Pharmacokinetic Variability and Target Attainment of Fluconazole in Critically Ill Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Sample Collection
2.3. Method of Analysis
2.4. Data Collection
2.5. Variability and Target Attainment
2.6. Statistical Analysis
3. Results
3.1. Study Population
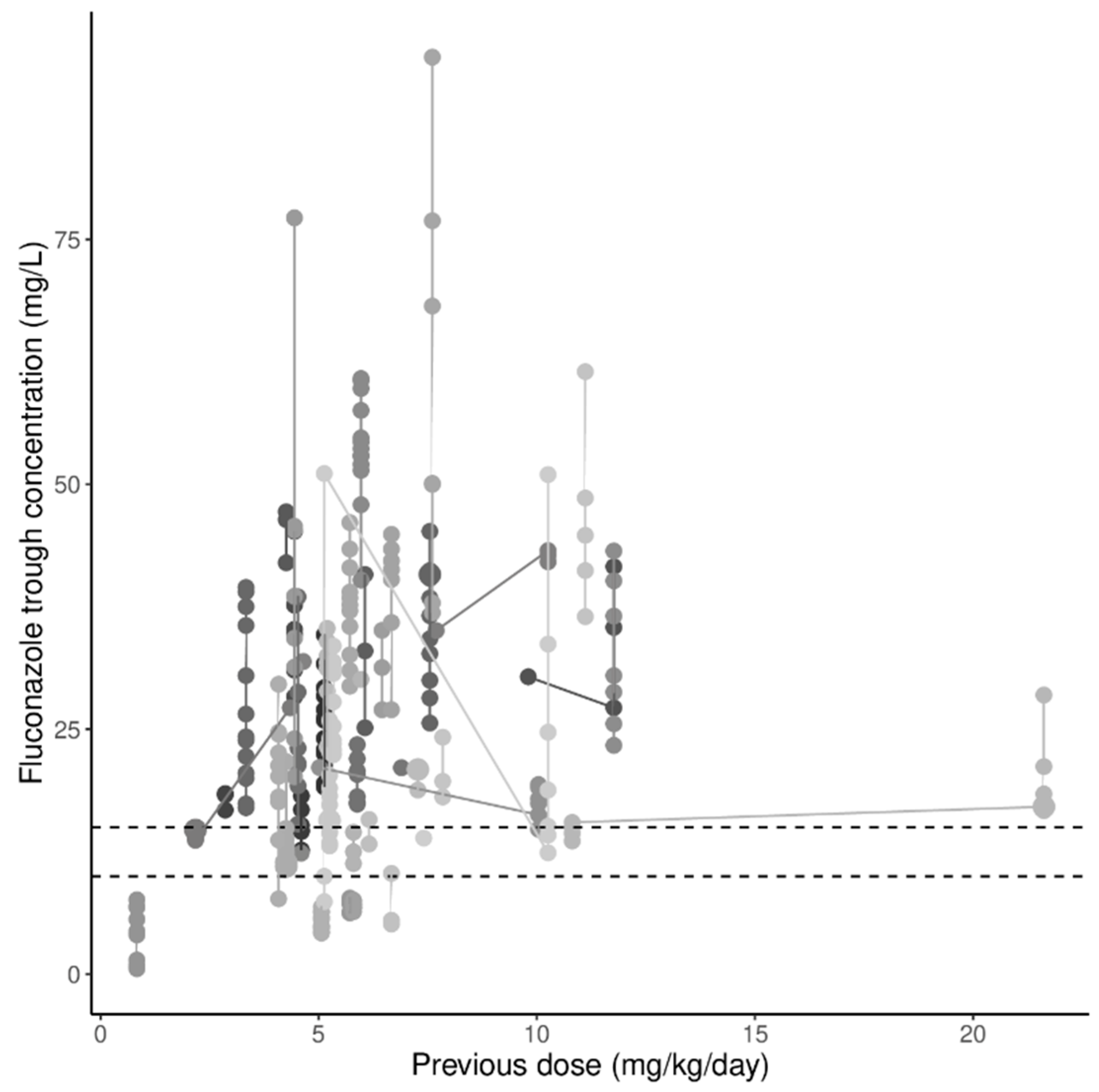
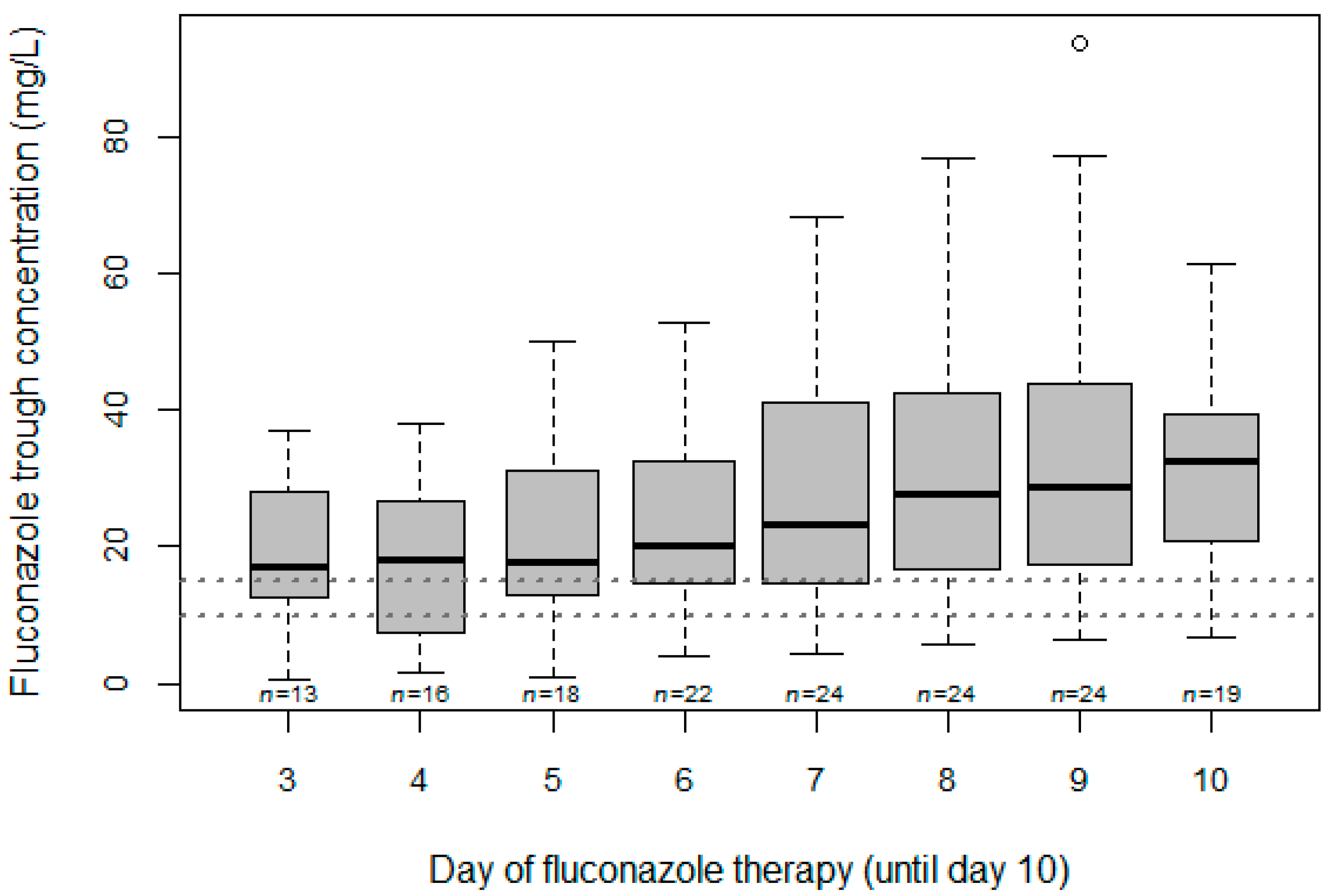
3.2. Variability and Target Attainment
3.3. Multivariate Analysis
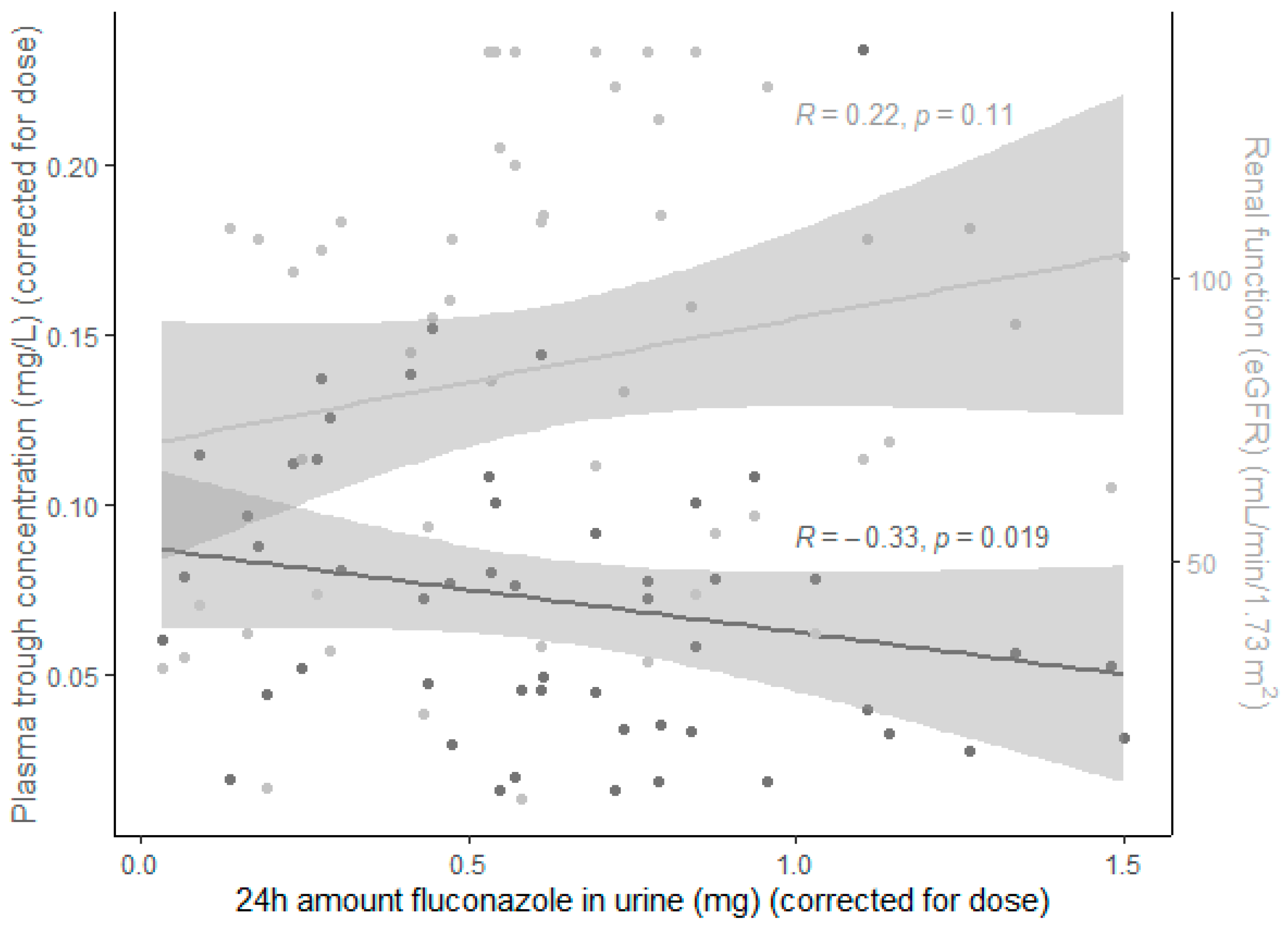
3.4. Correlation Plasma and Urine Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pfizer. Summary of Product Characteristics (SmPC) Diflucan. Available online: https://www.ema.europa.eu/en/documents/referral/diflucan-article-30-referral-annex-iii_en.pdf (accessed on 2 February 2012).
