The Influence of Above-Ground Herbivory on the Response of Arctic Soil Methanotrophs to Increasing CH4 Concentrations and Temperatures
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Site and Sampling
2.2. Soil Microcosms
2.3. CH4 Oxidation of Methylobacter Tundripaludum SV96
2.4. RNA Extraction
2.5. PmoA Transcript Numbers
2.6. Bioinformatics
2.6.1. Databases
2.6.2. Pipeline
2.6.3. Community Analyses
2.6.4. Redundancy Analysis (RDA)
2.6.5. Bioindicators
2.6.6. Phylogenetic Analysis
3. Results
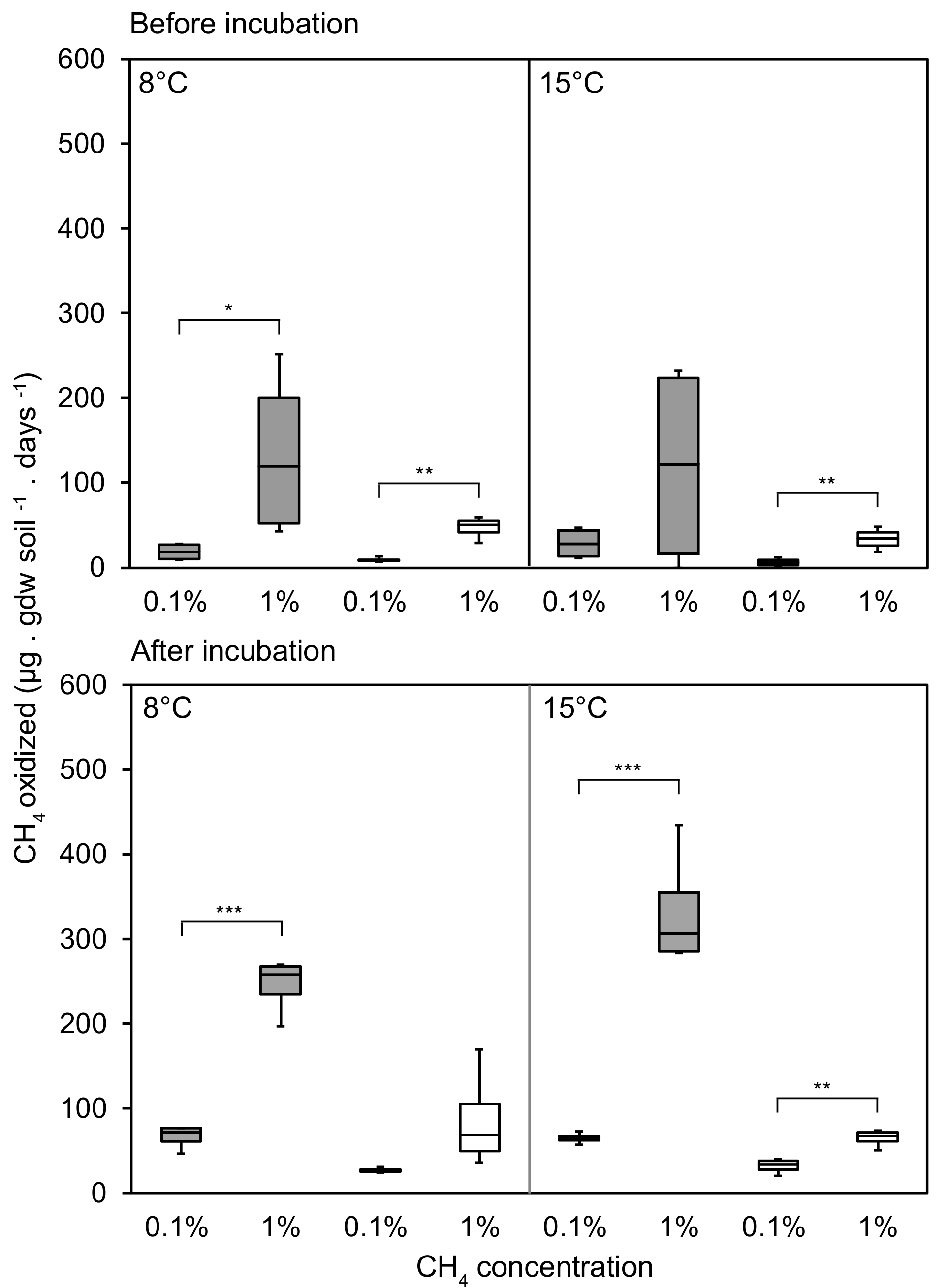
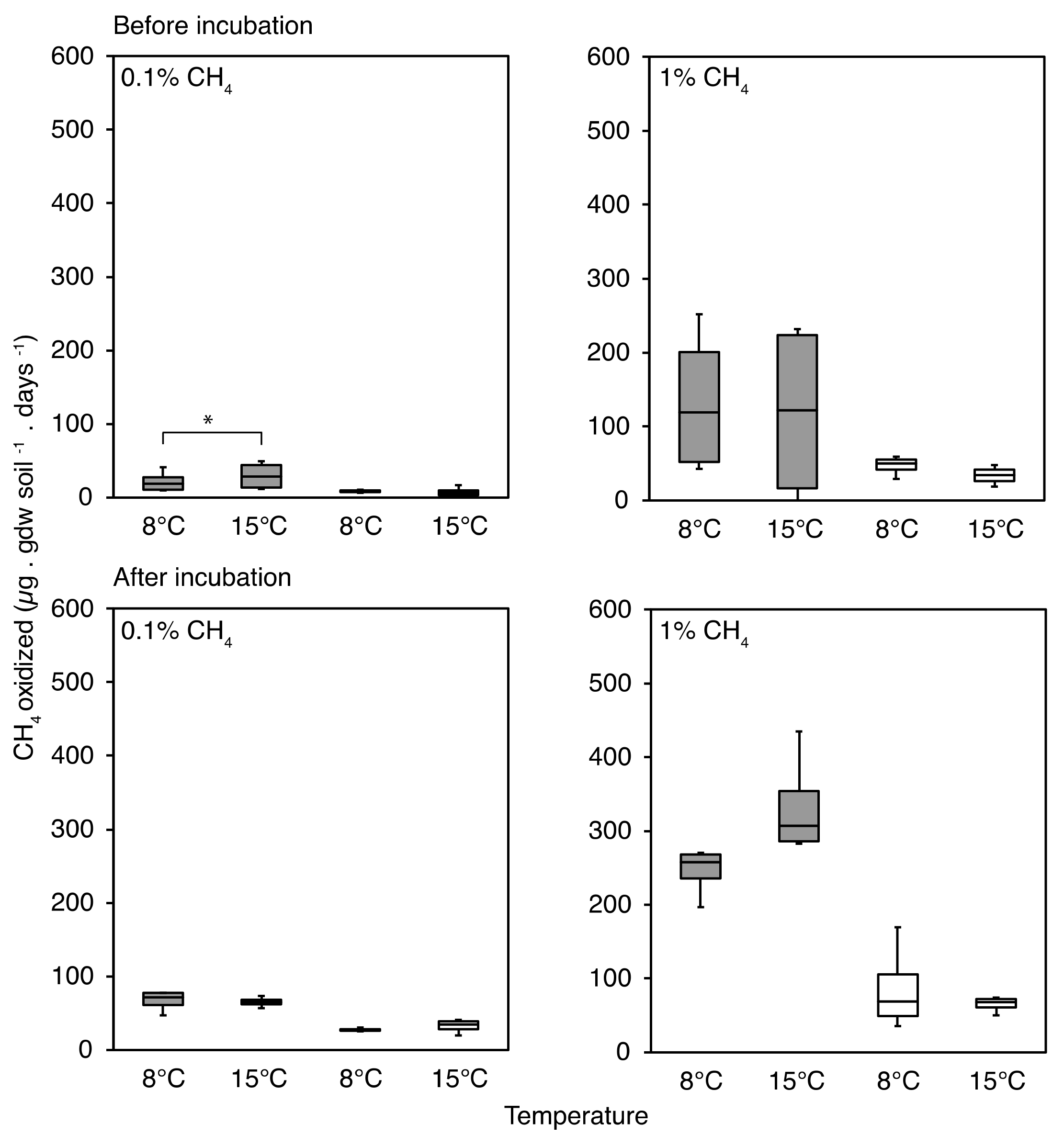
3.1. CH4 Oxidation Rates Changing in Response to CH4 Concentrations
3.2. CH4 Oxidation in Response to Temperature
3.3. CH4 Oxidation in a Pure Culture of M. Tundripaludum SV96 in Response to Temperature
3.4. MOB Community
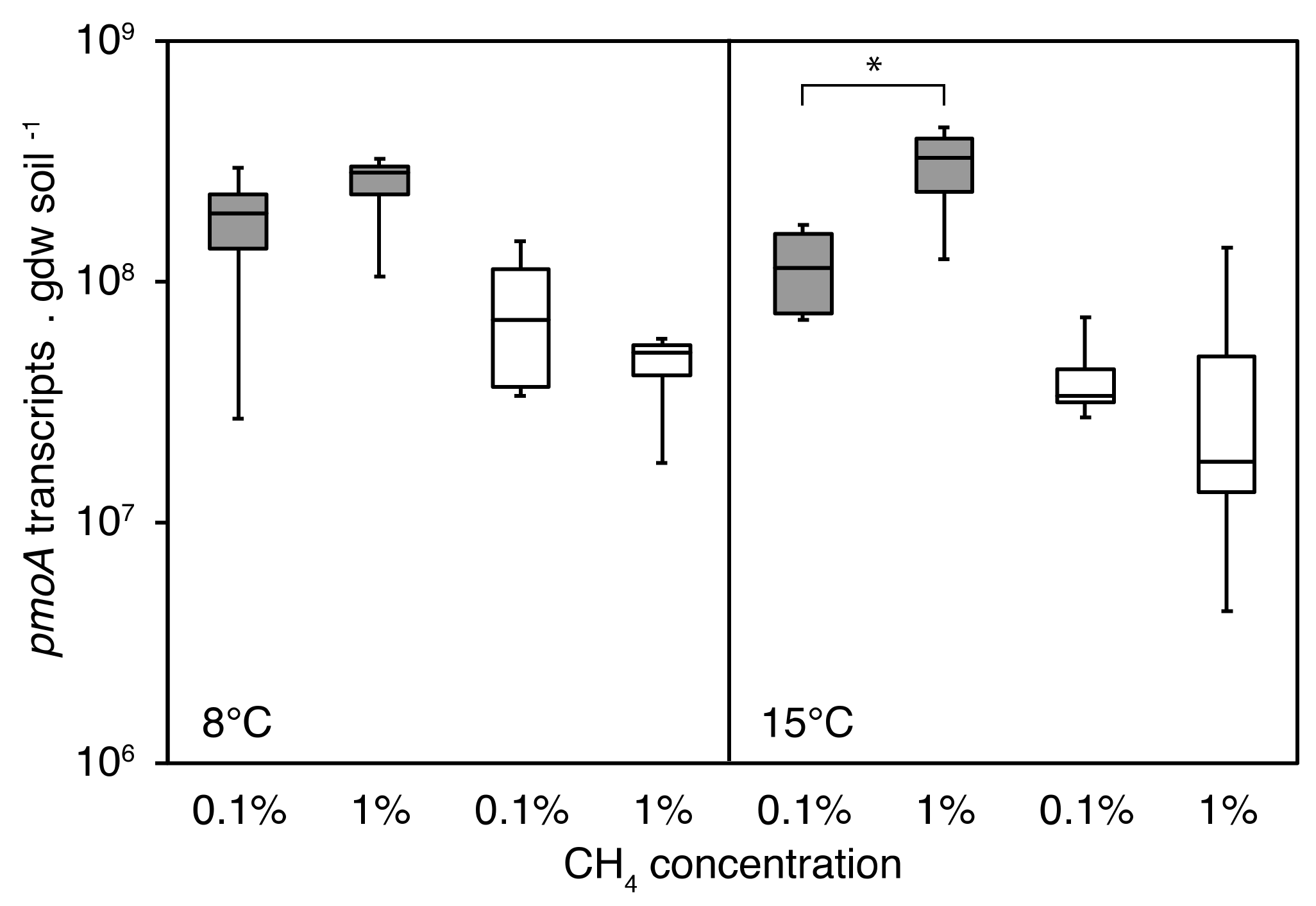
3.4.1. PmoA Transcript Numbers
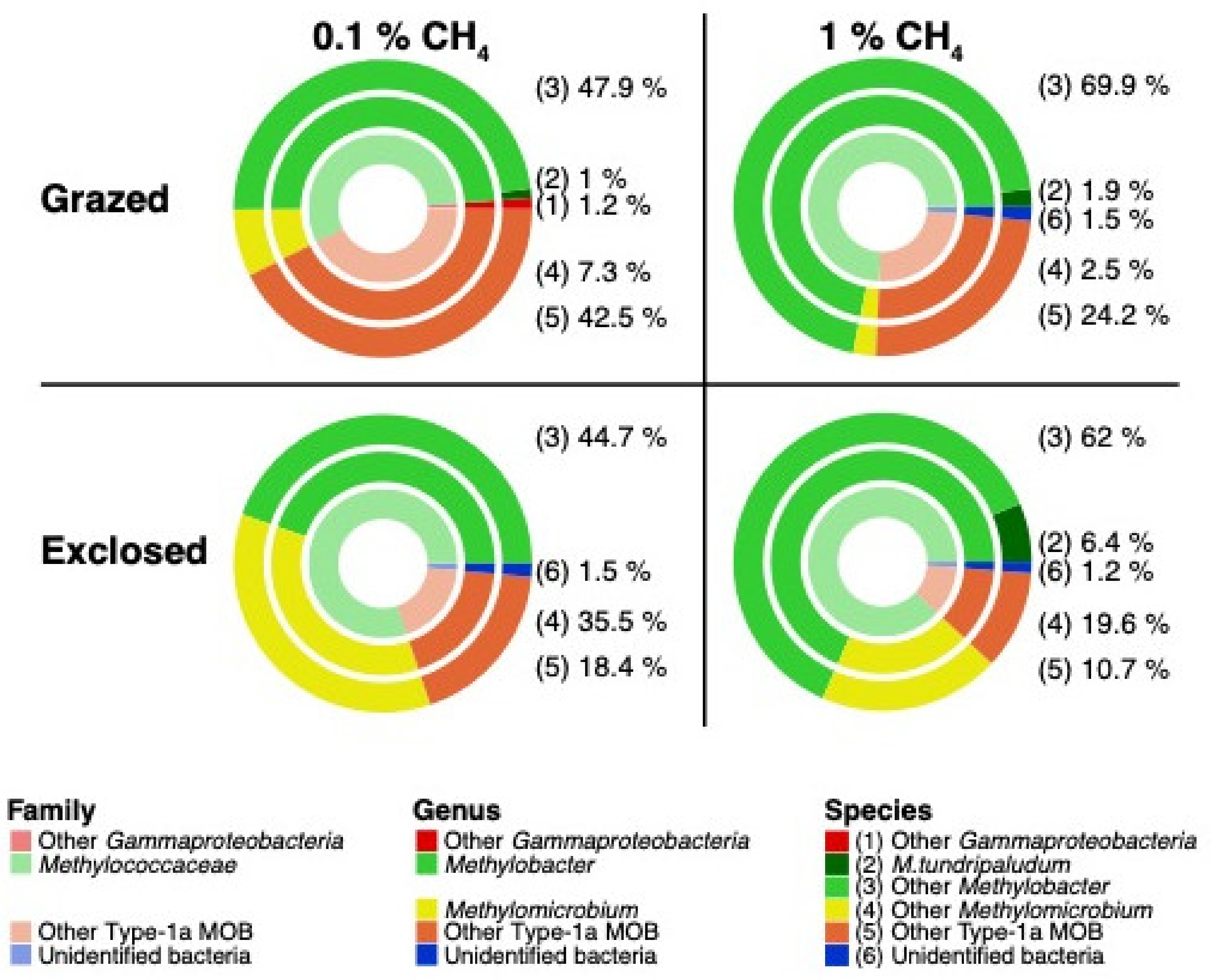
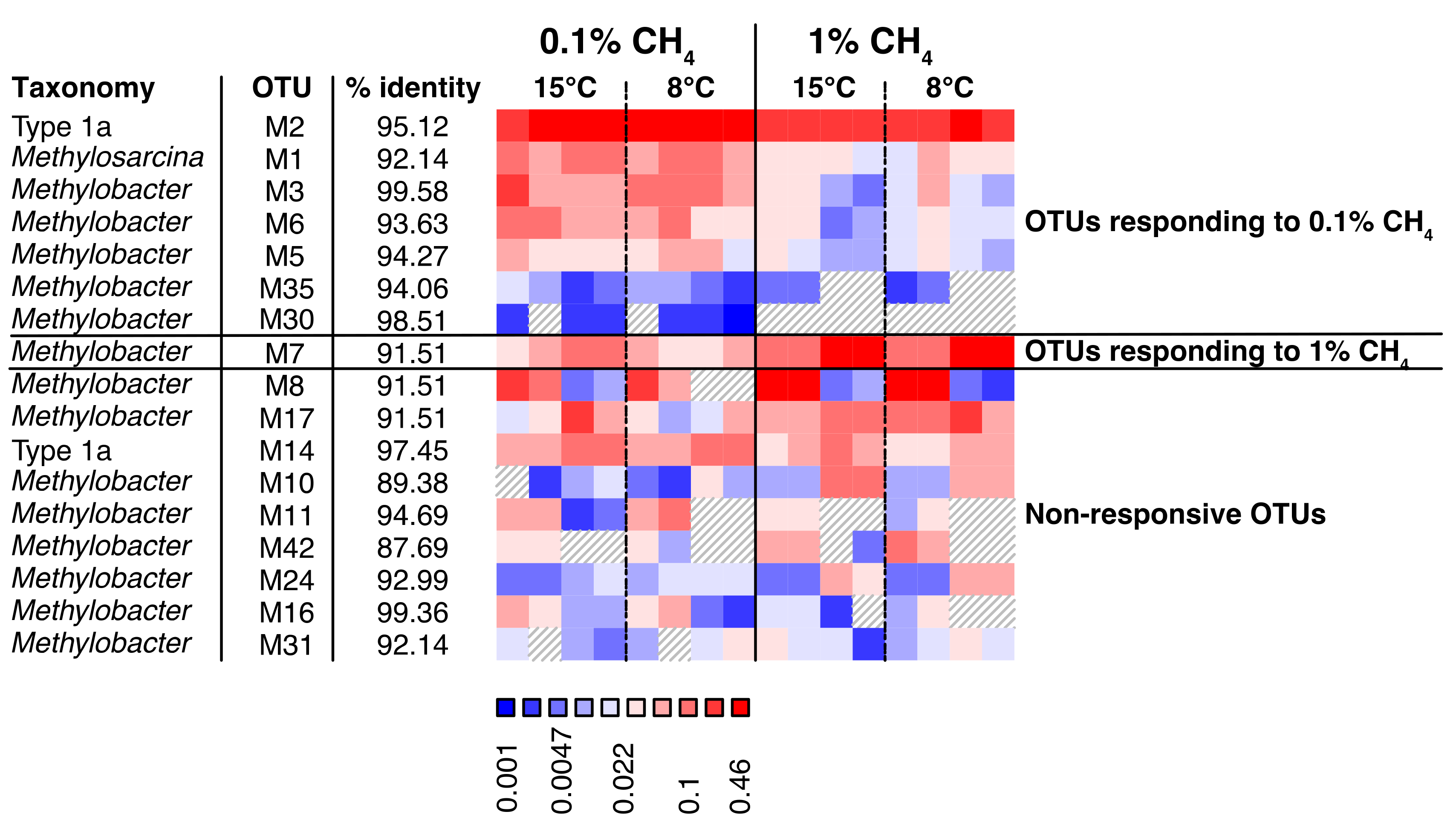
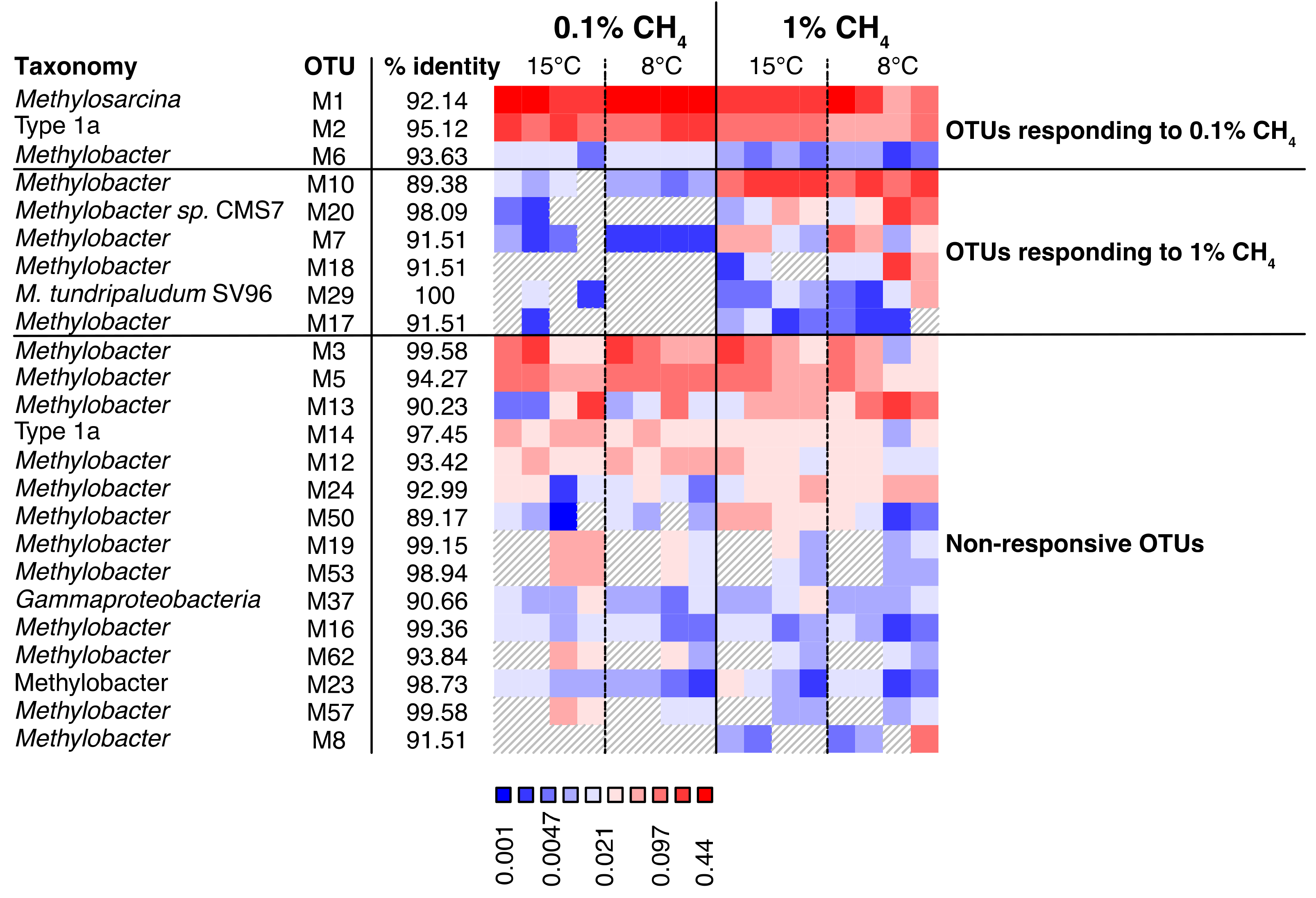
3.4.2. Transcriptionally Active MOB
4. Discussion
4.1. CH4 Response
4.1.1. Activity
4.1.2. MOB Transcript Numbers
4.1.3. MOB Transcript Composition
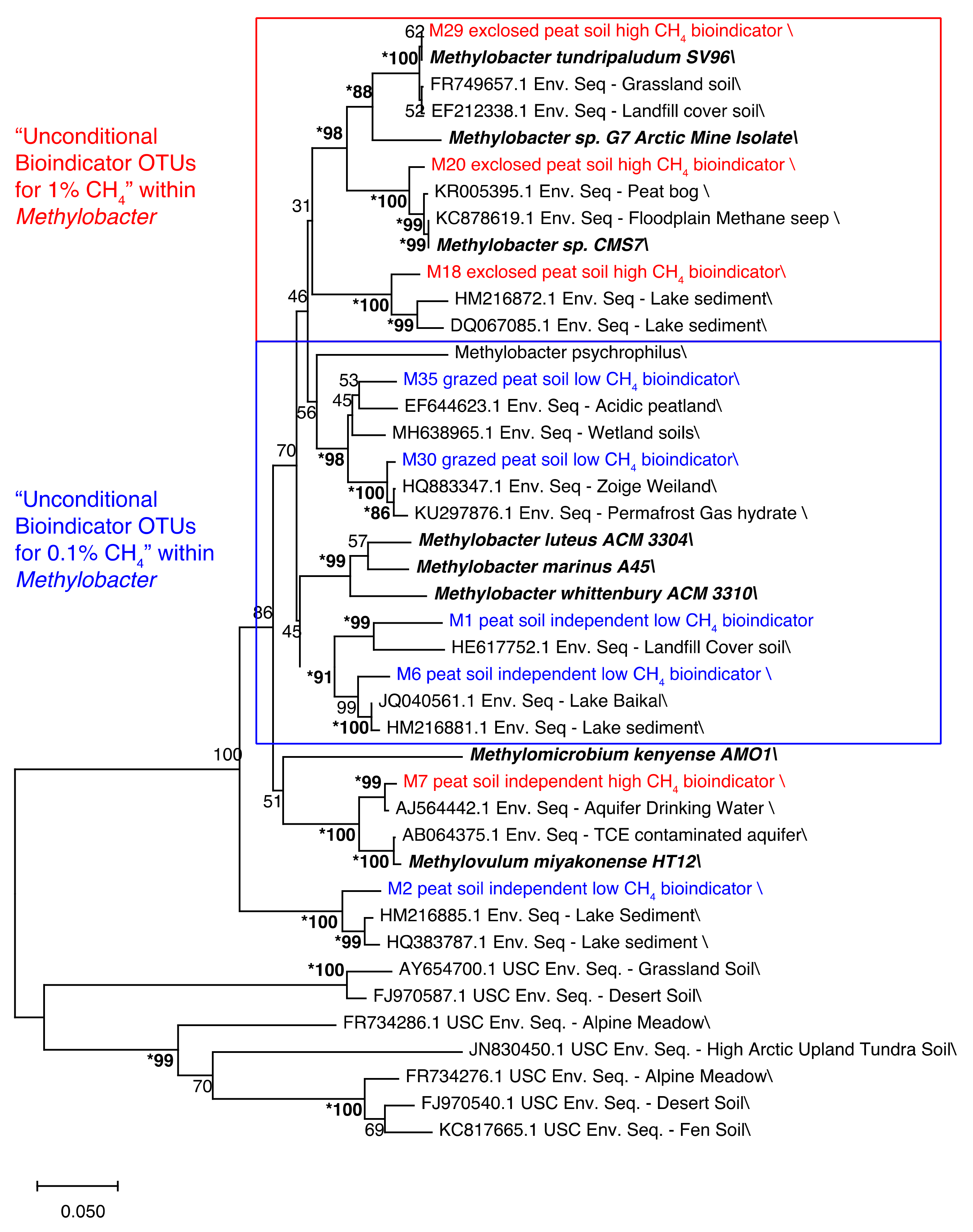
4.1.4. Phylogeny
4.2. Temperature Response
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kuijper, D.P.J.; Bakker, J.P.; Cooper, E.J.; Ubels, R.; Jónsdóttir, I.S.; Loonen, M.J.J.E. Intensive grazing by Barnacle geese depletes High Arctic seed bank. Can. J. Bot. 2006, 84, 995–1004. [Google Scholar] [CrossRef][Green Version]
- Jefferies, R.L.; Rockwell, R.F. Foraging geese, vegetation loss and soil degradation in an Arctic salt marsh. Appl. Veg. Sci. 2002, 5, 7–16. [Google Scholar] [CrossRef]
- Handa, I.T.; Harmsen, R.; Jefferies, R.L. Patterns of vegetation change and the recovery potential of degraded areas in a coastal marsh system of the Hudson Bay lowlands. J. Ecol. 2002, 90, 86–99. [Google Scholar] [CrossRef]
- Hessen, D.O.; Tombre, I.M.; van Geest, G.; Alfsnes, K. Global change and ecosystem connectivity: How geese link fields of central Europe to eutrophication of Arctic freshwaters. Ambio 2017, 46, 40–47. [Google Scholar] [CrossRef]
