 101031. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Yadav, M.; Sehrawat, N.; Alrehaili, J.; Anwer, R. Pathobiology of Multidrug Resistant Acinetobacterbaumannii: An update. Asian J. Biol. Life Sci. 2021, 10, 15–26. [Google Scholar] [CrossRef]
- Karakonstantis, S.; Gikas, A.; Astrinaki, E.; Kritsotakis, E.I. Excess mortality due to pandrug-resistant Acinetobacter baumannii infections in hospitalized patients. J. Hosp. Infect. 2020, 106, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Gautam, L.K.; Sharma, P.; Capalash, N. Attenuation of Acinetobacter baumannii virulence by inhibition of polyphosphate kinase 1 with repurposed drugs. Microbiol. Res. 2021, 242, 126627. [Google Scholar] [CrossRef] [PubMed]
- Usmani, Y.; Ahmed, A.; Faizi, S.; Versiani, M.A.; Shamshad, S.; Khan, S.; Simjee, S.U. Antimicrobial and biofilm inhibiting potential of an amide derivative [N-(2′, 4′-dinitrophenyl)-3beta-hydroxyurs-12-en-28-carbonamide] of ursolic acid by modulating membrane potential and quorum sensing against colistin resistant Acinetobacter baumannii. Microb. Pathog. 2021, 157, 104997. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singhal, L.; Arora, S.; Gautam, V.; Ray, P. Septicemia by Pseudomonas stutzeri Vb-3: First report from India. J. Patient Saf. Infect. Control. 2015, 3, 64–65. [Google Scholar] [CrossRef]
- Kumar, S.; Chaudhary, M.; Yadav, M.; Kumar, V. Global Surveillance Programs on Antimicrobial Resistance. Sustain. Agric. Rev. 2020, 46, 33–58. [Google Scholar] [CrossRef]
- Espinal, P.; Pantel, A.; Rolo, D.; Marti, S.; Lopez-Rojas, R.; Smani, Y.; Pachon, J.; Vila, J.; Lavigne, J.P. Relationship Between Different Resistance Mechanisms and Virulence in Acinetobacter baumannii. Microb. Drug Resist. 2019, 25, 752–760. [Google Scholar] [CrossRef]
- Saranathan, R.; Pagal, S.; Sawant, A.R.; Tomar, A.; Madhangi, M.; Sah, S.; Satti, A.; Arunkumar, K.P.; Prashanth, K. Disruption of tetR type regulator adeN by mobile genetic element confers elevated virulence in Acinetobacter baumannii. Virulence 2017, 8, 1316–1334. [Google Scholar] [CrossRef]
- Bassetti, M.; Labate, L.; Russo, C.; Vena, A.; Giacobbe, D.R. Therapeutic options for difficult-to-treat Acinetobacter baumannii infections: A 2020 perspective. Expert Opin. Pharmacother. 2021, 22, 167–177. [Google Scholar] [CrossRef]
- Hassan, A.; Ikram, A.; Raza, A.; Saeed, S.; Zafar Paracha, R.; Younas, Z.; Khadim, M.T. Therapeutic Potential of Novel Mastoparan-Chitosan Nanoconstructs Against Clinical MDR Acinetobacter baumannii: In silico, in vitro and in vivo Studies. Int. J. Nanomed. 2021, 16, 3755–3773. [Google Scholar] [CrossRef]
- Evans, B.A.; Hamouda, A.; Amyes, S.G. The Rise of Carbapenem-Resistant Acinetobacter baumannii. Curr. Pharm. Des. 2013, 19, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Garcia, I.; Fainstein, V.; LeBlanc, B.; Bodey, G.P. In vitro activities of new beta-lactam antibiotics against Acinetobacter spp. Antimicrob. Agents Chemother. 1983, 24, 297–299. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Joly-Guillou, M.L.; Bergogne-Berezin, E. Evolution of Acinetobacter calcoaceticus in the hospital milieu, from 1971 to 1984. Presse Med. 1985, 14, 2331–2335. [Google Scholar] [PubMed]
- Obana, Y.; Nishino, T.; Tanino, T. In-vitro and in-vivo activities of antimicrobial agents against Acinetobacter calcoaceticus. J. Antimicrob. Chemother. 1985, 15, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singhal, L.; Ray, P.; Gautam, V. Over-expression of RND and MATE efflux pumps contribute to decreased susceptibility in clinical isolates of carbapenem resistant Acinetobacter baumannii. Int. J. Pharm. Res. 2020, 12, 342–349. [Google Scholar] [CrossRef]
- Bonomo, R.A.; Szabo, D. Mechanisms of multidrug resistance in Acinetobacter species and Pseudomonas aeruginosa. Clin. Infect. Dis. 2006, 43 (Suppl. S2), S49–S56. [Google Scholar] [CrossRef]
- Naas, T.; Bogaerts, P.; Bauraing, C.; Degheldre, Y.; Glupczynski, Y.; Nordmann, P. Emergence of PER and VEB extended-spectrum beta-lactamases in Acinetobacter baumannii in Belgium. J. Antimicrob. Chemother. 2006, 58, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.H.; Lee, J.H.; Lee, J.J.; Park, K.S.; Karim, A.M.; Lee, C.R.; Jeong, B.C.; Lee, S.H. Structural basis for carbapenem-hydrolyzing mechanisms of carbapenemases conferring antibiotic resistance. Int. J. Mol. Sci. 2015, 16, 9654–9692. [Google Scholar] [CrossRef]
- Patel, G.; Bonomo, R.A. “Stormy waters ahead”: Global emergence of carbapenemases. Front. Microbiol. 2013, 4, 48. [Google Scholar] [CrossRef]
- Poirel, L.; Naas, T.; Nordmann, P. Diversity, epidemiology, and genetics of class D beta-lactamases. Antimicrob. Agents Chemother. 2010, 54, 24–38. [Google Scholar] [CrossRef]
- Diene, S.M.; Rolain, J.M. Carbapenemase genes and genetic platforms in Gram-negative bacilli: Enterobacteriaceae, Pseudomonas and Acinetobacter species. Clin. Microbiol. Infect. 2014, 20, 831–838. [Google Scholar] [CrossRef]
- Evans, B.A.; Amyes, S.G. OXA beta-lactamases. Clin. Microbiol Rev. 2014, 27, 241–263. [Google Scholar] [CrossRef] [PubMed]
- Paton, R.; Miles, R.S.; Hood, J.; Amyes, S.G.; Miles, R.S.; Amyes, S.G. ARI 1: Beta-lactamase-mediated imipenem resistance in Acinetobacter baumannii. Int. J. Antimicrob. Agents. 1993, 2, 81–87. [Google Scholar] [CrossRef]
- Bonnet, R.; Marchandin, H.; Chanal, C.; Sirot, D.; Labia, R.; De Champs, C.; Jumas-Bilak, E.; Sirot, J. Chromosome-encoded class D beta-lactamase OXA-23 in Proteus mirabilis. Antimicrob. Agents Chemother. 2002, 46, 2004–2006. [Google Scholar] [CrossRef] [PubMed]
- Bou, G.; Cervero, G.; Dominguez, M.A.; Quereda, C.; Martinez-Beltran, J. Characterization of a nosocomial outbreak caused by a multiresistant Acinetobacter baumannii strain with a carbapenem-hydrolyzing enzyme: High-level carbapenem resistance in A. baumannii is not due solely to the presence of beta-lactamases. J. Clin. Microbiol. 2000, 38, 3299–3305. [Google Scholar] [CrossRef]
- Poirel, L.; Cabanne, L.; Vahaboglu, H.; Nordmann, P. Genetic environment and expression of the extended-spectrum beta-lactamase blaPER-1 gene in gram-negative bacteria. Antimicrob. Agents Chemother. 2005, 49, 1708–1713. [Google Scholar] [CrossRef]
- D’Arezzo, S.; Capone, A.; Petrosillo, N.; Visca, P.; Ballardini, M.; Bartolini, S.; Bordi, E.; Di Stefano, A.; Galie, M.; Minniti, R.; et al. Epidemic multidrug-resistant Acinetobacter baumannii related to European clonal types I and II in Rome (Italy). Clin. Microbiol. Infect. 2009, 15, 347–357. [Google Scholar] [CrossRef]
