Fermentation Supernatants of Pleurotus eryngii Mushroom Ameliorate Intestinal Epithelial Barrier Dysfunction in Lipopolysaccharide-Induced Caco-2 Cells via Upregulation of Tight Junctions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fermentation Supernatants (FSs) Preparation
2.2. Bacterial Lipopolysaccharides (LPS)
2.3. Cell Lines
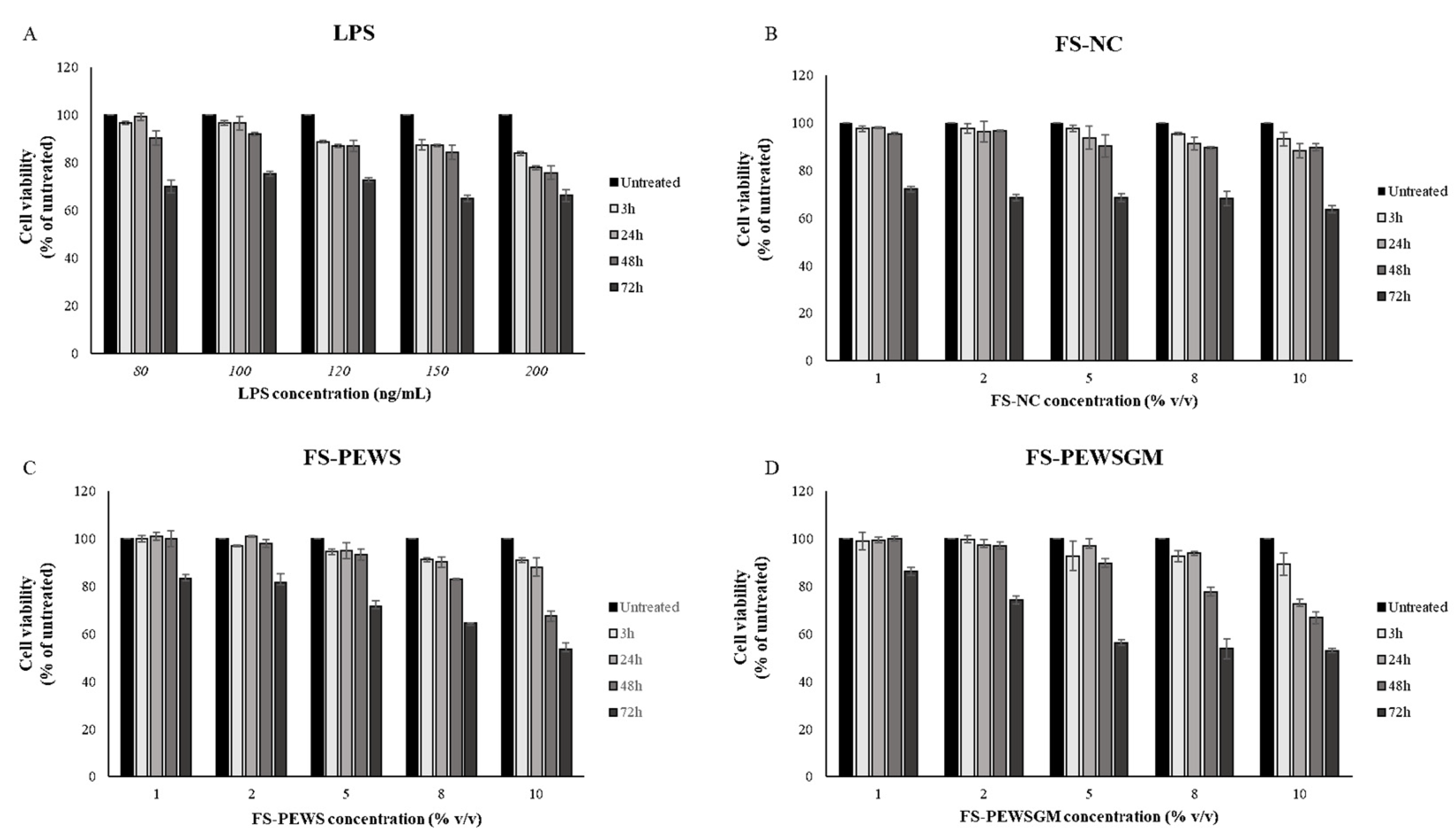
2.4. Cell Viability Assay
2.5. Challenge of Caco-2 Cells with FSs and LPS
- (1)
- Pre-incubation (FS/LPS): incubation of the Caco-2 cell line with FSs of PEWS or PEWSGM mushrooms for 48 h and then exposure to LPS for 24 h (protective effect),
- (2)
- Co-incubation (FS + LPS): simultaneous incubation of the Caco-2 cell line with the FSs of PEWS or PEWSGM mushrooms and the LPS for 48 h (protective effect),
- (3)
- Post-incubation (LPS/FS): exposure of the Caco-2 cell line to LPS for 24 h and then incubation with the FSs of PEWS or PEWSGM mushrooms for 48 h (reparative effect).