- Tombre, I.M.; Oudman, T.; Shimmings, P.; Griffin, L.; Prop, J. Northward range expansion in spring-staging barnacle geese is a response to climate change and population growth, mediated by individual experience. Glob. Chang. Biol. 2019, 25, 3680–3693. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Wardle, D.A. Herbivore-mediated linkages between aboveground and belowground communities. Ecology 2003, 84, 2258–2268. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setälä, H.; Van Der Putten, W.H.; Wall, D.H. Ecological linkages between aboveground and belowground biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef]
- Foley, K.M.; Beard, K.H.; Atwood, T.B.; Waring, B.G. Herbivory changes soil microbial communities and greenhouse gas fluxes in a high-latitude wetland. Microb. Ecol. 2021, 1–10. [Google Scholar] [CrossRef]
- Falk, J.M.; Schmidt, N.M.; Christensen, T.R.; Ström, L. Large herbivore grazing affects the vegetation structure and greenhouse gas balance in a high Arctic mire. Environ. Res. Lett. 2015, 10, 45001. [Google Scholar] [CrossRef]
- Winton, R.S.; Richardson, C.J. Top-down control of methane emission and nitrogen cycling by waterfowl. Ecology 2017, 98, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Kelsey, K.C.; Leffler, A.J.; Beard, K.H.; Schmutz, J.A.; Choi, R.T.; Welker, J.M. Interactions among vegetation, climate, and herbivory control greenhouse gas fluxes in a subarctic coastal wetland. J. Geophys. Res. Biogeosciences 2016, 121, 2960–2975. [Google Scholar] [CrossRef]
- Sjögersten, S.; van der Wal, R.; Loonen, M.J.J.E.; Woodin, S.J. Recovery of ecosystem carbon fluxes and storage from herbivory. Biogeochemistry 2011, 106, 357–370. [Google Scholar] [CrossRef]
- Bender, K.M.; Svenning, M.M.; Hu, Y.; Richter, A.; Schückel, J.; Jørgensen, B.; Liebner, S.; Tveit, A.T. Microbial responses to herbivory-induced vegetation changes in a high-Arctic peatland. Polar Biol. 2021, 44, 899–911. [Google Scholar] [CrossRef]