- Papa, A.; Koulourida, V.; Souliou, E. Molecular epidemiology of carbapenem-resistant Acinetobacter baumannii in a newly established Greek hospital. Microb. Drug Resist. 2009, 15, 257–260. [Google Scholar] [CrossRef]
- Donnarumma, F.; Sergi, S.; Indorato, C.; Mastromei, G.; Monnanni, R.; Nicoletti, P.; Pecile, P.; Cecconi, D.; Mannino, R.; Bencini, S.; et al. Molecular characterization of acinetobacter isolates collected in intensive care units of six hospitals in Florence, Italy, during a 3-year surveillance program: A population structure analysis. J. Clin. Microbiol. 2010, 48, 1297–1304. [Google Scholar] [CrossRef]
- Di Popolo, A.; Giannouli, M.; Triassi, M.; Brisse, S.; Zarrilli, R. Molecular epidemiological investigation of multidrug-resistant Acinetobacter baumannii strains in four Mediterranean countries with a multilocus sequence typing scheme. Clin. Microbiol. Infect. 2011, 17, 197–201. [Google Scholar] [CrossRef]
- Gogou, V.; Pournaras, S.; Giannouli, M.; Voulgari, E.; Piperaki, E.T.; Zarrilli, R.; Tsakris, A. Evolution of multidrug-resistant Acinetobacter baumannii clonal lineages: A 10 year study in Greece (2000–2009). J. Antimicrob. Chemother. 2011, 66, 2767–2772. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Nordmann, P. Genetic structures at the origin of acquisition and expression of the carbapenem-hydrolyzing oxacillinase gene blaOXA-58 in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2006, 50, 1442–1448. [Google Scholar] [CrossRef]
- Pournaras, S.; Markogiannakis, A.; Ikonomidis, A.; Kondyli, L.; Bethimouti, K.; Maniatis, A.N.; Legakis, N.J.; Tsakris, A. Outbreak of multiple clones of imipenem-resistant Acinetobacter baumannii isolates expressing OXA-58 carbapenemase in an intensive care unit. J. Antimicrob. Chemother. 2006, 57, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Tsakris, A.; Ikonomidis, A.; Poulou, A.; Spanakis, N.; Vrizas, D.; Diomidous, M.; Pournaras, S.; Markou, F. Clusters of imipenem-resistant Acinetobacter baumannii clones producing different carbapenemases in an intensive care unit. Clin. Microbiol. Infect. 2008, 14, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Patil, P.P.; Singhal, L.; Ray, P.; Patil, P.B.; Gautam, V. Molecular epidemiology of carbapenem-resistant Acinetobacter baumannii isolates reveals the emergence of blaOXA-23 and blaNDM-1 encoding international clones in India. Infect. Genet. Evol. 2019, 75, 103986. [Google Scholar] [CrossRef]
- Higgins, P.G.; Poirel, L.; Lehmann, M.; Nordmann, P.; Seifert, H. OXA-143, a novel carbapenem-hydrolyzing class D beta-lactamase in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2009, 53, 5035–5038. [Google Scholar] [CrossRef]
- Queenan, A.M.; Bush, K. Carbapenemases: The versatile beta-lactamases. Clin. Microbiol Rev. 2007, 20, 440–458, table of contents. [Google Scholar] [CrossRef]
- Heritier, C.; Poirel, L.; Lambert, T.; Nordmann, P. Contribution of acquired carbapenem-hydrolyzing oxacillinases to carbapenem resistance in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2005, 49, 3198–3202. [Google Scholar] [CrossRef]
- Piddock, L.J. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin. Microbiol. Rev. 2006, 19, 382–402. [Google Scholar] [CrossRef]
- Abdi, S.N.; Ghotaslou, R.; Ganbarov, K.; Mobed, A.; Tanomand, A.; Yousefi, M.; Asgharzadeh, M.; Kafil, H.S. Acinetobacter baumannii Efflux Pumps and Antibiotic Resistance. Infect. Drug Resist. 2020, 13, 423–434. [Google Scholar] [CrossRef]
- Andermahr, J.; Greb, A.; Hensler, T.; Helling, H.J.; Bouillon, B.; Sauerland, S.; Rehm, K.E.; Neugebauer, E. Pneumonia in multiple injured patients: A prospective controlled trial on early prediction using clinical and immunological parameters. Inflamm. Res. 2002, 51, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H. Multidrug efflux pumps of gram-negative bacteria. J. Bacteriol. 1996, 178, 5853–5859. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.T.; Gratwick, K.S.; Kollman, J.; Park, D.; Nies, D.H.; Goffeau, A.; Saier, M.H., Jr. The RND permease superfamily: An ancient, ubiquitous and diverse family that includes human disease and development proteins. J. Mol. Microbiol.Biotechnol. 1999, 1, 107–125. [Google Scholar]
- Zgurskaya, H.I.; Nikaido, H. Multidrug resistance mechanisms: Drug efflux across two membranes. Mol. Microbiol. 2000, 37, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Srikumar, R.; Kon, T.; Gotoh, N.; Poole, K. Expression of Pseudomonas aeruginosa multidrug efflux pumps MexA-MexB-OprM and MexC-MexD-OprJ in a multidrug-sensitive Escherichia coli strain. Antimicrob. Agents Chemother. 1998, 42, 65–71. [Google Scholar] [CrossRef]
- Gautam, V.; Kumar, S.; Patil, P.P.; Meletiadis, J.; Patil, P.B.; Mouton, J.W.; Sharma, M.; Daswal, A.; Singhal, L.; Ray, P.; et al. Exploring the Interplay of Resistance Nodulation Division Efflux Pumps, AmpC and OprD in Antimicrobial Resistance of Burkholderiacepacia Complex in Clinical Isolates. Microb. Drug Resist. 2020, 26, 1144–1152. [Google Scholar] [CrossRef]
- Kohler, T.; Michea-Hamzehpour, M.; Henze, U.; Gotoh, N.; Curty, L.K.; Pechere, J.C. Characterization of MexE-MexF-OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol. Microbiol. 1997, 23, 345–354. [Google Scholar] [CrossRef]
- Magnet, S.; Courvalin, P.; Lambert, T. Resistance-nodulation-cell division-type efflux pump involved in aminoglycoside resistance in Acinetobacter baumannii strain BM4454. Antimicrob. Agents Chemother. 2001, 45, 3375–3380. [Google Scholar] [CrossRef]
- Coyne, S.; Rosenfeld, N.; Lambert, T.; Courvalin, P.; Perichon, B. Overexpression of resistance-nodulation-cell division pump AdeFGH confers multidrug resistance in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2010, 54, 4389–4393. [Google Scholar] [CrossRef]
- Damier-Piolle, L.; Magnet, S.; Bremont, S.; Lambert, T.; Courvalin, P. AdeIJK, a resistance-nodulation-cell division pump effluxing multiple antibiotics in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2008, 52, 557–562. [Google Scholar] [CrossRef]
- Marchand, I.; Damier-Piolle, L.; Courvalin, P.; Lambert, T. Expression of the RND-type efflux pump AdeABC in Acinetobacter baumannii is regulated by the AdeRS two-component system. Antimicrob. Agents Chemother. 2004, 48, 3298–3304. [Google Scholar] [CrossRef]
- Rosenfeld, N.; Bouchier, C.; Courvalin, P.; Perichon, B. Expression of the resistance-nodulation-cell division pump AdeIJK in Acinetobacter baumannii is regulated by AdeN, a TetR-type regulator. Antimicrob. Agents Chemother. 2012, 56, 2504–2510. [Google Scholar] [CrossRef] [PubMed]
- Richet, H.; Fournier, P.E. Nosocomial infections caused by Acinetobacter baumannii: A major threat worldwide. Infect. Control. Hosp. Epidemiol. 2006, 27, 645–646. [Google Scholar] [CrossRef][Green Version]