2.6. RNA Extraction and cDNA Synthesis
2.7. Quantitative Real-Time PCR
2.8. Statistical Analysis
3. Results
3.1. Effect of FSs and LPS on Cell Viability in Caco-2 Cells
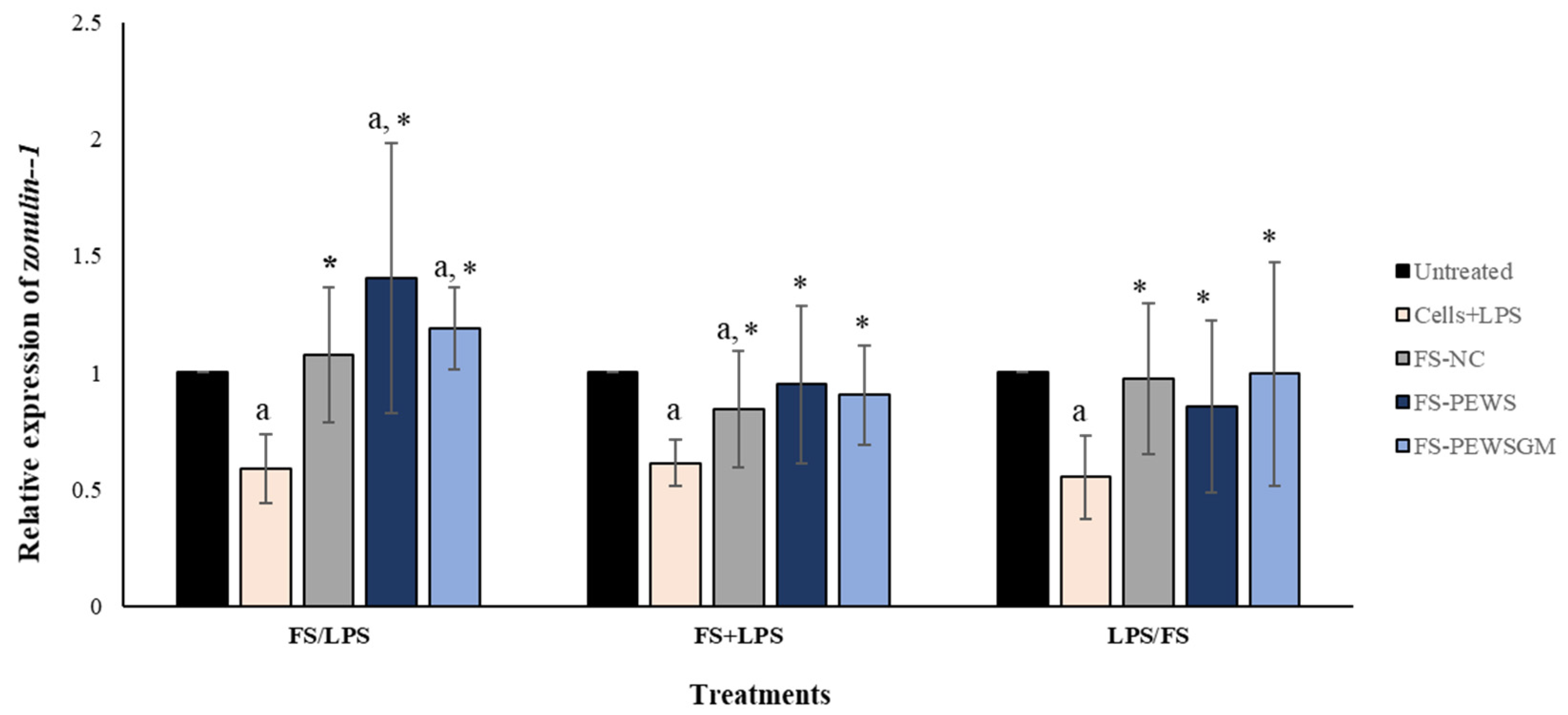
3.2. Effect of FS-PEWS and FS-PEWSGM on Zonulin-1 Expression Levels
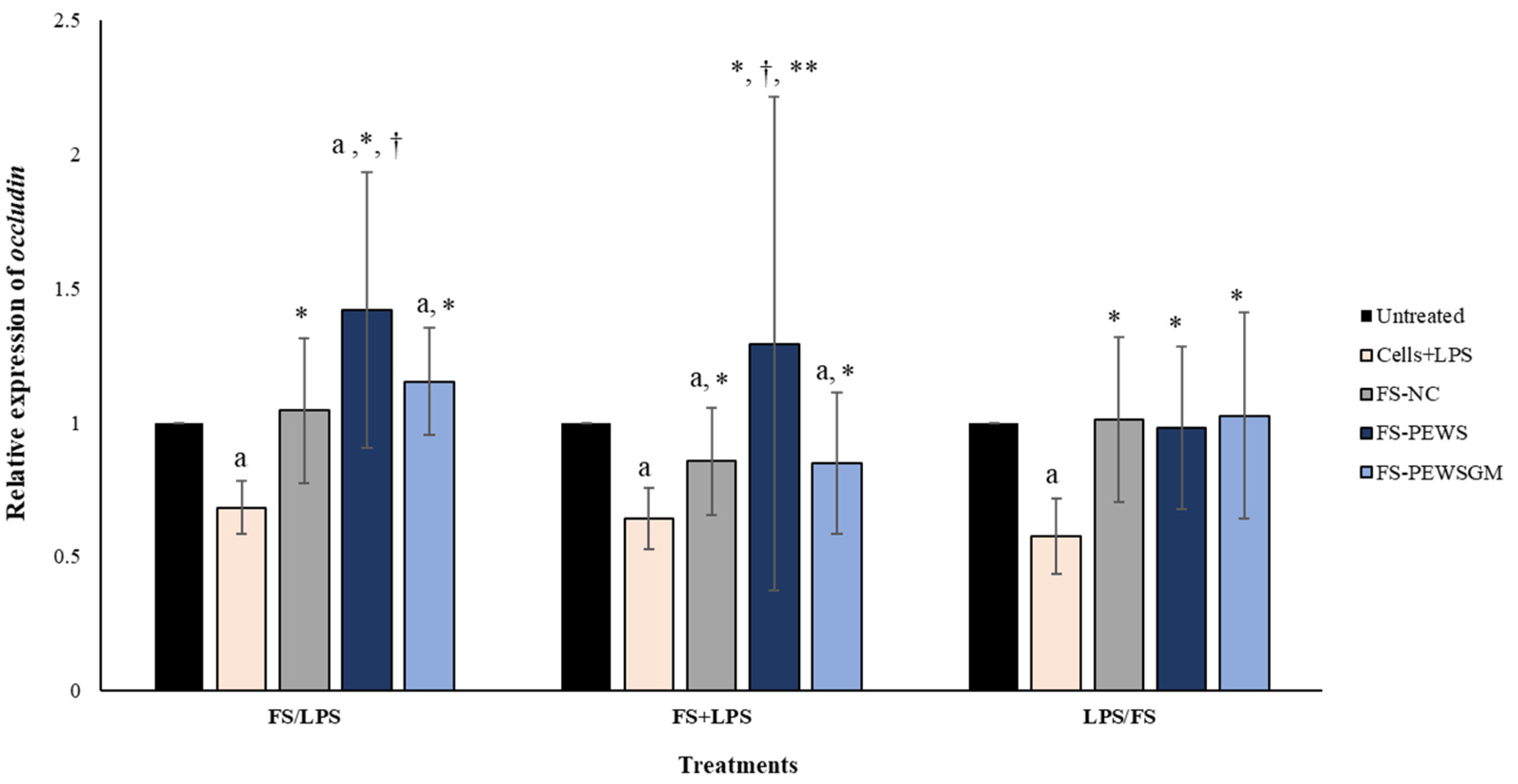
3.3. Effect of FS-PEWS and FS-PEWSGM on Occludin Expression Levels
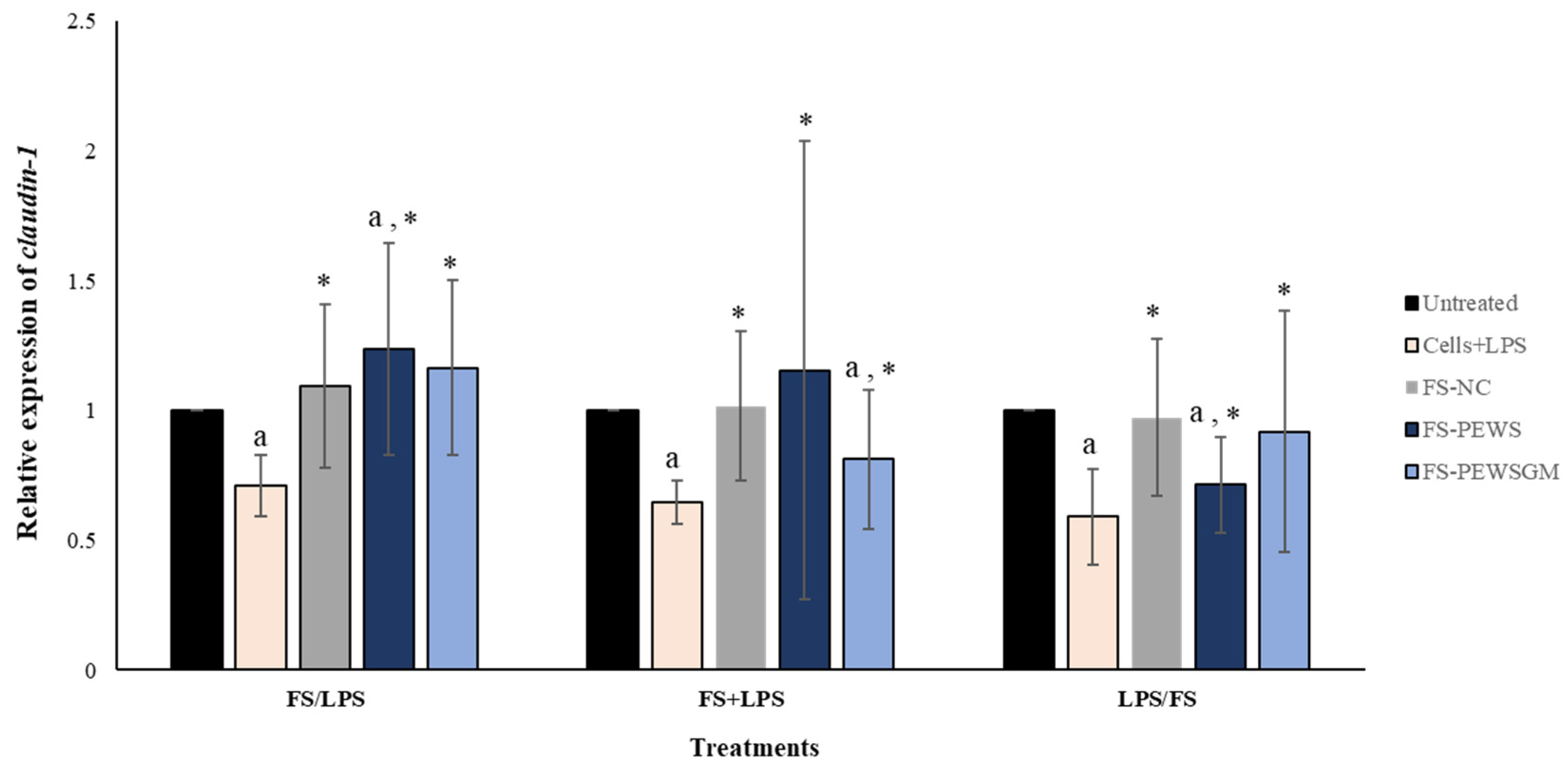
3.4. Effect of FS-PEWS and FS-PEWSGM on Claudin-1 Expression Levels
3.5. Effect of FSs on TJs Expression Levels per Volunteer
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, L.; Cui, K.; Mao, W.; Du, Y.; Yao, N.; Li, Z.; Zhao, H.; Ma, W. Weissella Cibaria Attenuated LPS-Induced Dysfunction of Intestinal Epithelial Barrier in a Caco-2 Cell Monolayer Model. Front. Microbiol. 2020, 11, 2039. [Google Scholar] [CrossRef]
- Uerlings, J.; Schroyen, M.; Willems, E.; Tanghe, S.; Bruggeman, G.; Bindelle, J.; Everaert, N. Differential Effects of Inulin or Its Fermentation Metabolites on Gut Barrier and Immune Function of Porcine Intestinal Epithelial Cells. J. Funct. Foods 2020, 67, 103855. [Google Scholar] [CrossRef]
- Ghosh, S.S.; Wang, J.; Yannie, P.J.; Ghosh, S. Intestinal Barrier Dysfunction, LPS Translocation, and Disease Development. J. Endocr. Soc. 2020, 4, bvz039. [Google Scholar] [CrossRef] [Green Version]
- Schoultz, I.; Keita, Å.V. The Intestinal Barrier and Current Techniques for the Assessment of Gut Permeability. Cells 2020, 9, 1909. [Google Scholar] [CrossRef]
- Vancamelbeke, M.; Vermeire, S. The Intestinal Barrier: A Fundamental Role in Health and Disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef]
- Ulluwishewa, D.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Wells, J.M.; Roy, N.C. Regulation of Tight Junction Permeability by Intestinal Bacteria and Dietary Components. J. Nutr. 2011, 141, 769–776. [Google Scholar] [CrossRef] [Green Version]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight Junctions: From Simple Barriers to Multifunctional Molecular Gates. Nat. Rev. Mol. Cell Biol. 2016, 17, 564–580. [Google Scholar] [CrossRef]
- Luissint, A.-C.; Parkos, C.A.; Nusrat, A. Inflammation and the Intestinal Barrier: Leukocyte-Epithelial Cell Interactions, Cell Junction Remodeling, and Mucosal Repair. Gastroenterology 2016, 151, 616–632. [Google Scholar] [CrossRef] [Green Version]
- Heinemann, U.; Schuetz, A. Structural Features of Tight-Junction Proteins. Int. J. Mol. Sci. 2019, 20, 6020. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Wu, T.; Tang, S.; Liang, F.; Fang, Y.; Cao, W.; Pan, S.; Xu, X. Fermented Blueberry Pomace Ameliorates Intestinal Barrier Function through the NF-ΚB-MLCK Signaling Pathway in High-Fat Diet Mice. Food Funct. 2020, 11, 3167–3179. [Google Scholar] [CrossRef]
- Mohebali, N.; Ekat, K.; Kreikemeyer, B.; Breitrück, A. Barrier Protection and Recovery Effects of Gut Commensal Bacteria on Differentiated Intestinal Epithelial Cells In Vitro. Nutrients 2020, 12, 2251. [Google Scholar] [CrossRef]
- Yang, H.-S.; Haj, F.G.; Lee, M.; Kang, I.; Zhang, G.; Lee, Y. Laminaria Japonica Extract Enhances Intestinal Barrier Function by Altering Inflammatory Response and Tight Junction-Related Protein in Lipopolysaccharide-Stimulated Caco-2 Cells. Nutrients 2019, 11, 1001. [Google Scholar] [CrossRef] [Green Version]
- Gibson, G.R.; Probert, H.M.; Loo, J.V.; Rastall, R.A.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Updating the Concept of Prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef] [Green Version]