- Cohen, J.; Screen, J.A.; Furtado, J.C.; Barlow, M.; Whittleston, D.; Coumou, D.; Francis, J.; Dethloff, K.; Entekhabi, D.; Overland, J.; et al. Recent Arctic amplification and extreme mid-latitude weather. Nat. Geosci. 2014, 7, 627–637. [Google Scholar] [CrossRef]
- Francis, J.A.; Vavrus, S.J.; Cohen, J. Amplified Arctic warming and mid-latitude weather: New perspectives on emerging connections. Wiley Interdiscip. Rev. Clim. Chang. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Serreze, M.C.; Barry, R.G. Processes and impacts of Arctic amplification: A research synthesis. Glob. Planet. Change 2011, 77, 85–96. [Google Scholar] [CrossRef]
- Tarnocai, C.; Canadell, J.G.; Schuur, E.A.G.; Kuhry, P.; Mazhitova, G.; Zimov, S. Soil organic carbon pools in the northern circumpolar permafrost region. Glob. Biogeochem. Cycles 2009, 23, 1–11. [Google Scholar] [CrossRef]
- Tveit, A.T.; Urich, T.; Frenzel, P.; Svenning, M.M. Metabolic and trophic interactions modulate methane production by Arctic peat microbiota in response to warming. Proc. Natl. Acad. Sci. USA 2015, 112, E2507–E2516. [Google Scholar] [CrossRef]
- Metje, M.; Frenzel, P. Methanogenesis and methanogenic pathways in a peat from subarctic permafrost. Environ. Microbiol. 2007, 9, 954–964. [Google Scholar] [CrossRef]
- Metje, M.; Frenzel, P. Effect of temperature on anaerobic ethanol oxidation and methanogenesis in acidic peat from a Northern Wetland. Appl. Environ. Microbiol. 2005, 71, 8191–8200. [Google Scholar] [CrossRef] [PubMed]
- Høj, L.; Olsen, R.A.; Torsvik, V.L. Archaeal communities in High Arctic wetlands at Spitsbergen, Norway (78° N) as characterized by 16S rRNA gene fingerprinting. FEMS Microbiol. Ecol. 2005, 53, 89–101. [Google Scholar] [CrossRef][Green Version]
- Brune, A.; Frenzel, P.; Cypionka, H. Life at the oxic-anoxic interface: Microbial activities and adaptations. FEMS Microbiol. Rev. 2000, 24, 691–710. [Google Scholar] [CrossRef]
- Reim, A.; Lüke, C.; Krause, S.; Pratscher, J.; Frenzel, P. One millimetre makes the difference: High-resolution analysis of methane-oxidizing bacteria and their specific activity at the oxic-anoxic interface in a flooded paddy soil. ISME J. 2012, 6, 2128–2139. [Google Scholar] [CrossRef] [PubMed]