- Su, X.Z.; Chen, J.; Mizushima, T.; Kuroda, T.; Tsuchiya, T. AbeM, an H+-coupled Acinetobacter baumannii multidrug efflux pump belonging to the MATE family of transporters. Antimicrob. Agents Chemother. 2005, 49, 4362–4364. [Google Scholar] [CrossRef]
- Cerqueira, G.M.; Peleg, A.Y. Insights into Acinetobacter baumannii pathogenicity. IUBMB Life 2011, 63, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Bamunuarachchi, N.I.; Khan, F.; Kim, Y.M. Inhibition of Virulence Factors and Biofilm Formation of Acinetobacter baumannii by Naturally-derived and Synthetic Drugs. Curr. Drug Targets 2021, 22, 734–759. [Google Scholar] [CrossRef]
- Uppalapati, S.R.; Sett, A.; Pathania, R. The Outer Membrane Proteins OmpA, CarO, and OprD of Acinetobacter baumannii Confer a Two-Pronged Defense in Facilitating Its Success as a Potent Human Pathogen. Front. Microbiol. 2020, 11, 589234. [Google Scholar] [CrossRef]
- Jahangiri, A.; Rasooli, I.; Owlia, P.; Imani Fooladi, A.A.; Salimian, J. Highly conserved exposed immunogenic peptides of Omp34 against Acinetobacter baumannii: An innovative approach. J. Microbiol. Methods 2018, 144, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Nie, D.; Hu, Y.; Chen, Z.; Li, M.; Hou, Z.; Luo, X.; Mao, X.; Xue, X. Outer membrane protein A (OmpA) as a potential therapeutic target for Acinetobacter baumannii infection. J. Biomed. Sci. 2020, 27, 26. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xun, M.; Han, J. A bovine myeloid antimicrobial peptide (BMAP-28) and its analogs kill pan-drug-resistant Acinetobacter baumannii by interacting with outer membrane protein A (OmpA). Medicine 2018, 97, e12832. [Google Scholar] [CrossRef]
- Nafarieh, T.; Bandehpour, M.; Hashemi, A.; Taheri, S.; Yardel, V.; Jamaati, H.; Moosavi, S.M.; Mosaffa, N. Identification of antigens from nosocomial Acinetobacter baumannii clinical isolates in sera from ICU staff and infected patients using the antigenome technique. World J. Microbiol. Biotechnol. 2017, 33, 189. [Google Scholar] [CrossRef] [PubMed]
- Roszkowiak, J.; Jajor, P.; Gula, G.; Gubernator, J.; Zak, A.; Drulis-Kawa, Z.; Augustyniak, D. Interspecies Outer Membrane Vesicles (OMVs) Modulate the Sensitivity of Pathogenic Bacteria and Pathogenic Yeasts to Cationic Peptides and Serum Complement. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef]
- Habier, J.; May, P.; Heintz-Buschart, A.; Ghosal, A.; Wienecke-Baldacchino, A.K.; Nolte-’t Hoen, E.N.M.; Wilmes, P.; Fritz, J.V. Extraction and Analysis of RNA Isolated from Pure Bacteria-Derived Outer Membrane Vesicles. Methods Mol. Biol. 2018, 1737, 213–230. [Google Scholar] [CrossRef]
- Jan, A.T. Outer Membrane Vesicles (OMVs) of Gram-negative Bacteria: A Perspective Update. Front. Microbiol. 2017, 8, 1053. [Google Scholar] [CrossRef]
- Fulsundar, S.; Domingues, S.; Nielsen, K.M. Vesicle-Mediated Gene Transfer in Acinetobacter baumannii. Methods Mol. Biol. 2019, 1946, 87–94. [Google Scholar] [CrossRef]
- Yang, C.H.; Su, P.W.; Moi, S.H.; Chuang, L.Y. Biofilm Formation in Acinetobacter baumannii: Genotype-Phenotype Correlation. Molecules 2019, 24, 1849. [Google Scholar] [CrossRef] [PubMed]
- Pakharukova, N.; Tuittila, M.; Paavilainen, S.; Malmi, H.; Parilova, O.; Teneberg, S.; Knight, S.D.; Zavialov, A.V. Structural basis for Acinetobacter baumannii biofilm formation. Proc. Natl. Acad. Sci. USA 2018, 115, 5558–5563. [Google Scholar] [CrossRef] [PubMed]
- Gaddy, J.A.; Tomaras, A.P.; Actis, L.A. The Acinetobacter baumannii 19606 OmpA protein plays a role in biofilm formation on abiotic surfaces and in the interaction of this pathogen with eukaryotic cells. Infect. Immun. 2009, 77, 3150–3160. [Google Scholar] [CrossRef]
- Mancilla-Rojano, J.; Castro-Jaimes, S.; Ochoa, S.A.; Bobadilla Del Valle, M.; Luna-Pineda, V.M.; Bustos, P.; Laris-Gonzalez, A.; Arellano-Galindo, J.; Parra-Ortega, I.; Hernandez-Castro, R.; et al. Whole-Genome Sequences of Five Acinetobacter baumannii Strains From a Child With Leukemia M2. Front. Microbiol. 2019, 10, 132. [Google Scholar] [CrossRef]
- Saipriya, K.; Swathi, C.H.; Ratnakar, K.S.; Sritharan, V. Quorum-sensing system in Acinetobacter baumannii: A potential target for new drug development. J. Appl. Microbiol. 2020, 128, 15–27. [Google Scholar] [CrossRef]
- Alvarez-Fraga, L.; Vazquez-Ucha, J.C.; Martinez-Guitian, M.; Vallejo, J.A.; Bou, G.; Beceiro, A.; Poza, M. Pneumonia infection in mice reveals the involvement of the feoA gene in the pathogenesis of Acinetobacter baumannii. Virulence 2018, 9, 496–509. [Google Scholar] [CrossRef]
- Flannery, A.; Le Berre, M.; Pier, G.B.; O’Gara, J.P.; Kilcoyne, M. Glycomics Microarrays Reveal Differential In Situ Presentation of the Biofilm Polysaccharide Poly-N-acetylglucosamine on Acinetobacter baumannii and Staphylococcus aureus Cell Surfaces. Int. J. Mol. Sci. 2020, 21, 2465. [Google Scholar] [CrossRef]
- Costa, D.M.; Johani, K.; Melo, D.S.; Lopes, L.K.O.; Lopes Lima, L.K.O.; Tipple, A.F.V.; Hu, H.; Vickery, K. Biofilm contamination of high-touched surfaces in intensive care units: Epidemiology and potential impacts. Lett. Appl. Microbiol. 2019, 68, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Zeighami, H.; Valadkhani, F.; Shapouri, R.; Samadi, E.; Haghi, F. Virulence characteristics of multidrug resistant biofilm forming Acinetobacter baumannii isolated from intensive care unit patients. BMC Infect. Dis. 2019, 19, 629. [Google Scholar] [CrossRef]
- Lin, M.F.; Lin, Y.Y.; Lan, C.Y. A method to assess influence of different medical tubing on biofilm formation by Acinetobacter baumannii. J. Microbiol. Methods 2019, 160, 84–86. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Zeng, W.; Xu, Y.; Liao, W.; Xu, W.; Zhou, T.; Cao, J.; Chen, L. Bloodstream infections caused by ST2 Acinetobacter baumannii: Risk factors, antibiotic regimens, and virulence over 6 years period in China. Antimicrob. Resist. Infect. Control. 2021, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.N.; Kazi, M.I.; Biboy, J.; Gray, J.; Bovermann, H.; Ausman, J.; Boutte, C.C.; Vollmer, W.; Boll, J.M. Septal Class A Penicillin-Binding Protein Activity and ld-Transpeptidases Mediate Selection of Colistin-Resistant Lipooligosaccharide-Deficient Acinetobacter baumannii. MBio 2021, 12, e02185-20. [Google Scholar] [CrossRef]
- Boll, J.M.; Crofts, A.A.; Peters, K.; Cattoir, V.; Vollmer, W.; Davies, B.W.; Trent, M.S. A penicillin-binding protein inhibits selection of colistin-resistant, lipooligosaccharide-deficient Acinetobacter baumannii. Proc. Natl. Acad. Sci. USA 2016, 113, E6228–E6237. [Google Scholar] [CrossRef]