- Mitsou, E.K.; Saxami, G.; Stamoulou, E.; Kerezoudi, E.; Terzi, E.; Koutrotsios, G.; Bekiaris, G.; Zervakis, G.I.; Mountzouris, K.C.; Pletsa, V.; et al. Effects of Rich in Β-Glucans Edible Mushrooms on Aging Gut Microbiota Characteristics: An In Vitro Study. Molecules 2020, 25, 2806. [Google Scholar] [CrossRef]
- Vlassopoulou, M.; Yannakoulia, M.; Pletsa, V.; Zervakis, G.I.; Kyriacou, A. Effects of Fungal Beta-Glucans on Health – a Systematic Review of Randomized Controlled Trials. Food Funct. 2021, 12, 3366–3380. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, J.; Fu, Q.; Fu, X.; Shu, T.; Bi, Y.; Song, B. Antitumor Activity of a Polysaccharide from Pleurotus eryngii on Mice Bearing Renal Cancer. Carbohydr. Polym. 2013, 95, 615–620. [Google Scholar] [CrossRef]
- Xue, Z. Antitumor and Immunomodulatory Activity of Pleurotus eryngii Extract. J. Food Biochem. 2015, 39, 19–27. [Google Scholar] [CrossRef]
- Mishra, K.K.; Pal, R.S.; ArunKumar, R.; Chandrashekara, C.; Jain, S.K.; Bhatt, J.C. Antioxidant Properties of Different Edible Mushroom Species and Increased Bioconversion Efficiency of Pleurotus eryngii Using Locally Available Casing Materials. Food Chem. 2013, 138, 1557–1563. [Google Scholar] [CrossRef]
- Krupodorova, T.; Rybalko, S.; Barshteyn, V. Antiviral Activity of Basidiomycete Mycelia against Influenza Type A (Serotype H1N1) and Herpes Simplex Virus Type 2 in Cell Culture. Virol. Sin. 2014, 29, 284–290. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Kalogeropoulos, N.; Kaliora, A.C.; Zervakis, G.I. Toward an Increased Functionality in Oyster (Pleurotus) Mushrooms Produced on Grape Marc or Olive Mill Wastes Serving as Sources of Bioactive Compounds. J. Agric. Food Chem. 2018, 66, 5971–5983. [Google Scholar] [CrossRef]
- Wang, J.; Ji, H.; Wang, S.; Liu, H.; Zhang, W.; Zhang, D.; Wang, Y. Probiotic Lactobacillus Plantarum Promotes Intestinal Barrier Function by Strengthening the Epithelium and Modulating Gut Microbiota. Front. Microbiol. 2018, 9, 1953. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Yu, Z.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Surface Components and Metabolites of Probiotics for Regulation of Intestinal Epithelial Barrier. Microb. Cell Fact. 2020, 19. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms Regulating Intestinal Barrier Integrity and Its Pathological Implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Liu, L.; Dou, X.; Wang, C.; Zhang, W.; Gao, K.; Liu, J.; Wang, H. Lactobacillus Reuteri ZJ617 Maintains Intestinal Integrity via Regulating Tight Junction, Autophagy and Apoptosis in Mice Challenged with Lipopolysaccharide. Oncotarget 2017, 8, 77489–77499. [Google Scholar] [CrossRef]
- Wongkrasant, P.; Pongkorpsakol, P.; Ariyadamrongkwan, J.; Meesomboon, R.; Satitsri, S.; Pichyangkura, R.; Barrett, K.E.; Muanprasat, C. A Prebiotic Fructo-Oligosaccharide Promotes Tight Junction Assembly in Intestinal Epithelial Cells via an AMPK-Dependent Pathway. Biomed. Pharmacother. 2020, 129, 110415. [Google Scholar] [CrossRef]
- Wu, R.Y.; Abdullah, M.; Määttänen, P.; Pilar, A.V.C.; Scruten, E.; Johnson-Henry, K.C.; Napper, S.; O’Brien, C.; Jones, N.L.; Sherman, P.M. Protein Kinase C δ Signaling Is Required for Dietary Prebiotic-Induced Strengthening of Intestinal Epithelial Barrier Function. Sci. Rep. 2017, 7, 40820. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.T.; Seifert, N.; Richard, N.; Raederstorff, D.; Steinert, R.E.; Prudence, K.; Mohajeri, M.H. The Effects of Fermentation Products of Prebiotic Fibres on Gut Barrier and Immune Functions in Vitro. PeerJ 2018, 6, e5288. [Google Scholar] [CrossRef] [PubMed]