- Knief, C. Diversity and habitat preferences of cultivated and uncultivated aerobic methanotrophic bacteria evaluated based on pmoA as molecular marker. Front. Microbiol. 2015, 6, 1346. [Google Scholar] [CrossRef]
- Wartiainen, I.; Hestnes, A.G.; Svenning, M.M. Methanotrophic diversity in high arctic wetlands on the islands of Svalbard (Norway)-denaturing gradient gel electrophoresis analysis of soil DNA and enrichment cultures. Can. J. Microbiol. 2003, 49, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Martineau, C.; Whyte, L.G.; Greer, C.W. Stable isotope probing analysis of the diversity and activity of Methanotrophic bacteria in soils from the Canadian high Arctic. Appl. Environ. Microbiol. 2010, 76, 5773–5784. [Google Scholar] [CrossRef] [PubMed]
- Rainer, E.M.; Seppey, C.V.W.; Tveit, A.T.; Svenning, M.M. Methanotroph populations and CH4 oxidation potentials in High Arctic peat are altered by herbivory induced vegetation change. FEMS Microbiol. Ecol. 2020, 96, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tveit, A.T.; Kiss, A.; Winkel, M.; Horn, F.; Hájek, T.; Svenning, M.M.; Wagner, D.; Liebner, S. Environmental patterns of brown moss- and Sphagnum-associated microbial communities. Sci. Rep. 2020, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Dedysh, S.N.; Didriksen, A.; Danilova, O.V.; Belova, S.E.; Liebner, S.; Svenning, M.M. Methylocapsa palsarum sp. nov., a methanotroph isolated from a subarctic discontinuous permafrost ecosystem. Int. J. Syst. Evol. Microbiol. 2015, 65, 3618–3624. [Google Scholar] [CrossRef]
- Dedysh, S.N.; Berestovskaya, Y.Y.; Vasylieva, L.V.; Belova, S.E.; Khmelenina, V.N.; Suzina, N.E.; Trotsenko, Y.A.; Liesack, W.; Zavarzin, G.A. Methylocella tundrae sp. nov., a novel methanotrophic bacterium from acidic tundra peatlands. Int. J. Syst. Evol. Microbiol. 2004, 54, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Belova, S.E.; Oshkin, I.Y.; Glagolev, M.V.; Lapshina, E.D.; Maksyutov, S.S.; Dedysh, S.N. Methanotrophic bacteria in cold seeps of the floodplains of northern rivers. Microbiology 2013, 82, 743–750. [Google Scholar] [CrossRef]