- Bhagwat, S.S.; Periasamy, H.; Takalkar, S.S.; Palwe, S.R.; Khande, H.N.; Patel, M.V. The Novel beta-Lactam Enhancer Zidebactam Augments the In Vivo Pharmacodynamic Activity of Cefepime in a Neutropenic Mouse Lung Acinetobacter baumannii Infection Model. Antimicrob. Agents Chemother. 2019, 63, e02146-18. [Google Scholar] [CrossRef]
- Russo, T.A.; MacDonald, U.; Beanan, J.M.; Olson, R.; MacDonald, I.J.; Sauberan, S.L.; Luke, N.R.; Schultz, L.W.; Umland, T.C. Penicillin-binding protein 7/8 contributes to the survival of Acinetobacter baumannii in vitro and in vivo. J. Infect. Dis. 2009, 199, 513–521. [Google Scholar] [CrossRef]
- Monogue, M.L.; Sakoulas, G.; Nizet, V.; Nicolau, D.P. Humanized Exposures of a beta-Lactam-beta-Lactamase Inhibitor, Tazobactam, versus Non-beta-Lactam-beta-Lactamase Inhibitor, Avibactam, with or without Colistin, against Acinetobacter baumannii in Murine Thigh and Lung Infection Models. Pharmacology 2018, 101, 255–261. [Google Scholar] [CrossRef]
- Katsube, T.; Echols, R.; Wajima, T. Pharmacokinetic and Pharmacodynamic Profiles of Cefiderocol, a Novel Siderophore Cephalosporin. Clin. Infect. Dis. 2019, 69, S552–S558. [Google Scholar] [CrossRef]
- Delgado-Valverde, M.; Conejo, M.D.C.; Serrano, L.; Fernandez-Cuenca, F.; Pascual, A. Activity of cefiderocol against high-risk clones of multidrug-resistant Enterobacterales, Acinetobacter baumannii, Pseudomonas aeruginosa and Stenotrophomonas maltophilia. J. Antimicrob. Chemother. 2020, 75, 1840–1849. [Google Scholar] [CrossRef]
- Miethke, M.; Marahiel, M.A. Siderophore-based iron acquisition and pathogen control. Microbiol. Mol. Biol. Rev. 2007, 71, 413–451. [Google Scholar] [CrossRef]
- Katsube, T.; Echols, R.; Arjona Ferreira, J.C.; Krenz, H.K.; Berg, J.K.; Galloway, C. Cefiderocol, a Siderophore Cephalosporin for Gram-Negative Bacterial Infections: Pharmacokinetics and Safety in Subjects with Renal Impairment. J. Clin. Pharmacol. 2017, 57, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, H.; Song, W.Y.; Kim, H.J. Total Syntheses of Fimsbactin A and B and Their Stereoisomers to Probe the Stereoselectivity of the Fimsbactin Uptake Machinery in Acinetobacter baumannii. Org. Lett. 2020, 22, 2806–2810. [Google Scholar] [CrossRef]
- Goldberg, J.A.; Kumar, V.; Spencer, E.J.; Hoyer, D.; Marshall, S.H.; Hujer, A.M.; Hujer, K.M.; Bethel, C.R.; Papp-Wallace, K.M.; Perez, F.; et al. A gamma-lactam siderophore antibiotic effective against multidrug-resistant Pseudomonas aeruginosa, Klebsiella pneumoniae, and Acinetobacter spp. Eur. J. Med. Chem. 2021, 220, 113436. [Google Scholar] [CrossRef] [PubMed]
- Pfefferle, K.; Lopalco, P.; Breisch, J.; Siemund, A.; Corcelli, A.; Averhoff, B. In vivo synthesis of monolysocardiolipin and cardiolipin by Acinetobacter baumannii phospholipase D and effect on cationic antimicrobial peptide resistance. Environ. Microbiol. 2020, 22, 5300–5308. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.K.; Adams, F.G.; Brown, M.H. Diversity and Function of Capsular Polysaccharide in Acinetobacter baumannii. Front. Microbiol. 2018, 9, 3301. [Google Scholar] [CrossRef]
- Lee, C.R.; Lee, J.H.; Park, M.; Park, K.S.; Bae, I.K.; Kim, Y.B.; Cha, C.J.; Jeong, B.C.; Lee, S.H. Biology of Acinetobacter baumannii: Pathogenesis, Antibiotic Resistance Mechanisms, and Prospective Treatment Options. Front. Cell Infect. Microbiol. 2017, 7, 55. [Google Scholar] [CrossRef]
- Isler, B.; Doi, Y.; Bonomo, R.A.; Paterson, D.L. New Treatment Options against Carbapenem-Resistant Acinetobacter baumannii Infections. Antimicrob. Agents Chemother. 2019, 63, e01110-18. [Google Scholar] [CrossRef]
- Ramirez, M.S.; Bonomo, R.A.; Tolmasky, M.E. Carbapenemases: Transforming Acinetobacter baumannii into a Yet More Dangerous Menace. Biomolecules 2020, 10, 720. [Google Scholar] [CrossRef] [PubMed]
- Ciginskiene, A.; Dambrauskiene, A.; Rello, J.; Adukauskiene, D. Ventilator-Associated Pneumonia due to Drug-Resistant Acinetobacter baumannii: Risk Factors and Mortality Relation with Resistance Profiles, and Independent Predictors of In-Hospital Mortality. Medicina 2019, 55, 49. [Google Scholar] [CrossRef]
- Khalili, H.; Shojaei, L.; Mohammadi, M.; Beigmohammadi, M.T.; Abdollahi, A.; Doomanlou, M. Meropenem/colistin versus meropenem/ampicillin-sulbactam in the treatment of carbapenem-resistant pneumonia. J. Comp. Eff. Res. 2018, 7, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, P.; Poirel, L. Epidemiology and Diagnostics of Carbapenem Resistance in Gram-negative Bacteria. Clin. Infect. Dis. 2019, 69, S521–S528. [Google Scholar] [CrossRef]
- Hamidian, M.; Nigro, S.J. Emergence, molecular mechanisms and global spread of carbapenem-resistant Acinetobacter baumannii. Microb. Genom. 2019, 5, e000306. [Google Scholar] [CrossRef] [PubMed]
- Levy-Blitchtein, S.; Roca, I.; Plasencia-Rebata, S.; Vicente-Taboada, W.; Velasquez-Pomar, J.; Munoz, L.; Moreno-Morales, J.; Pons, M.J.; Del Valle-Mendoza, J.; Vila, J. Emergence and spread of carbapenem-resistant Acinetobacter baumannii international clones II and III in Lima, Peru. Emerg. Microbes Infect. 2018, 7, 119. [Google Scholar] [CrossRef]
- Karakonstantis, S.; Kritsotakis, E.I.; Gikas, A. Treatment options for K. pneumoniae, P. aeruginosa and A. baumannii co-resistant to carbapenems, aminoglycosides, polymyxins and tigecycline: An approach based on the mechanisms of resistance to carbapenems. Infection 2020, 48, 835–851. [Google Scholar] [CrossRef]
- Mei, H.; Yang, T.; Wang, J.; Wang, R.; Cai, Y. Efficacy and safety of tigecycline in treatment of pneumonia caused by MDR Acinetobacter baumannii: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2019, 74, 3423–3431. [Google Scholar] [CrossRef]
- Lyu, C.; Zhang, Y.; Liu, X.; Wu, J.; Zhang, J. Clinical efficacy and safety of polymyxins based versus non-polymyxins based therapies in the infections caused by carbapenem-resistant Acinetobacter baumannii: A systematic review and meta-analysis. BMC Infect. Dis. 2020, 20, 296. [Google Scholar] [CrossRef]
- Jones, R.N.; Flonta, M.; Gurler, N.; Cepparulo, M.; Mendes, R.E.; Castanheira, M. Resistance surveillance program report for selected European nations (2011). Diagn. Microbiol. Infect. Dis. 2014, 78, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Beganovic, M.; Daffinee, K.E.; Luther, M.K.; LaPlante, K.L. Minocycline Alone and in Combination with Polymyxin B, Meropenem, and Sulbactam against Carbapenem-Susceptible and -Resistant Acinetobacter baumannii in an In Vitro Pharmacodynamic Model. Antimicrob. Agents Chemother. 2021, 65, e01680-20. [Google Scholar] [CrossRef]