- Gijsen, M.; Wilmer, A.; Meyfroidt, G.; Wauters, J.; Spriet, I. Can augmented renal clearance be detected using estimators of glomerular filtration rate? Crit. Care 2020, 24, 359. [Google Scholar] [CrossRef]
- Smith, B.S.; Yogaratnam, D.; Levasseur-Franklin, K.E.; Forni, A.; Fong, J. Introduction to drug pharmacokinetics in the critically ill patient. Chest 2012, 141, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Alobaid, A.S.; Wallis, S.C.; Jarrett, P.; Starr, T.; Stuart, J.; Lassig-Smith, M.; Mejia, J.L.; Roberts, M.S.; Sinnollareddy, M.G.; Roger, C.; et al. Effect of Obesity on the Population Pharmacokinetics of Fluconazole in Critically Ill Patients. Antimicrob. Agents Chemother. 2016, 60, 6550–6557. [Google Scholar] [CrossRef]
- Gharibian, K.N.; Mueller, B.A. Fluconazole dosing predictions in critically-ill patients receiving prolonged intermittent renal replacement therapy: A Monte Carlo simulation approach. Clin. Nephrol. 2016, 86, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Roberts, J.A.; Lipman, J.; Tett, S.E.; Deldot, M.E.; Kirkpatrick, C.M. Population pharmacokinetics of fluconazole in critically ill patients receiving continuous venovenous hemodiafiltration: Using Monte Carlo simulations to predict doses for specified pharmacodynamic targets. Antimicrob. Agents Chemother. 2011, 55, 5868–5873. [Google Scholar] [CrossRef] [PubMed]
- Lopez, N.D.; Phillips, K.M. Fluconazole pharmacokinetics in a morbidly obese, critically ill patient receiving continuous venovenous hemofiltration. Pharmacotherapy 2014, 34, e162–e168. [Google Scholar] [CrossRef]
- Sinnollareddy, M.G.; Roberts, J.A.; Lipman, J.; Akova, M.; Bassetti, M.; De Waele, J.J.; Kaukonen, K.M.; Koulenti, D.; Martin, C.; Montravers, P.; et al. Pharmacokinetic variability and exposures of fluconazole, anidulafungin, and caspofungin in intensive care unit patients: Data from multinational Defining Antibiotic Levels in Intensive care unit (DALI) patients Study. Crit. Care 2015, 19, 33. [Google Scholar] [CrossRef] [PubMed]
- Muilwijk, E.W.; de Lange, D.W.; Schouten, J.A.; Wasmann, R.E.; Ter Heine, R.; Burger, D.M.; Colbers, A.; Haas, P.J.; Verweij, P.E.; Pickkers, P.; et al. Suboptimal Dosing of Fluconazole in Critically Ill Patients: Time To Rethink Dosing. Antimicrob. Agents Chemother. 2020, 64, e00984-20. [Google Scholar] [CrossRef]
- Boonstra, J.M.; Märtson, A.G.; Sandaradura, I.; Kosterink, J.G.W.; van der Werf, T.S.; Marriott, D.J.E.; Zijlstra, J.G.; Touw, D.J.; Alffenaar, J.W.C. Optimization of Fluconazole Dosing for the Prevention and Treatment of Invasive Candidiasis Based on the Pharmacokinetics of Fluconazole in Critically Ill Patients. Antimicrob. Agents Chemother. 2021, 65, e01554-20. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2016, 62, e1–e50. [Google Scholar] [CrossRef]
- Chen, J.; Tian, S.; Han, X.; Chu, Y.; Wang, Q.; Zhou, B.; Shang, H. Is the superbug fungus really so scary? A systematic review and meta-analysis of global epidemiology and mortality of Candida auris. BMC Infect Dis 2020, 20, 827. [Google Scholar] [CrossRef]
- Cornely, O.A.; Bassetti, M.; Calandra, T.; Garbino, J.; Kullberg, B.J.; Lortholary, O.; Meersseman, W.; Akova, M.; Arendrup, M.C.; Arikan-Akdagli, S.; et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: Non-neutropenic adult patients. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2012, 18 (Suppl. 7), 19–37. [Google Scholar] [CrossRef]
- Martin-Loeches, I.; Antonelli, M.; Cuenca-Estrella, M.; Dimopoulos, G.; Einav, S.; De Waele, J.J.; Garnacho-Montero, J.; Kanj, S.S.; Machado, F.R.; Montravers, P.; et al. ESICM/ESCMID task force on practical management of invasive candidiasis in critically ill patients. Intensive Care Med. 2019, 45, 789–805. [Google Scholar] [CrossRef]
- Kett, D.H.; Shorr, A.F.; Reboli, A.C.; Reisman, A.L.; Biswas, P.; Schlamm, H.T. Anidulafungin compared with fluconazole in severely ill patients with candidemia and other forms of invasive candidiasis: Support for the 2009 IDSA treatment guidelines for candidiasis. Crit. Care (Lond. Engl.) 2011, 15, R253. [Google Scholar] [CrossRef]
- López-Cortés, L.E.; Almirante, B.; Cuenca-Estrella, M.; Garnacho-Montero, J.; Padilla, B.; Puig-Asensio, M.; Ruiz-Camps, I.; Rodríguez-Baño, J. Empirical and targeted therapy of candidemia with fluconazole versus echinocandins: A propensity score-derived analysis of a population-based, multicentre prospective cohort. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2016, 22, 733.e731–733.e738. [Google Scholar] [CrossRef]
- Debruyne, D.; Ryckelynck, J.P. Clinical pharmacokinetics of fluconazole. Clin. Pharmacokinet. 1993, 24, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Wade, K.C.; Benjamin, D.K., Jr.; Kaufman, D.A.; Ward, R.M.; Smith, P.B.; Jayaraman, B.; Adamson, P.C.; Gastonguay, M.R.; Barrett, J.S. Fluconazole dosing for the prevention or treatment of invasive candidiasis in young infants. Pediatr. Infect Dis. J. 2009, 28, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Pea, F.; Righi, E.; Cojutti, P.; Carnelutti, A.; Baccarani, U.; Soardo, G.; Bassetti, M. Intra-abdominal penetration and pharmacodynamic exposure to fluconazole in three liver transplant patients with deep-seated candidiasis. J. Antimicrob. Chemother. 2014, 69, 2585–2586. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Rationale for EUCAST Clinical Breakpoints. Fluconazole. Version 3.0. 2020. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Rationale_documents/Fluconazole_RD_v3.0_final_18_02.pdf (accessed on 21 September 2021).