- Wartiainen, I.; Hestnes, A.G.; McDonald, I.R.; Svenning, M.M. Methylobacter tundripaludum sp. nov., a methane-oxidizing bacterium from Arctic wetland soil on the Svalbard islands, Norway (78° N). Int. J. Syst. Evol. Microbiol. 2006, 56, 109–113. [Google Scholar] [CrossRef]
- Berestovskaya, Y.Y.; Vasil’eva, L.V.; Chestnykh, O.V.; Zavarzin, G.A. Methanotrophs of the psychrophilic microbial community of the Russian arctic tundra. Microbiology 2002, 71, 460–466. [Google Scholar] [CrossRef]
- Einola, J.K.M.; Kettunen, R.H.; Rintala, J.A. Responses of methane oxidation to temperature and water content in cover soil of a boreal landfill. Soil Biol. Biochem. 2007, 39, 1156–1164. [Google Scholar] [CrossRef]
- Zheng, J.; RoyChowdhury, T.; Yang, Z.; Gu, B.; Wullschleger, S.D.; Graham, D.E. Impacts of temperature and soil characteristics on methane production and oxidation in Arctic tundra. Biogeosciences 2018, 15, 6621–6635. [Google Scholar] [CrossRef]
- King, G.M.; Adamsen, A.P.S. Effects of temperature on methane consumption in a forest soil and in pure cultures of the methanotroph Methylomonas rubra. Appl. Environ. Microbiol. 1992, 58, 2758–2763. [Google Scholar] [CrossRef]
- Kevbrina, M.V.; Okhapkina, A.A.; Akhlynin, D.S.; Kravchenko, I.K.; Nozhevnikova, A.N.; Gal’chenko, V.F. Growth of mesophilic methanotrophs at low temperatures. Microbiology 2001, 70, 384–391. [Google Scholar] [CrossRef]
- Lofton, D.D.; Whalen, S.C.; Hershey, A.E. Effect of temperature on methane dynamics and evaluation of methane oxidation kinetics in shallow Arctic Alaskan lakes. Hydrobiologia 2014, 721, 209–222. [Google Scholar] [CrossRef]
- Zeng, L.; Tian, J.; Chen, H.; Wu, N.; Yan, Z.; Du, L.; Shen, Y.; Wang, X. Changes in methane oxidation ability and methanotrophic community composition across different climatic zones. J. Soils Sediments 2019, 19, 533–543. [Google Scholar] [CrossRef]
- Solheim, B.; Endal, A.; Vigstad, H. Nitrogen fixation in Arctic vegetation and soils from Svalbard, Norway. Polar Biol. 1996, 16, 35–40. [Google Scholar] [CrossRef]