- Van den Abbeele, P.; Taminiau, B.; Pinheiro, I.; Duysburgh, C.; Jacobs, H.; Pijls, L.; Marzorati, M. Arabinoxylo-Oligosaccharides and Inulin Impact Inter-Individual Variation on Microbial Metabolism and Composition, Which Immunomodulates Human Cells. J. Agric. Food Chem. 2018, 66, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Nielsen, D.S.G.; Forssten, S.D.; Knudsen, K.E.B.; Saarinen, M.T.; Ouwehand, A.C.; Purup, S. Effects of Colonic Fermentation Products of Polydextrose, Lactitol and Xylitol on Intestinal Barrier Repair In Vitro. Appl. Sci. 2021, 11, 4174. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Saxami, G.; Karapetsas, A.; Lamprianidou, E.; Kotsianidis, I.; Chlichlia, A.; Tassou, C.; Zoumpourlis, V.; Galanis, A. Two Potential Probiotic Lactobacillus Strains Isolated from Olive Microbiota Exhibit Adhesion and Anti-Proliferative Effects in Cancer Cell Lines. J. Funct. Foods 2016, 24, 461–471. [Google Scholar] [CrossRef]
- Chen, M.; Liu, Y.; Xiong, S.; Wu, M.; Li, B.; Ruan, Z.; Hu, X. Dietary L-Tryptophan Alleviated LPS-Induced Intestinal Barrier Injury by Regulating Tight Junctions in a Caco-2 Cell Monolayer Model. Food Funct. 2019, 10, 2390–2398. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.C.; Bistritz, L.; Meddings, J.B. Alterations in Intestinal Permeability. Gut 2006, 55, 1512–1520. [Google Scholar] [CrossRef] [Green Version]
- Groschwitz, K.R.; Hogan, S.P. Intestinal Barrier Function: Molecular Regulation and Disease Pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, R.K.; Samak, G. Protection and Restitution of Gut Barrier by Probiotics: Nutritional and Clinical Implications. Curr. Nutr. Food Sci. 2013, 9, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Camilleri, M. Human Intestinal Barrier: Effects of Stressors, Diet, Prebiotics, and Probiotics. Clin. Transl. Gastroenterol. 2021, 12, e00308. [Google Scholar] [CrossRef]
- Ducray, H.A.G.; Globa, L.; Pustovyy, O.; Morrison, E.; Vodyanoy, V.; Sorokulova, I. Yeast Fermentate Prebiotic Improves Intestinal Barrier Integrity during Heat Stress by Modulation of the Gut Microbiota in Rats. J. Appl. Microbiol. 2019, 127, 1192–1206. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Han, D.; Ye, H.; Tao, S.; Pi, Y.; Zhao, J.; Chen, L.; Wang, J. Short Administration of Combined Prebiotics Improved Microbial Colonization, Gut Barrier, and Growth Performance of Neonatal Piglets. ACS Omega 2020, 5, 20506–20516. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.-D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal Permeability—A New Target for Disease Prevention and Therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzman, J.R.; Conlin, V.S.; Jobin, C. Diet, Microbiome, and the Intestinal Epithelium: An Essential Triumvirate? Biomed. Res. Int. 2013, 2013, 425146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, L.; Li, Z.-R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate Enhances the Intestinal Barrier by Facilitating Tight Junction Assembly via Activation of AMP-Activated Protein Kinase in Caco-2 Cell Monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef]