- van der Elst, K.C.; Pereboom, M.; van den Heuvel, E.R.; Kosterink, J.G.; Schölvinck, E.H.; Alffenaar, J.W. Insufficient fluconazole exposure in pediatric cancer patients and the need for therapeutic drug monitoring in critically ill children. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2014, 59, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.; Brüggemann, R.; Padoin, C.; Maertens, J.; Marchetti, O.; Groll, A.; Johnson, E.; Arendrup, M. Triazole Antifungal Therapeutic Drug Monitoring. In Proceedings of the ECIL 6 Meeting, Sophia Antipolis, France, 11–12 September 2015. [Google Scholar]
- Mistretta, V.; Dubois, N.; Denooz, R.; Charlier, C. Simultaneous determination of seven azole antifungal drugs in serum by ultra-high pressure liquid chromatography and diode array detection. Acta Clin. Belg. 2014, 69, 53–61. [Google Scholar] [CrossRef]
- ClinCalc.com. APACHE II Calculator. Available online: https://clincalc.com/IcuMortality/APACHEII.aspx (accessed on 19 July 2019).
- Ceesay, M.M.; Couchman, L.; Smith, M.; Wade, J.; Flanagan, R.J.; Pagliuca, A. Triazole antifungals used for prophylaxis and treatment of invasive fungal disease in adult haematology patients: Trough serum concentrations in relation to outcome. Med. Mycol. 2016, 54, 691–698. [Google Scholar] [CrossRef] [PubMed]
- El-Yazigi, A.; Ellis, M.; Ernst, P.; Hussein, R.; Baillie, F.J. Effect of repeated dosing on the pharmacokinetics of oral fluconazole in bone marrow transplant patients. J. Clin. Pharmacol. 1997, 37, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Bergner, R.; Hoffmann, M.; Riedel, K.D.; Mikus, G.; Henrich, D.M.; Haefeli, W.E.; Uppenkamp, M.; Walter-Sack, I. Fluconazole dosing in continuous veno-venous haemofiltration (CVVHF): Need for a high daily dose of 800 mg. Nephrol Dial. Transpl. 2006, 21, 1019–1023. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matana, A.; Zaninović Jurjević, T.; Matana Kaštelan, Z. Can the difference in serum concentration of urea and cystatin C be used in diagnosis and prognosis of heart failure? Med. Hypotheses 2014, 83, 401–403. [Google Scholar] [CrossRef] [PubMed]
- Gijsen, M.; Huang, C.Y.; Flechet, M.; Van Daele, R.; Declercq, P.; Debaveye, Y.; Meersseman, P.; Meyfroidt, G.; Wauters, J.; Spriet, I. Development and External Validation of an Online Clinical Prediction Model for Augmented Renal Clearance in Adult Mixed Critically Ill Patients: The Augmented Renal Clearance Predictor. Crit. Care Med. 2020, 48, e1260–e1268. [Google Scholar] [CrossRef]
- Leroy, G.; Lambiotte, F.; Thévenin, D.; Lemaire, C.; Parmentier, E.; Devos, P.; Leroy, O. Evaluation of “Candida score” in critically ill patients: A prospective, multicenter, observational, cohort study. Ann. Intensive Care 2011, 1, 50. [Google Scholar] [CrossRef]
- Brammer, K.W.; Coakley, A.J.; Jezequel, S.G.; Tarbit, M.H. The disposition and metabolism of [14C]fluconazole in humans. Drug Metab. Dispos. Biol. Fate Chem. 1991, 19, 764–767. [Google Scholar] [PubMed]