- Santella, B.; Folliero, V.; Pirofalo, G.M.; Serretiello, E.; Zannella, C.; Moccia, G.; Santoro, E.; Sanna, G.; Motta, O.; De Caro, F.; et al. Sepsis-A Retrospective Cohort Study of Bloodstream Infections. Antibiotics 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Santella, B.; Serretiello, E.; De Filippis, A.; Veronica, F.; Iervolino, D.; Dell’Annunziata, F.; Manente, R.; Valitutti, F.; Santoro, E.; Pagliano, P.; et al. Lower Respiratory Tract Pathogens and Their Antimicrobial Susceptibility Pattern: A 5-Year Study. Antibiotics 2021, 10, 851. [Google Scholar] [CrossRef] [PubMed]
- Assimakopoulos, S.F.; Karamouzos, V.; Lefkaditi, A.; Sklavou, C.; Kolonitsiou, F.; Christofidou, M.; Fligou, F.; Gogos, C.; Marangos, M. Triple combination therapy with high-dose ampicillin/sulbactam, high-dose tigecycline and colistin in the treatment of ventilator-associated pneumonia caused by pan-drug resistant Acinetobacter baumannii: A case series study. Infez. Med. 2019, 27, 11–16. [Google Scholar] [PubMed]
- Lenhard, J.R.; Thamlikitkul, V.; Silveira, F.P.; Garonzik, S.M.; Tao, X.; Forrest, A.; Soo Shin, B.; Kaye, K.S.; Bulitta, J.B.; Nation, R.L.; et al. Polymyxin-resistant, carbapenem-resistant Acinetobacter baumannii is eradicated by a triple combination of agents that lack individual activity. J. Antimicrob. Chemother. 2017, 72, 1415–1420. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Xie, X.; Wu, X.; Li, X.; Zhao, Z.; Luo, S.; Wan, Z.; Liu, J.; Fu, L. In vitro and in vivo assessment of the antibacterial activity of colistin alone and in combination with other antibiotics against Acinetobacter baumannii and Escherichia coli. J Glob. Antimicrob. Resist. 2020, 20, 351–359. [Google Scholar] [CrossRef]
- Rodriguez, C.H.; Brune, A.; Nastro, M.; Vay, C.; Famiglietti, A. In vitro synergistic activity of the sulbactam/avibactam combination against extensively drug-resistant Acinetobacter baumannii. J. Med. Microbiol. 2020, 69, 928–931. [Google Scholar] [CrossRef]
- Chen, J.; Yang, Y.; Xiang, K.; Li, D.; Liu, H. Combined Rifampin and Sulbactam Therapy for Multidrug-Resistant Acinetobacter baumannii Ventilator-Associated Pneumonia in Pediatric Patients. J. Anesth. Perioper. Med. 2018, 5, 176–185. [Google Scholar] [CrossRef]
- Zusman, O.; Altunin, S.; Koppel, F.; DishonBenattar, Y.; Gedik, H.; Paul, M. Polymyxin monotherapy or in combination against carbapenem-resistant bacteria: Systematic review and meta-analysis. J. Antimicrob. Chemother. 2017, 72, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Azimi, L.; Tahbaz, S.V.; Alaghehbandan, R.; Alinejad, F.; Lari, A.R. Synergistic Effect of Tazobactam on Amikacin MIC in Acinetobacter baumannii Isolated from Burn Patients in Tehran, Iran. Curr. Pharm. Biotechnol. 2020, 21, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Montero, A.; Ariza, J.; Corbella, X.; Domenech, A.; Cabellos, C.; Ayats, J.; Tubau, F.; Borraz, C.; Gudiol, F. Antibiotic combinations for serious infections caused by carbapenem-resistant Acinetobacter baumannii in a mouse pneumonia model. J. Antimicrob. Chemother. 2004, 54, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.J.; Kim, J.O.; Lee, H.; Yoon, E.J.; Jeong, S.H.; Yong, D.; Lee, K. In vitro antimicrobial synergy of colistin with rifampicin and carbapenems against colistin-resistant Acinetobacter baumannii clinical isolates. Diagn. Microbiol. Infect. Dis. 2016, 86, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Lyu, Y.; Li, Y. Trends in Drug Resistance of Acinetobacter baumannii over a 10-year Period: Nationwide Data from the China Surveillance of Antimicrobial Resistance Program. Chin. Med. J. 2017, 130, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Garnacho-Montero, J.; Timsit, J.F. Managing Acinetobacter baumannii infections. Curr.Opin. Infect. Dis. 2019, 32, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Eljaaly, K.; Alharbi, A.; Alshehri, S.; Ortwine, J.K.; Pogue, J.M. Plazomicin: A Novel Aminoglycoside for the Treatment of Resistant Gram-Negative Bacterial Infections. Drugs 2019, 79, 243–269. [Google Scholar] [CrossRef]
- Huband, M.D.; Mendes, R.E.; Pfaller, M.A.; Lindley, J.M.; Strand, G.J.; Benn, V.J.; Zhang, J.; Li, L.; Zhang, M.; Tan, X.; et al. In Vitro Activity of KBP-7072, a Novel Third-Generation Tetracycline, against 531 Recent Geographically Diverse and Molecularly Characterized Acinetobacter baumannii Species Complex Isolates. Antimicrob. Agents Chemother. 2020, 64, e02375-19. [Google Scholar] [CrossRef]
- Ren, J.; Li, X.; Wang, L.; Liu, M.; Zheng, K.; Wang, Y. Risk Factors and Drug Resistance of the MDR Acinetobacter baumannii in Pneumonia Patients in ICU. Open Med. 2019, 14, 772–777. [Google Scholar] [CrossRef]
- Geisinger, E.; Mortman, N.J.; Dai, Y.; Cokol, M.; Syal, S.; Farinha, A.; Fisher, D.G.; Tang, A.Y.; Lazinski, D.W.; Wood, S.; et al. Antibiotic susceptibility signatures identify potential antimicrobial targets in the Acinetobacter baumannii cell envelope. Nat. Commun. 2020, 11, 4522. [Google Scholar] [CrossRef]
- Patrier, J.; Timsit, J.F. Carbapenem use in critically ill patients. Curr.Opin. Infect. Dis. 2020, 33, 86–91. [Google Scholar] [CrossRef]
- Kumar, S.; Patil, P.P.; Midha, S.; Ray, P.; Patil, P.B.; Gautam, V. Genome Sequence of Acinetobacter baumannii Strain 5021_13, Isolated from Cerebrospinal Fluid. Genome Announc. 2015, 3, e01213-15. [Google Scholar] [CrossRef]
- Kumar, S.; Patil, P.P.; Midha, S.; Ray, P.; Patil, P.B.; Gautam, V. Genome Sequence of Acinetobacter baumannii Strain 10441_14 Belonging to ST451, Isolated from India. Genome Announc. 2015, 3, e01322-15. [Google Scholar] [CrossRef]
- Bian, X.; Liu, X.; Feng, M.; Bergen, P.J.; Li, J.; Chen, Y.; Zheng, H.; Song, S.; Zhang, J. Enhanced bacterial killing with colistin/sulbactam combination against carbapenem-resistant Acinetobacter baumannii. Int. J. Antimicrob. Agents. 2021, 57, 106271. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed Ahmed, M.A.E.; Zhong, L.L.; Shen, C.; Yang, Y.; Doi, Y.; Tian, G.B. Colistin and its role in the Era of antibiotic resistance: An extended review (2000–2019). Emerg. Microbes Infect. 2020, 9, 868–885. [Google Scholar] [CrossRef] [PubMed]