- Wang, G.; Sun, W.; Pei, X.; Jin, Y.; Wang, H.; Tao, W.; Xiao, Z.; Liu, L.; Wang, M. Galactooligosaccharide Pretreatment Alleviates Damage of the Intestinal Barrier and Inflammatory Responses in LPS-Challenged Mice. Food Funct. 2021, 12, 1569–1579. [Google Scholar] [CrossRef]
- Sheng, K.; He, S.; Sun, M.; Zhang, G.; Kong, X.; Wang, J.; Wang, Y. Synbiotic Supplementation Containing Bifidobacterium Infantis and Xylooligosaccharides Alleviates Dextran Sulfate Sodium-Induced Ulcerative Colitis. Food Funct. 2020, 11, 3964–3974. [Google Scholar] [CrossRef]
- Orlando, A.; Linsalata, M.; Notarnicola, M.; Tutino, V.; Russo, F. Lactobacillus GG Restoration of the Gliadin Induced Epithelial Barrier Disruption: The Role of Cellular Polyamines. BMC Microbiol. 2014, 14, 19. [Google Scholar] [CrossRef] [Green Version]
- Proszkowiec-Weglarz, M.; Schreier, L.L.; Kahl, S.; Miska, K.B.; Russell, B.; Elsasser, T.H. Effect of Delayed Feeding Post-Hatch on Expression of Tight Junction- and Gut Barrier-Related Genes in the Small Intestine of Broiler Chickens during Neonatal Development. Poult. Sci. 2020, 99, 4714–4729. [Google Scholar] [CrossRef]
- Lee, B.; Moon, K.M.; Kim, C.Y. Tight Junction in the Intestinal Epithelium: Its Association with Diseases and Regulation by Phytochemicals. J. Immunol. Res. 2018, 2018, 2645465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Sadi, R.; Khatib, K.; Guo, S.; Ye, D.; Youssef, M.; Ma, T. Occludin Regulates Macromolecule Flux across the Intestinal Epithelial Tight Junction Barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G1054–G1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbari, P.; Braber, S.; Alizadeh, A.; Verheijden, K.A.; Schoterman, M.H.; Kraneveld, A.D.; Garssen, J.; Fink-Gremmels, J. Galacto-Oligosaccharides Protect the Intestinal Barrier by Maintaining the Tight Junction Network and Modulating the Inflammatory Responses after a Challenge with the Mycotoxin Deoxynivalenol in Human Caco-2 Cell Monolayers and B6C3F1 Mice. J. Nutr. 2015, 145, 1604–1613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Hernandez, V.; Quiros, M.; Nusrat, A. Intestinal Epithelial Claudins: Expression and Regulation in Homeostasis and Inflammation. Ann. N. Y. Acad. Sci. 2017, 1397, 66–79. [Google Scholar] [CrossRef]
- Zhang, C.; Li, S.; Zhang, J.; Hu, C.; Che, G.; Zhou, M.; Jia, L. Antioxidant and Hepatoprotective Activities of Intracellular Polysaccharide from Pleurotus eryngii SI-04. Int. J. Biol. Macromol. 2016, 91, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Mao, D.; Yong, Y.; Li, J.; Wei, H.; Lu, L. Hepatoprotective and Hypolipidemic Effects of Water-Soluble Polysaccharidic Extract of Pleurotus eryngii. Food Chem. 2012, 130, 687–694. [Google Scholar] [CrossRef]
- Chen, J.; Yong, Y.; Xia, X.; Wang, Z.; Liang, Y.; Zhang, S.; Lu, L. The Excreted Polysaccharide of Pleurotus eryngii Inhibits the Foam-Cell Formation via down-Regulation of CD36. Carbohydr. Polym. 2014, 112, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Kawai, J.; Andoh, T.; Ouchi, K.; Inatomi, S. Pleurotus eryngii Ameliorates Lipopolysaccharide-Induced Lung Inflammation in Mice. Evid. Based Complement. Altern. Med. 2014, 2014, 532389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, X.; Hu, H.; Xiao, X.; Chen, D.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Luo, J.; Luo, Y.; et al. Lentinan Administration Relieves Gut Barrier Dysfunction Induced by Rotavirus in a Weaned Piglet Model. Food Funct. 2019, 10, 2094–2101. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, W.; Wang, L.; Yu, C.; Zhang, G.; Zhu, H.; Wang, C.; Zhao, S.; Hu, C.-A.A.; Liu, Y. Lentinan Modulates Intestinal Microbiota and Enhances Barrier Integrity in a Piglet Model Challenged with Lipopolysaccharide. Food Funct. 2019, 10, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Whitley, C.S.; Haribabu, B.; Jala, V.R. Regulation of Intestinal Barrier Function by Microbial Metabolites. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 1463–1482. [Google Scholar] [CrossRef]
- Alam, A.; Neish, A. Role of Gut Microbiota in Intestinal Wound Healing and Barrier Function. Tissue Barriers 2018, 6, 1539595. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Mainali, R.; Ahmadi, S.; Wang, S.; Singh, R.; Kavanagh, K.; Kitzman, D.W.; Kushugulova, A.; Marotta, F.; Yadav, H. Gut Microbiome and Aging: Physiological and Mechanistic Insights. Nutr. Healthy Aging 2018, 4, 267–285. [Google Scholar] [CrossRef] [Green Version]
- Qi, Y.; Goel, R.; Kim, S.; Richards, E.M.; Carter, C.S.; Pepine, C.J.; Raizada, M.K.; Buford, T.W. Intestinal Permeability Biomarker Zonulin Is Elevated in Healthy Aging. J. Am. Med. Dir. Assoc. 2017, 18, 810.e1–810.e4. [Google Scholar] [CrossRef]
| Description | Abbreviation |
|---|---|
| FS of P. eryngii mushrooms cultivated in a wheat straw substrate | FS-PEWS |
| FS of P. eryngii mushrooms cultivated in a wheat straw and grape marc (ratio 1:1, w/w) substrate | FS-PEWSGM |
| FS of negative control (gut microbiota of each donor with no carbohydrate source) | FS-NC |
| Gene | Primer Sequences (5′-3′) | Reference |
|---|---|---|
| b-actin F | GCGCGGCTACAGCTTCA | [31] |
| b-actin R | CTTAATGTCACGCACGATTTCC | [31] |
| Zonulin-1 F | TTCACGCAGTTACGAGCAAG | [32] |
| Zonulin-1 R | TTGGTGTTTGAAGGCAGAGC | [32] |
| Occludin F | ACAAGCGGTTTTATCCAGAGTC | [32] |
| Occludin R | GTCATCCACAGGCGAAGTTAAT | [32] |
| Claudin-1 F | TGGTCAGGCTCTCTTCACTG | [32] |
| Claudin-1 R | TTGGATAGGGCCTTGGTGTT | [32] |
| Pre-Incubation | ||||
| TJs Genes | ||||
| Treatment | zonulin-1 | occludin | claudin-1 | |
| Volunteer 1 | Cells + LPS | 0.508 ± 0.265 | 0.740 ± 0.064 | 0.788 ± 0.018 a |
| FS-NC | 1.230 ± 0.085 | 1.325 ± 0.703 | 1.603 ± 0.364 | |
| FS-PEWS | 1.040 ± 0.014 | 1.275 ± 0.007 a,* | 1.200 ± 0.141 | |
| FS-PEWSGM | 1.230 ±0.057 | 1.245 ± 0.276 | 1.080 ± 0.127 | |
| Volunteer 2 | Cells + LPS | 0.638 ± 0.216 | 0.728 ± 0.219 | 0.540 ± 0.163 |
| FS-NC | 0.998 ± 0.258 | 0.930 ± 0.240 | 0.905 ± 0.071 | |
| FS-PEWS | 1.180 ± 0.014 a | 1.025 ± 0.106 | 1.050 ± 0.184 | |
| FS-PEWSGM | 1.230 ± 0.014 a | 1.305 ± 0.035 | 1.085 ± 0.092 | |
| Volunteer 3 | Cells + LPS | 0.623 ± 0.138 | 0.668 ± 0.152 | 0.685 ± 0.170 |
| FS-NC | 1.083 ± 0.407 | 0.788 ± 0.322 | 0.958 ± 0.463 | |
| FS-PEWS | 1.150 ± 0.113 * | 1.220 ± 0.085 | 1.300 ± 0.014 a | |
| FS-PEWSGM | 1.060 ± 0.014 | 1.010 ± 0.000 | 1.055 ± 0.078 | |
| Volunteer 4 | Cells + LPS | 0.645 ± 0.035 a | 0.760 ± 0.071 | 0.800 ± 0.028 |
| FS-NC | 1.323 ± 0.668 | 1.113 ± 0.392 | 1.008 ± 0.527 | |
| FS-PEWS | 1.820 ± 0.990 | 2.370 ± 0.325 | 2.100 ± 0.410 | |
| FS-PEWSGM | 1.025 ± 0.050 | 1.025 ± 0.092 | 0.940 ± 0.085 | |
| Volunteer 5 | Cells + LPS | 0.503 ± 0.103 † | 0.650 ± 0.014 a | 0.818 ± 0.095 |
| FS-NC | 1.255 ± 0.099 * | 1.105 ± 0.198 | 1.173 ± 0.279 | |
| FS-PEWS | 1.295 ± 0.120 * | 1.900 ± 0.467 | 1.280 ± 0.057 * | |
| FS-PEWSGM | 1.235 ± 0.163 * | 1.150 ± 0.014 a | 1.850 ± 0.453 | |