- Graef, C.; Hestnes, A.G.; Svenning, M.M.; Frenzel, P. The active methanotrophic community in a wetland from the High Arctic. Environ. Microbiol. Rep. 2011, 3, 466–472. [Google Scholar] [CrossRef]
- Tveit, A.; Schwacke, R.; Svenning, M.M.; Urich, T. Organic carbon transformations in high-Arctic peat soils: Key functions and microorganisms. ISME J. 2013, 7, 299–311. [Google Scholar] [CrossRef]
- Niemann, H.; Steinle, L.; Blees, J.; Bussmann, I.; Treude, T.; Krause, S.; Elvert, M.; Lehmann, M.F. Toxic effects of lab-grade butyl rubber stoppers on aerobic methane oxidation. Limnol. Oceanogr. Methods 2015, 13, 40–52. [Google Scholar] [CrossRef]
- Tveit, A.T.; Schmider, T.; Hestnes, A.G.; Lindgren, M.; Didriksen, A.; Svenning, M.M. Simultaneous oxidation of atmospheric methane, carbon monoxide and hydrogen for bacterial growth. Microorganisms 2021, 9, 153. [Google Scholar] [CrossRef] [PubMed]
- Whittenbury, R.; Phillips, K.C.; Wilkinson, J.F. Enrichment, Isolation and Some Properties of Methane-utilizing Bacteria. J. Gen. Microbiol. 1970, 61, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Tays, C.; Guarnieri, M.T.; Sauvageau, D.; Stein, L.Y. Combined effects of carbon and nitrogen source to optimize growth of proteobacterial methanotrophs. Front. Microbiol. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Urich, T.; Lanzén, A.; Qi, J.; Huson, D.H.; Schleper, C.; Schuster, S.C. Simultaneous assessment of soil microbial community structure and function through analysis of the meta-transcriptome. PLoS ONE 2008, 3, e2527. [Google Scholar] [CrossRef]
- Costello, A.M.; Lidstrom, M.E. Molecular characterization of functional and phylogenetic genes from natural populations of methanotrophs in lake sediments. Appl. Environ. Microbiol. 1999, 65, 5066–5074. [Google Scholar] [CrossRef]
- Holmes, A.J.; Costello, A.; Lidstrom, M.E.; Murrell, J.C. Evidence that particulate methane monooxygenasre and ammonia monooxygenase may be related. FEMS Microbiol. Lett. 1995, 132, 203–208. [Google Scholar] [CrossRef]
- Henneberger, R.; Lüke, C.; Mosberger, L.; Schroth, M.H. Structure and function of methanotrophic communities in a landfill-cover soil. FEMS Microbiol. Ecol. 2012, 81, 52–65. [Google Scholar] [CrossRef]