- Giannini, E.G.; Testa, R.; Savarino, V. Liver enzyme alteration: A guide for clinicians. Cmaj 2005, 172, 367–379. [Google Scholar] [CrossRef]
- Matsumoto, K.; Ueno, K.; Yoshimura, H.; Morii, M.; Takada, M.; Sawai, T.; Mitsutake, K.; Shibakawa, M. Fluconazole-induced convulsions at serum trough concentrations of approximately 80 microg/mL. Ther. Drug Monit. 2000, 22, 635–636. [Google Scholar] [CrossRef]
- Tortorano, A.M.; Kibbler, C.; Peman, J.; Bernhardt, H.; Klingspor, L.; Grillot, R. Candidaemia in Europe: Epidemiology and resistance. Int. J. Antimicrob. Agents 2006, 27, 359–366. [Google Scholar] [CrossRef]
- Trouvé, C.; Blot, S.; Hayette, M.P.; Jonckheere, S.; Patteet, S.; Rodriguez-Villalobos, H.; Symoens, F.; Van Wijngaerden, E.; Lagrou, K. Epidemiology and reporting of candidaemia in Belgium: A multi-centre study. Eur. J. Clin. Microbiol. Infect Dis. 2017, 36, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Sandaradura, I.; Wojciechowski, J.; Marriott, D.J.E.; Day, R.O.; Stocker, S.; Reuter, S.E. Model-Optimized Fluconazole Dose Selection for Critically Ill Patients Improves Early Pharmacodynamic Target Attainment without the Need for Therapeutic Drug Monitoring. Antimicrob. Agents Chemother. 2021, 65, e02019-20. [Google Scholar] [CrossRef] [PubMed]
| Patient Characteristics (n = 43) | |
|---|---|
| Age (years), median (IQR) | 66 (58–70) |
| Sex (male), n (%) | 28 (65) |
| Body weight (kg), median (IQR) | 70 (61–87) |
| BMI (kg/m2), median (IQR) | 25 (21–28) |
| APACHE II score (n = 41), | |
| median (IQR) | 18 (14–24) |
| n (%), < 15 | 11 (27) |
| ≥15 | 30 (73) |
| Length of ICU-stay (days), median (IQR) | 22 (14–37) |
| Number of patients who received a loading dose, n (%) | |
| Yes | 25 (58) |
| No | 14 (33) |
| Unknown (transfer to other hospital) | 4 (9) |
| Number of patients that received a loading dose (yes/no), n (%) | |
| Yes | 18 (78) |
| No | 5 (22) |
| Number of patients with at least one subtherapeutic | 17 (40) |
| (<15 mg/L) fluconazole trough concentration, n (%) | |
| Fluconazole Concentrations (n = 288) | |
| Number of samples per patient, median (IQR) | 7 (3–10) |
| Fluconazole Cmin (mg/L), median (IQR) | 22.9 (14.7–35.2) |
| Fluconazole dose (mg/kg), median (IQR) | 5.3 (4.5–7.0) |
| Fluconazole dose (mg), median (IQR) | 400 (400–400) |
| Fluconazole dose (mg), n (%) | |
| 50 | 9 (3) |
| 200 | 28 (10) |
| 400 | 206 (72) |
| 600 | 1 (0.35) |
| 800 | 40 (14) |
| 1000 | 1 (0.35) |
| 1200 | 3 (1) |
| Administration route (PO), n (%) | 28 (10) |
| SOFA score on day of sampling (n = 273), median (IQR) | 7 (4–11) |
| Variability | |
| %CV intrasubject | 28.3 |
| %CV intersubject | 50.5 |
| Renal Function During Sample Collection | Samples (n = 288) |
|---|---|
| eGFR (CKD-Epi) (mL/min/1.73 m2), median (IQR) | 81 (41–101) |
| CrCL (Cockcroft-Gault) (mL/min), median (IQR) | 83 (47–118) |
| Serum creatinine concentration (mg/dL), median (IQR) | 0.91 (0.66–1.52) |