| Volunteer 6 | Cells + LPS | 0.505 ± 0.339 | 0.575 ± 0.163 † | 0.678 ± 0.067 |
| FS-NC | 0.770 ± 0.092 | 0.790 ± 0.141 * | 0.993 ± 0.145 | |
| FS-PEWS | 1.010 ± 0.198 | 0.890 ± 0.057 | 0.910 ± 0.297 | |
| FS-PEWSGM | 1.010 ± 0.071 † | 1.020 ± 0.014 | 0.840 ± 0.085 * | |
| Volunteer 7 | Cells + LPS | 0.560 ± 0.127 | 0.643 ± 0.060 | 0.640 ± 0.134 |
| FS-NC | 0.985 ± 0.262 | 1.130 ± 0.170 | 1.050 ± 0.262 | |
| FS-PEWS | 2.505 ± 0.559 | 1.065 ± 0.035 | 0.790 ± 0.014 a | |
| FS-PEWSGM | 1.165 ± 0.050 | 0.950 ± 0.042 * | 1.320 ± 0.226 | |
| Volunteer 8 | Cells + LPS | 0.625 ± 0.028 a,† | 0.698 ± 0.004 a | 0.743 ± 0.039 |
| FS-NC | 0.960 ± 0.042 * | 1.180 ± 0.092 | 1.070 ± 0.156 | |
| FS-PEWS | 1.220 ± 0.014 a,*,† | 1.605 ± 0.134 | 1.255 ± 0.092 * | |
| FS-PEWSGM | 1.550 ± 0.071 * | 1.510 ± 0.042 a,* | 1.150 ± 0.198 | |
| Co-Incubation | ||||
| Treatment | zonulin-1 | occludin | claudin-1 | |
| Volunteer 1 | Cells + LPS | 0.615 ± 0.064 | 0.665 ± 0.078 | 0.625 ± 0.064 |
| FS-NC | 0.878 ± 0.279 | 0.703 ± 0.032 a | 0.905 ± 0.148 | |
| FS-PEWS | 0.750 ± 0.042 | 0.770 ± 0.099 | 0.675 ± 0.064 | |
| FS-PEWSGM | 1.255 ± 0.050 * | 0.720 ± 0.156 | 1.050 ± 0.057 | |
| Volunteer 2 | Cells + LPS | 0.618 ± 0.018 a | 0.740 ± 0.000 | 0.603 ± 0.131 |
| FS-NC | 0.595 ± 0.042 * | 0.853 ± 0.053 | 0.890 ± 0.410 | |
| FS-PEWS | 0.745 ± 0.050 * | 0.840 ± 0.042 | 0.810 ± 0.099 | |
| FS-PEWSGM | 0.760 ± 0.240 | 0.770 ± 0.198 | 0.880 ± 0.028 | |
| Volunteer 3 | Cells + LPS | 0.568 ± 0.244 | 0.518 ± 0.081 | 0.600 ± 0.156 |
| FS-NC | 0.838 ± 0.315 | 0.745 ± 0.297 | 0.805 ± 0.240 | |
| FS-PEWS | 0.980 ± 0.042 | 1.000 ± 0.014 | 0.835 ± 0.106 | |
| FS-PEWSGM | 0.650 ± 0.085 | 0.605 ± 0.035 a | 0.660 ± 0.085 | |
| Volunteer 4 | Cells + LPS | 0.610 ± 0.042 a | 0.568 ± 0.117 | 0.633 ± 0.117 † |
| FS-NC | 1.248 ± 0.230 | 0.990 ± 0.297 | 1.493 ± 0.194 * | |
| FS-PEWS | 1.000 ± 0.099 ** | 1.250 ± 0.127 * | 1.050 ± 0.071 *,** | |
| FS-PEWSGM | 0.705 ± 0.120 | 0.510 ± 0.396 | 0.215 ± 0.007 a | |
| Volunteer 5 | Cells + LPS | 0.568 ± 0.046 a | 0.600 ± 0.191 † | 0.698 ± 0.095 |
| FS-NC | 0.820 ± 0.410 | 1.058 ± 0.223 * | 1.195 ± 0.332 | |
| FS-PEWS | 0.715 ± 0.064 | 1.545 ± 0.050 a | 1.610 ± 0.269 | |
| FS-PEWSGM | 0.965 ± 0.205 | 1.005 ± 0.078 | 0.840 ± 0.000 | |
| Volunteer 6 | Cells + LPS | 0.620 ± 0.071 | 0.583 ± 0.039 a | 0.720 ± 0.042 |
| FS-NC | 0.645 ± 0.085 | 0.630 ± 0.064 ** | 0.738 ± 0.145 | |
| FS-PEWS | 1.585 ± 0.728 | 2.850 ± 2.503 | 2.660 ± 2.663 | |
| FS-PEWSGM | 1.040 ± 0.028 * | 1.225 ± 0.007 a,* | 0.960 ± 0.071 | |
| Volunteer 7 | Cells + LPS | 0.605 ± 0.148 | 0.778 ± 0.152 | 0.680 ± 0.028 a,† |
| FS-NC | 0.843 ± 0.004 a | 0.890 ± 0.113 | 1.043 ± 0.018 * | |
| FS-PEWS | 0.825 ± 0.205 | 1.055 ± 0.276 | 0.935 ± 0.035 | |
| FS-PEWSGM | 0.910 ± 0.057 | 1.005 ± 0.007 | 1.045 ± 0.148 | |
| Volunteer 8 | Cells + LPS | 0.698 ± 0.173 | 0.688 ± 0.039 | 0.600 ± 0.028 a,† |
| FS-NC | 0.883 ± 0.011 a | 0.983 ± 0.138 | 1.058 ± 0.018 * | |
| FS-PEWS | 1.000 ± 0.042 | 1.035 ± 0.050 * | 0.665 ± 0.035 a,* | |
| FS-PEWSGM | 0.945 ± 0.021 | 0.950 ± 0.099 | 0.845 ± 0.078 | |
| Post-incubation | ||||
| Treatment | zonulin-1 | occludin | claudin-1 | |
| Volunteer 1 | Cell + LPS | 0.550 ± 0.219 | 0.645 ± 0.064 | 0.710 ± 0.099 |
| FS-NC | 1.028 ± 0.385 | 1.053 ± 0.336 | 1.110 ± 0.148 | |
| FS-PEWS | 0.890 ± 0.042 | 0.900 ± 0.141 | 0.795 ± 0.021 a,** | |
| FS-PEWSGM | 1.675 ± 0.163 * | 1.290 ± 0.141 | 1.685 ± 0.021 a | |
| Volunteer 2 | Cell + LPS | 0.710 ± 0.219 | 0.658 ± 0.117 | 0.545 ± 0.445 |