- Chamoun, S.; Welander, J.; Martis-Thiele, M.M.; Ntzouni, M.; Claesson, C.; Vikstrom, E.; Turkina, M.V. Colistin Dependence in Extensively Drug-Resistant Acinetobacter baumannii Strain Is Associated with ISAjo2 and ISAba13 Insertions and Multiple Cellular Responses. Int. J. Mol. Sci. 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Halstead, D.C.; Abid, J.; Dowzicky, M.J. Antimicrobial susceptibility among Acinetobacter calcoaceticus-baumannii complex and Enterobacteriaceae collected as part of the Tigecycline Evaluation and Surveillance Trial. J. Infect. 2007, 55, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Makris, D.; Petinaki, E.; Tsolaki, V.; Manoulakas, E.; Mantzarlis, K.; Apostolopoulou, O.; Sfyras, D.; Zakynthinos, E. Colistin versus Colistin Combined with Ampicillin-Sulbactam for Multiresistant Acinetobacter baumannii Ventilator-associated Pneumonia Treatment: An Open-label Prospective Study. Indian J. Crit. Care Med. 2018, 22, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Chusri, S.; Sakarunchai, I.; Kositpantawong, N.; Panthuwong, S.; Santimaleeworagun, W.; Pattharachayakul, S.; Singkhamanan, K.; Doi, Y. Outcomes of adjunctive therapy with intrathecal or intraventricular administration of colistin for post-neurosurgical meningitis and ventriculitis due to carbapenem-resistant Acinetobacter baumannii. Int. J. Antimicrob. Agents 2018, 51, 646–650. [Google Scholar] [CrossRef]
- Katip, W.; Uitrakul, S.; Oberdorfer, P. The effectiveness and nephrotoxicity of loading dose colistin combined with or without meropenem for the treatment of carbapenem-resistant A. baumannii. Int. J. Infect. Dis. 2020, 97, 391–395. [Google Scholar] [CrossRef]
- Bian, X.; Liu, X.; Chen, Y.; Chen, D.; Li, J.; Zhang, J. Dose Optimization of Colistin Combinations against Carbapenem-Resistant Acinetobacter baumannii from Patients with Hospital-Acquired Pneumonia in China by Using an In Vitro Pharmacokinetic/Pharmacodynamic Model. Antimicrob. Agents Chemother. 2019, 63, e01989-18. [Google Scholar] [CrossRef] [PubMed]
- Hussain, K.; Salat, M.S.; Ambreen, G.; Mughal, A.; Idrees, S.; Sohail, M.; Iqbal, J. Intravenous vs intravenous plus aerosolized colistin for treatment of ventilator-associated pneumonia—A matched case-control study in neonates. Expert Opin. Drug Saf. 2020, 19, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Mangal, S.; Park, H.; Zeng, L.; Yu, H.H.; Lin, Y.W.; Velkov, T.; Denman, J.A.; Zemlyanov, D.; Li, J.; Zhou, Q.T. Composite particle formulations of colistin and meropenem with improved in-vitro bacterial killing and aerosolization for inhalation. Int.J. Pharm. 2018, 548, 443–453. [Google Scholar] [CrossRef]
- Liu, J.; Shu, Y.; Zhu, F.; Feng, B.; Zhang, Z.; Liu, L.; Wang, G. Comparative efficacy and safety of combination therapy with high-dose sulbactam or colistin with additional antibacterial agents for multiple drug-resistant and extensively drug-resistant Acinetobacter baumannii infections: A systematic review and network meta-analysis. J. Glob. Antimicrob. Resist. 2021, 24, 136–147. [Google Scholar] [CrossRef]
- Chen, L.; Lin, J.; Lu, H.; Zhang, X.; Wang, C.; Liu, H.; Li, J.; Cao, J.; Zhou, T. Deciphering colistin heteroresistance in Acinetobacter baumannii clinical isolates from Wenzhou, China. J. Antibiot. 2020, 73, 463–470. [Google Scholar] [CrossRef]
- Shafiee, F.; NajiEsfahani, S.S.; Hakamifard, A.; Soltani, R. In vitro synergistic effect of colistin and ampicillin/sulbactam with several antibiotics against clinical strains of multi-drug resistant Acinetobacter baumannii. Indian J. Med. Microbiol. 2021. [Google Scholar] [CrossRef]
- Lv, Q.; Deng, Y.; Zhu, X.; Ruan, J.; Chen, L. Effectiveness of Cefoperazone-sulbactam alone and Combined with Tigecycline in the Treatment of Multi-drug Resistant Acinetobacter baumannii Pulmonary Infection. J. Coll. Phys. Surg. Pak. 2020, 30, 332–334. [Google Scholar] [CrossRef]
- Wang, Y.C.; Kuo, S.C.; Yang, Y.S.; Lee, Y.T.; Chiu, C.H.; Chuang, M.F.; Lin, J.C.; Chang, F.Y.; Chen, T.L. Individual or Combined Effects of Meropenem, Imipenem, Sulbactam, Colistin, and Tigecycline on Biofilm-Embedded Acinetobacter baumannii and Biofilm Architecture. Antimicrob. Agents Chemother. 2016, 60, 4670–4676. [Google Scholar] [CrossRef] [PubMed]
- Boral, B.; Unaldi, O.; Ergin, A.; Durmaz, R.; Eser, O.K. A prospective multicenter study on the evaluation of antimicrobial resistance and molecular epidemiology of multidrug-resistant Acinetobacter baumannii infections in intensive care units with clinical and environmental features. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 19. [Google Scholar] [CrossRef]
- Xu, N.; Wang, G.; Leng, Y.; Dong, X.; Chen, F.; Xing, Q. Sulbactam enhances the in vitro activity of sitafloxacin against extensively-drug resistant Acinetobacter baumannii. Exp. Ther. Med. 2018, 16, 3485–3491. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xie, J.; Chen, L.; Meng, T.; Liu, L.; Hao, R.; Dong, H.; Wang, X.; Dong, Y. Treatment efficacy of tigecycline in comparison to cefoperazone/ sulbactam alone or in combination therapy for carbapenenm-resistant Acinetobacter baumannii infections. Pak. J. Pharm. Sci. 2020, 33, 161–168. [Google Scholar]
- Adnan, S.; Paterson, D.L.; Lipman, J.; Roberts, J.A. Ampicillin/sulbactam: Its potential use in treating infections in critically ill patients. Int. J. Antimicrob. Agents 2013, 42, 384–389. [Google Scholar] [CrossRef]
- Yang, Y.; Fu, Y.; Lan, P.; Xu, Q.; Jiang, Y.; Chen, Y.; Ruan, Z.; Ji, S.; Hua, X.; Yu, Y. Molecular Epidemiology and Mechanism of Sulbactam Resistance in Acinetobacter baumannii Isolates with Diverse Genetic Backgrounds in China. Antimicrob. Agents Chemother. 2018, 62, e01947-17. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, J.; Wu, L.; Zhang, D.; Fu, L.; Xue, X. Comparison of the treatment efficacy between tigecycline plus high-dose cefoperazone-sulbactam and tigecycline monotherapy against ventilator-associated pneumonia caused by extensively drug-resistant Acinetobacter baumannii. Int. J. Clin. Pharmacol. Ther. 2018, 56, 120–129. [Google Scholar] [CrossRef]
- Yang, Y.S.; Chen, H.Y.; Hsu, W.J.; Chou, Y.C.; Perng, C.L.; Shang, H.S.; Hsiao, Y.T.; Sun, J.R. Overexpression of AdeABC efflux pump associated with tigecycline resistance in clinical Acinetobacter nosocomialis isolates. Clin. Microbiol. Infect. 2019, 25, e1–e512. [Google Scholar] [CrossRef] [PubMed]
- Foong, W.E.; Wilhelm, J.; Tam, H.K.; Pos, K.M. Tigecycline efflux in Acinetobacter baumannii is mediated by TetA in synergy with RND-type efflux transporters. J. Antimicrob. Chemother. 2020, 75, 1135–1139. [Google Scholar] [CrossRef] [PubMed]
- Jean, S.S.; Hsieh, T.C.; Hsu, C.W.; Lee, W.S.; Bai, K.J.; Lam, C. Comparison of the clinical efficacy between tigecycline plus extended-infusion imipenem and sulbactam plus imipenem against ventilator-associated pneumonia with pneumonic extensively drug-resistant Acinetobacter baumannii bacteremia, and correlation of clinical efficacy with in vitro synergy tests. J. Microbiol. Immunol. Infect. 2016, 49, 924–933. [Google Scholar] [CrossRef]