- Wen, X.; Yang, S.; Liebner, S. Evaluation and update of cutoff values for methanotrophic pmoA gene sequences. Arch. Microbiol. 2016, 198, 629–636. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Mahé, F.; Rognes, T.; Quince, C.; de Vargas, C.; Dunthorn, M. Swarm: Robust and fast clustering method for amplicon-based studies. PeerJ 2014, 2, e593. [Google Scholar] [CrossRef]
- Pearson, W.R. Flexible Sequence Similarity Searching with the FASTA3 Program Package. In Methods in Molecular Biology; Misener, S., Krawetz, S.A., Eds.; Humana Press: Totowa, NJ, USA, 2000; pp. 185–219. [Google Scholar]
- Anderson, M.J.; Ellingsen, K.E.; McArdle, B.H. Multivariate dispersion as a measure of beta diversity. Ecol. Lett. 2006, 9, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. 2018. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 24 January 2018).
- Roberts, D. Package’labdsv’. 2016. Available online: https://cran.r-project.org/web/packages/labdsv/labdsv.pdf (accessed on 24 January 2016).
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Mayr, M.J.; Zimmermann, M.; Dey, J.; Brand, A.; Wehrli, B.; Bürgmann, H. Growth and rapid succession of methanotrophs effectively limit methane release during lake overturn. Commun. Biol. 2020, 3, 1–9. [Google Scholar] [CrossRef]
- Cai, Y.; Zheng, Y.; Bodelier, P.L.E.; Conrad, R.; Jia, Z. Conventional methanotrophs are responsible for atmospheric methane oxidation in paddy soils. Nat. Commun. 2016, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Siljanen, H.M.P.; Saari, A.; Bodrossy, L.; Martikainen, P.J. Seasonal variation in the function and diversity of methanotrophs in the littoral wetland of a boreal eutrophic lake. FEMS Microbiol. Ecol. 2012, 80, 548–555. [Google Scholar] [CrossRef]
- Tveit, A.T.; Hestnes, A.G.; Robinson, S.L.; Schintlmeister, A.; Dedysh, S.N.; Jehmlich, N.; Von Bergen, M.; Herbold, C.; Wagner, M.; Richter, A.; et al. Widespread soil bacterium that oxidizes atmospheric methane. Proc. Natl. Acad. Sci. USA 2019, 116, 8515–8524. [Google Scholar] [CrossRef] [PubMed]