| Urea concentration (mg/dL), median (IQR) | 51 (34–97) |
| Measured 24 h clearance (mL/min), median (IQR), n = 190 | 63 (23–101) |
| Number of samples while ARC (based on CKD-Epi), n (%) | 94 (33) |
| Number of samples on CVVH, n (%) Fluconazole concentration (mg/L), median (IQR) Fluconazole dose (mg), median (IQR) Fluconazole dose (mg/kg), median (IQR) | 37 (13) 14.9 (7.4–17.5) 800 (400–800) 6.7 (5.8–10.3) |
| Number of samples on IHD, n (%) | 9 (3) |
| Significant Covariate | p-Value | Beta-Value |
|---|---|---|
| Multivariate analysis 1: ARC as CrCL24h-1 > 130 mL/min (n = 190) | ||
| Dose previous administration (mg/kg) | 0.010 | 1.30 |
| CVVH | <0.0001 | −25.96 |
| ARC | 0.002 | −9.17 |
| Multivariate analysis 2: ARC as eGFR (CKD-Epi) > 96.5 mL/min/1.73 m2 (n = 288) | ||
| Dose previous administration (mg/kg) | <0.0001 | 1.26 |
| Mode of administration | 0.033 | 5.79 |
| CVVH | 0.006 | −10.56 |
| ARC | 0.002 | −2.59 |
| Alkaline phosphatase | 0.013 | 0.04 |
| Loading Dose | ||||
|---|---|---|---|---|
| Yes (n = 161 *) | No (n = 90 *) | |||
| Cmin < 15 mg/L | Cmin > 80 mg/L | Cmin < 15 mg/L | Cmin > 80 mg/L | |
| Day 3 | 4/11 (36%) | 0/11 (0%) | 1/2 (50%) | 0/2 (0%) |
| Day 4 | 4/12 (33%) | 0/12 (0%) | 2/4 (50%) | 0/4 (0%) |
| Day 5 | 3/13 (23%) | 0/13 (0%) | 4/5 (80%) | 0/5 (0%) |
| Day 6 | 4/15 (27%) | 0/15 (0%) | 2/7 (29%) | 0/7 (0%) |
| Day 7 | 4/18 (22%) | 0/18 (0%) | 2/5 (40%) | 0/5 (0%) |
| Day 8 | 2/17 (12%) | 0/17 (0%) | 2/6 (33%) | 0/6 (0%) |
| Day 9 | 1/17 (6%) | 1/17 (6%) | 2/6 (33%) | 0/6 (0%) |
| Day 10 | 0/11 (0%) | 0/11 (0%) | 2/6 (33%) | 0/6 (0%) |
| Day > 10 | 4/47 (9%) | 0/47 (0%) | 12/49 (25%) | 0/49 (0%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Daele, R.; Wauters, J.; Lagrou, K.; Denooz, R.; Hayette, M.-P.; Gijsen, M.; Brüggemann, R.J.; Debaveye, Y.; Spriet, I. Pharmacokinetic Variability and Target Attainment of Fluconazole in Critically Ill Patients. Microorganisms 2021, 9, 2068. https://doi.org/10.3390/microorganisms9102068
Van Daele R, Wauters J, Lagrou K, Denooz R, Hayette M-P, Gijsen M, Brüggemann RJ, Debaveye Y, Spriet I. Pharmacokinetic Variability and Target Attainment of Fluconazole in Critically Ill Patients. Microorganisms. 2021; 9(10):2068. https://doi.org/10.3390/microorganisms9102068
Chicago/Turabian StyleVan Daele, Ruth, Joost Wauters, Katrien Lagrou, Raphaël Denooz, Marie-Pierre Hayette, Matthias Gijsen, Roger J. Brüggemann, Yves Debaveye, and Isabel Spriet. 2021. "Pharmacokinetic Variability and Target Attainment of Fluconazole in Critically Ill Patients" Microorganisms 9, no. 10: 2068. https://doi.org/10.3390/microorganisms9102068
APA StyleVan Daele, R., Wauters, J., Lagrou, K., Denooz, R., Hayette, M.-P., Gijsen, M., Brüggemann, R. J., Debaveye, Y., & Spriet, I. (2021). Pharmacokinetic Variability and Target Attainment of Fluconazole in Critically Ill Patients. Microorganisms, 9(10), 2068. https://doi.org/10.3390/microorganisms9102068