- Abdallah, M.; Alsaleh, H.; Baradwan, A.; Alfawares, R.; Alobaid, A.; Rasheed, A.; Soliman, I. Intraventricular Tigecycline as a Last Resort Therapy in a Patient with Difficult-to-Treat Healthcare-Associated Acinetobacter baumannii Ventriculitis: A Case Report. SN Compr. Clin. Med. 2020, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Gautam, L.; Kaur, R.; Kumar, S.; Bansal, A.; Gautam, V.; Singh, M.; Ray, P. Pseudomonas oleovorans Sepsis in a Child: The First Reported Case in India. Jpn. J. Infect. Dis. 2015, 68, 254–255. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, X.; Xu, P.; Zhu, Y.; Wang, K.; Xiang, D.; Wang, F.; Banh, H.L. Clinical experience with tigecycline in the treatment of hospital-acquired pneumonia caused by multidrug resistant Acinetobacter baumannii. BMC Pharmacol. Toxicol. 2019, 20, 19. [Google Scholar] [CrossRef] [PubMed]
- Shankar, C.; Pragasam, A.K.; Veeraraghavan, B.; Amladi, A. Bad bug, no test: Tigecycline susceptibility testing challenges and way forward. Indian J. Med. Microbiol. 2019, 37, 91–94. [Google Scholar] [CrossRef]
- Li, M.X.; Li, N.; Zhu, L.Q.; Liu, W. Optimization of tigecycline dosage regimen for different infections in the patients with hepatic or renal impairment. J. Chemother. 2020, 32, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Serretiello, E.; Folliero, V.; Santella, B.; Giordano, G.; Santoro, E.; De Caro, F.; Pagliano, P.; Ferro, M.; Aliberti, S.M.; Capunzo, M.; et al. Trend of Bacterial Uropathogens and Their Susceptibility Pattern: Study of Single Academic High-Volume Center in Italy (2015–2019). Int. J. Microbiol. 2021, 2021, 5541706. [Google Scholar] [CrossRef]
- Brust, K.; Evans, A.; Plemmons, R. Tigecycline in treatment of multidrug-resistant Gram-negative bacillus urinary tract infections: A systematic review. J. Antimicrob. Chemother. 2014, 69, 2606–2610. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Polat, M.; Ozkaya-Parlakay, A. Tigecycline salvage therapy for ventriculoperitoneal shunt meningitis due to extensively drug-resistant Acinetobacter baumannii. Eur. J. Pediatr. 2019, 178, 117–118. [Google Scholar] [CrossRef]
- Falghoush, A.; Beyenal, H.; Call, D.R. Sequential Hypertonic-Hypotonic Treatment Enhances Efficacy of Antibiotic against Acinetobacter baumannii Biofilm Communities. Antibiotics 2020, 9, 832. [Google Scholar] [CrossRef]
- Huang, Q.; Zhou, Q.; Ju, T.; Xu, H.; Wang, W. Meropenem and Amikacin for Management of Post-Neurosurgical Infections from Acinetobacter baumannii. Surg Infect. 2019, 20, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.S.; Ko, K.S. Eradication of persister cells of Acinetobacter baumannii through combination of colistin and amikacin antibiotics. J. Antimicrob. Chemother. 2019, 74, 1277–1283. [Google Scholar] [CrossRef]
- Anderson, S.E.; Sherman, E.X.; Weiss, D.S.; Rather, P.N. Aminoglycoside Heteroresistance in Acinetobacter baumannii AB5075. mSphere. 2018, 3, e00271-18. [Google Scholar] [CrossRef]
- Kwon, H.I.; Kim, S.; Oh, M.H.; Shin, M.; Lee, J.C. Distinct role of outer membrane protein A in the intrinsic resistance of Acinetobacter baumannii and Acinetobacter nosocomialis. Infect. Genet. Evol. 2019, 67, 33–37. [Google Scholar] [CrossRef]
- Juhas, M.; Widlake, E.; Teo, J.; Huseby, D.L.; Tyrrell, J.M.; Polikanov, Y.S.; Ercan, O.; Petersson, A.; Cao, S.; Aboklaish, A.F.; et al. In vitro activity of apramycin against multidrug-, carbapenem- and aminoglycoside-resistant Enterobacteriaceae and Acinetobacter baumannii. J. Antimicrob. Chemother. 2019, 74, 944–952. [Google Scholar] [CrossRef]
- Gaurav, A.; Gupta, V.; Shrivastava, S.K.; Pathania, R. Mechanistic insights into synergy between nalidixic acid and tetracycline against clinical isolates of Acinetobacter baumannii and Escherichia coli. Commun. Biol. 2021, 4, 542. [Google Scholar] [CrossRef]
- Fragkou, P.C.; Poulakou, G.; Blizou, A.; Blizou, M.; Rapti, V.; Karageorgopoulos, D.E.; Koulenti, D.; Papadopoulos, A.; Matthaiou, D.K.; Tsiodras, S. The Role of Minocycline in the Treatment of Nosocomial Infections Caused by Multidrug, Extensively Drug and Pandrug Resistant Acinetobacter baumannii: A Systematic Review of Clinical Evidence. Microorganisms. 2019, 7, 159. [Google Scholar] [CrossRef] [PubMed]
- Tarazi, Z.; Sabet, M.; Dudley, M.N.; Griffith, D.C. Pharmacodynamics of Minocycline against Acinetobacter baumannii in a Rat Pneumonia Model. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [PubMed]
- Iregui, A.; Landman, D.; Quale, J. Activity of Omadacycline and Other Tetracyclines Against Contemporary Gram-Negative Pathogens from New York City Hospitals. Microb. Drug Resist. 2021, 27, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Beheshti, M.; Ardebili, A.; Beheshti, F.; Lari, A.R.; Siyadatpanah, A.; Pournajaf, A.; Gautam, D.; Dolma, K.G.; Nissapatorn, V. Tetracycline resistance mediated by tet efflux pumps in clinical isolates of Acinetobacter baumannii. Rev. Inst. Med. Trop. Sao Paulo. 2020, 62, e88. [Google Scholar] [CrossRef]
- WHO. Guidelines for the Prevention and Control of Carbapenem-Resistant Enterobacteriaceae, Acinetobacter baumannii and Pseudomonas aeruginosa in Health Care Facilities. In WHO Guidelines Approved by the Guidelines Review Committee; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Teerawattanapong, N.; Kengkla, K.; Dilokthornsakul, P.; Saokaew, S.; Apisarnthanarak, A.; Chaiyakunapruk, N. Prevention and Control of Multidrug-Resistant Gram-Negative Bacteria in Adult Intensive Care Units: A Systematic Review and Network Meta-analysis. Clin. Infect. Dis. 2017, 64, S51–S60. [Google Scholar] [CrossRef]
- Warde, E.; Davies, E.; Ward, A. Control of a multidrug-resistant Acinetobacter baumannii outbreak. Br. J. Nurs. 2019, 28, 242–248. [Google Scholar] [CrossRef]
- Valencia-Martin, R.; Gonzalez-Galan, V.; Alvarez-Marin, R.; Cazalla-Foncueva, A.M.; Aldabo, T.; Gil-Navarro, M.V.; Alonso-Araujo, I.; Martin, C.; Gordon, R.; Garcia-Nunez, E.J.; et al. A multimodal intervention program to control a long-term Acinetobacter baumannii endemic in a tertiary care hospital. Antimicrob. Resist. Infect. Control. 2019, 8, 199. [Google Scholar] [CrossRef]
- Gramatniece, A.; Silamikelis, I.; Zahare, I.; Urtans, V.; Dimina, E.; Saule, M.; Balode, A.; Radovica-Spalvina, I.; Klovins, J.; Fridmanis, D.; et al. Control of Acinetobacter baumannii outbreak in the neonatal intensive care unit in Latvia: Whole-genome sequencing powered investigation and closure of the ward. Antimicrob. Resist. Infect. Control. 2019, 8, 84. [Google Scholar] [CrossRef]