- Kits, K.D.; Sedlacek, C.J.; Lebedeva, E.V.; Han, P.; Bulaev, A.; Pjevac, P.; Daebeler, A.; Romano, S.; Albertsen, M.; Stein, L.Y.; et al. Kinetic analysis of a complete nitrifier reveals an oligotrophic lifestyle. Nature 2017, 549, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.J.E.; Wanek, W.; Zappe, A.; Richter, A.; Svenning, M.M.; Schleper, C.; Urich, T. Nitrification rates in Arctic soils are associated with functionally distinct populations of ammonia-oxidizing archaea. ISME J. 2013, 7, 1620–1631. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, P.; Thomas, S.C.; Heinrich, P.L.; Koch, G.W.; Schwartz, E.; Hungate, B.A. Effect of temperature on metabolic activity of intact microbial communities: Evidence for altered metabolic pathway activity but not for increased maintenance respiration and reduced carbon use efficiency. Soil Biol. Biochem. 2011, 43, 2023–2031. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rainer, E.M.; Seppey, C.V.W.; Hammer, C.; Svenning, M.M.; Tveit, A.T. The Influence of Above-Ground Herbivory on the Response of Arctic Soil Methanotrophs to Increasing CH4 Concentrations and Temperatures. Microorganisms 2021, 9, 2080. https://doi.org/10.3390/microorganisms9102080
Rainer EM, Seppey CVW, Hammer C, Svenning MM, Tveit AT. The Influence of Above-Ground Herbivory on the Response of Arctic Soil Methanotrophs to Increasing CH4 Concentrations and Temperatures. Microorganisms. 2021; 9(10):2080. https://doi.org/10.3390/microorganisms9102080
Chicago/Turabian StyleRainer, Edda M., Christophe V. W. Seppey, Caroline Hammer, Mette M. Svenning, and Alexander T. Tveit. 2021. "The Influence of Above-Ground Herbivory on the Response of Arctic Soil Methanotrophs to Increasing CH4 Concentrations and Temperatures" Microorganisms 9, no. 10: 2080. https://doi.org/10.3390/microorganisms9102080
APA StyleRainer, E. M., Seppey, C. V. W., Hammer, C., Svenning, M. M., & Tveit, A. T. (2021). The Influence of Above-Ground Herbivory on the Response of Arctic Soil Methanotrophs to Increasing CH4 Concentrations and Temperatures. Microorganisms, 9(10), 2080. https://doi.org/10.3390/microorganisms9102080