| FS-NC | 0.983 ± 0.117 | 0.900 ± 0.071 | 0.848 ± 0.124 | |
| FS-PEWS | 0.785 ± 0.064 | 0.930 ± 0.141 | 0.635 ± 0.078 | |
| FS-PEWSGM | 0.805 ± 0.064 | 0.715 ± 0.346 | 0.475 ± 0.078 | |
| Volunteer 3 | Cell + LPS | 0.588 ± 0.025 a | 0.608 ± 0.315 | 0.498 ± 0.216 † |
| FS-NC | 0.733 ± 0.308 | 0.643 ± 0.265 | 0.670 ± 0.198 * | |
| FS-PEWS | 0.670 ± 0.141 | 1.085 ± 0.007 a,** | 0.765 ± 0.177 | |
| FS-PEWSGM | 0.675 ± 0.106 | 0.510 ± 0.028 a | 0.570 ± 0.085 | |
| Volunteer 4 | Cell + LPS | 0.463 ± 0.194 | 0.483 ± 0.039 a | 0.608 ± 0.166 |
| FS-NC | 1.185 ± 0.431 | 1.015 ± 0.078 | 1.183 ± 0.046 | |
| FS-PEWS | 0.690 ± 0.999 | 0.575 ± 0.120 † | 0.565 ± 0.078 ** | |
| FS-PEWSGM | 0.720 ± 0.028 a | 1.135 ± 0.120 | 0.950 ± 0.042 | |
| Volunteer 5 | Cell + LPS | 0.300 ± 0.156 | 0.425 ± 0.014 a | 0.440 ± 0.021 a |
| FS-NC | 1.048 ± 0.866 | 0.835 ± 0.445 | 0.875 ± 0.629 | |
| FS-PEWS | 0.310 ± 0.014 a | 0.655 ± 0.035 a,* | 0.500 ± 0.255 | |
| FS-PEWSGM | 1.825 ± 0.318 * | 1.605 ± 0.544 | 1.505 ± 0.530 | |
| Volunteer 6 | Cell + LPS | 0.670 ± 0.191 | 0.703 ± 0.173 | 0.640 ± 0.071 |
| FS-NC | 0.968 ± 0.018 | 1.413 ± 0.039 a | 1.250 ± 0.297 | |
| FS-PEWS | 0.950 ± 0.085 | 1.030 ± 0.198 * | 0.930 ± 0.156 | |
| FS-PEWSGM | 0.780 ± 0.226 | 1.075 ± 0.262 | 0.800 ± 0.226 | |
| Volunteer 7 | Cell + LPS | 0.495 ± 0.042 a | 0.555 ± 0.113 | 0.518 ± 0.053 a |
| FS-NC | 0.895 ± 0.304 | 1.120 ± 0.467 | 0.698 ± 0.237 | |
| FS-PEWS | 1.615 ± 0.247 †,** | 1.467 ± 0.290 ** | 0.865 ± 0.219 † | |
| FS-PEWSGM | 0.750 ± 0.269 | 0.915 ± 0.233 | 0.685 ± 0.375 | |
| Volunteer 8 | Cell + LPS | 0.650 ± 0.163 | 0.543 ± 0.110 | 0.760 ± 0.212 |
| FS-NC | 0.958 ± 0.124 | 1.110 ± 0.269 | 1.155 ± 0.106 | |
| FS-PEWS | 0.920 ± 0.071 | 1.195 ± 0.134 | 0.645 ± 0.092 | |
| FS-PEWSGM | 0.725 ± 0.191 | 0.960 ± 0.198 * | 0.680 ± 0.141 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saxami, G.; Kerezoudi, E.N.; Mitsou, E.K.; Koutrotsios, G.; Zervakis, G.I.; Pletsa, V.; Kyriacou, A. Fermentation Supernatants of Pleurotus eryngii Mushroom Ameliorate Intestinal Epithelial Barrier Dysfunction in Lipopolysaccharide-Induced Caco-2 Cells via Upregulation of Tight Junctions. Microorganisms 2021, 9, 2071. https://doi.org/10.3390/microorganisms9102071
Saxami G, Kerezoudi EN, Mitsou EK, Koutrotsios G, Zervakis GI, Pletsa V, Kyriacou A. Fermentation Supernatants of Pleurotus eryngii Mushroom Ameliorate Intestinal Epithelial Barrier Dysfunction in Lipopolysaccharide-Induced Caco-2 Cells via Upregulation of Tight Junctions. Microorganisms. 2021; 9(10):2071. https://doi.org/10.3390/microorganisms9102071
Chicago/Turabian StyleSaxami, Georgia, Evangelia N. Kerezoudi, Evdokia K. Mitsou, Georgios Koutrotsios, Georgios I. Zervakis, Vasiliki Pletsa, and Adamantini Kyriacou. 2021. "Fermentation Supernatants of Pleurotus eryngii Mushroom Ameliorate Intestinal Epithelial Barrier Dysfunction in Lipopolysaccharide-Induced Caco-2 Cells via Upregulation of Tight Junctions" Microorganisms 9, no. 10: 2071. https://doi.org/10.3390/microorganisms9102071
APA StyleSaxami, G., Kerezoudi, E. N., Mitsou, E. K., Koutrotsios, G., Zervakis, G. I., Pletsa, V., & Kyriacou, A. (2021). Fermentation Supernatants of Pleurotus eryngii Mushroom Ameliorate Intestinal Epithelial Barrier Dysfunction in Lipopolysaccharide-Induced Caco-2 Cells via Upregulation of Tight Junctions. Microorganisms, 9(10), 2071. https://doi.org/10.3390/microorganisms9102071