- Patil, P.P.; Mali, S.; Midha, S.; Gautam, V.; Dash, L.; Kumar, S.; Shastri, J.; Singhal, L.; Patil, P.B. Genomics Reveals a Unique Clone of Burkholderiacenocepacia Harboring an Actively Excising Novel Genomic Island. Front. Microbiol. 2017, 8, 590. [Google Scholar] [CrossRef] [PubMed]
- Raorane, C.J.; Lee, J.H.; Kim, Y.G.; Rajasekharan, S.K.; Garcia-Contreras, R.; Lee, J. Antibiofilm and Antivirulence Efficacies of Flavonoids and Curcumin Against Acinetobacter baumannii. Front. Microbiol. 2019, 10, 990. [Google Scholar] [CrossRef]
- Corral, J.; Perez-Varela, M.; Barbe, J.; Aranda, J. Direct interaction between RecA and a CheW-like protein is required for surface-associated motility, chemotaxis and the full virulence of Acinetobacter baumannii strain ATCC 17978. Virulence 2020, 11, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhu, Y.; Han, J.; Lin, Y.W.; Aichem, M.; Wang, J.; Chen, K.; Velkov, T.; Schreiber, F.; Li, J. Genome-Scale Metabolic Modeling Reveals Metabolic Alterations of Multidrug-Resistant Acinetobacter baumannii in a Murine Bloodstream Infection Model. Microorganisms 2020, 8, 1793. [Google Scholar] [CrossRef]
- Kumar, S.; Singhal, L.; Ray, P.; Gautam, V. In vitro and in vivo fitness of clinical isolates of carbapenem-resistant and -susceptible Acinetobacter baumannii. Indian J. Med. Microbiol. 2020, 38, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Labrador-Herrera, G.; Perez-Pulido, A.J.; Alvarez-Marin, R.; Casimiro-Soriguer, C.S.; Cebrero-Cangueiro, T.; Moran-Barrio, J.; Pachon, J.; Viale, A.M.; Pachon-Ibanez, M.E. Virulence role of the outer membrane protein CarO in carbapenem-resistant Acinetobacter baumannii. Virulence 2020, 11, 1727–1737. [Google Scholar] [CrossRef]
- Martinez-Guitian, M.; Vazquez-Ucha, J.C.; Alvarez-Fraga, L.; Conde-Perez, K.; Vallejo, J.A.; Perina, A.; Bou, G.; Poza, M.; Beceiro, A. Global Transcriptomic Analysis During Murine Pneumonia Infection Reveals New Virulence Factors in Acinetobacter baumannii. J. Infect. Dis. 2021, 223, 1356–1366. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.M.; Samir, R.; Saber, F.R.; Ahmed, S.R.; Farag, M.A. Pimenta Oil as A Potential Treatment for Acinetobacter baumannii Wound Infection: In Vitro and In Vivo Bioassays in Relation to Its Chemical Composition. Antibiotics 2020, 9, 679. [Google Scholar] [CrossRef] [PubMed]
- Skerniskyte, J.; Krasauskas, R.; Pechoux, C.; Kulakauskas, S.; Armalyte, J.; Suziedeliene, E. Surface-Related Features and Virulence Among Acinetobacter baumannii Clinical Isolates Belonging to International Clones I and II. Front. Microbiol. 2018, 9, 3116. [Google Scholar] [CrossRef]
- Monem, S.; Furmanek-Blaszk, B.; Lupkowska, A.; Kuczynska-Wisnik, D.; Stojowska-Swedrzynska, K.; Laskowska, E. Mechanisms Protecting Acinetobacter baumannii against Multiple Stresses Triggered by the Host Immune Response, Antibiotics and Outside-Host Environment. Int. J. Mol. Sci. 2020, 21, 5498. [Google Scholar] [CrossRef]
- Harris, G.; KuoLee, R.; Xu, H.H.; Chen, W. Mouse Models of Acinetobacter baumannii Infection. Curr. Protoc. Microbiol. 2017, 46, 6G.3.1–6G.3.23. [Google Scholar] [CrossRef]
- Grygorcewicz, B.; Roszak, M.; Golec, P.; Sleboda-Taront, D.; Lubowska, N.; Gorska, M.; Jursa-Kulesza, J.; Rakoczy, R.; Wojciuk, B.; Dolegowska, B. Antibiotics Act with vB_AbaP_AGC01 Phage against Acinetobacter baumannii in Human Heat-Inactivated Plasma Blood and Galleria mellonella Models. Int. J. Mol. Sci. 2020, 21, 4390. [Google Scholar] [CrossRef]
- Maslova, E.; Shi, Y.; Sjoberg, F.; Azevedo, H.S.; Wareham, D.W.; McCarthy, R.R. An Invertebrate Burn Wound Model That Recapitulates the Hallmarks of Burn Trauma and Infection Seen in Mammalian Models. Front. Microbiol. 2020, 11, 998. [Google Scholar] [CrossRef]
- Chen, Y.W.; Ton-That, H. Corynebacterium diphtheriae Virulence Analyses Using a Caenorhabditis elegans Model. Curr. Protoc. Microbiol. 2020, 58, e109. [Google Scholar] [CrossRef]
- Kaito, C.; Murakami, K.; Imai, L.; Furuta, K. Animal infection models using non-mammals. Microbiol. Immunol. 2020, 64, 585–592. [Google Scholar] [CrossRef]
- Perez-Varela, M.; Tierney, A.R.P.; Kim, J.S.; Vazquez-Torres, A.; Rather, P. Characterization of RelA in Acinetobacter baumannii. J. Bacteriol. 2020, 202, e00045-20. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.M.; Pei, J.; Gomez, G.; Rice-Ficht, A.; Ficht, T.A.; de Figueiredo, P. A Tractable Drosophila Cell System Enables Rapid Identification of Acinetobacter baumannii Host Factors. Front. Cell Infect. Microbiol. 2020, 10, 240. [Google Scholar] [CrossRef]
- Abdelli, N.; Peng, L.; Keping, C. Silkworm, Bombyx mori, as an alternative model organism in toxicological research. Environ. Sci. Pollut. Res. Int. 2018, 25, 35048–35054. [Google Scholar] [CrossRef] [PubMed]
- Martin-Gonzalez, J.; Montero-Bullon, J.F.; Lacal, J. Dictyosteliumdiscoideum as a non-mammalian biomedical model. Microb. Biotechnol. 2021, 14, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Olsowski, M.; Hoffmann, F.; Hain, A.; Kirchhoff, L.; Theegarten, D.; Todt, D.; Steinmann, E.; Buer, J.; Rath, P.M.; Steinmann, J. Exophiala dermatitidis isolates from various sources: Using alternative invertebrate host organisms (Caenorhabditis elegans and Galleria mellonella) to determine virulence. Sci. Rep. 2018, 8, 12747. [Google Scholar] [CrossRef]
- Balla, K.M.; Troemel, E.R. Caenorhabditis elegans as a model for intracellular pathogen infection. Cell Microbiol. 2013, 15, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Berg, M.; Dierking, K.; Felix, M.A.; Shapira, M.; Samuel, B.S.; Schulenburg, H. Caenorhabditis elegans as a Model for Microbiome Research. Front. Microbiol. 2017, 8, 485. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, S.; Anwer, R.; Azzi, A. Virulence Potential and Treatment Options of Multidrug-Resistant (MDR) Acinetobacter baumannii. Microorganisms 2021, 9, 2104. https://doi.org/10.3390/microorganisms9102104
Kumar S, Anwer R, Azzi A. Virulence Potential and Treatment Options of Multidrug-Resistant (MDR) Acinetobacter baumannii. Microorganisms. 2021; 9(10):2104. https://doi.org/10.3390/microorganisms9102104
Chicago/Turabian StyleKumar, Sunil, Razique Anwer, and Arezki Azzi. 2021. "Virulence Potential and Treatment Options of Multidrug-Resistant (MDR) Acinetobacter baumannii" Microorganisms 9, no. 10: 2104. https://doi.org/10.3390/microorganisms9102104
APA StyleKumar, S., Anwer, R., & Azzi, A. (2021). Virulence Potential and Treatment Options of Multidrug-Resistant (MDR) Acinetobacter baumannii. Microorganisms, 9(10), 2104. https://doi.org/10.3390/microorganisms9102104






