Goat Paratuberculosis: Experimental Model for the Evaluation of Mycobacterium Persistence in Raw Milk Cheese
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Manufacturing Process
2.3. Sampling
2.4. Biomolecular Assays
2.5. Cultural, Physical and Chemical Assays
2.5.1. MAP Cultural Assay
2.5.2. LAB Cultural Assay
2.5.3. Mesophilic Lactococci Cultural Assay
2.5.4. Water Activity (Aw) and pH Assays
2.6. Statistical Analysis
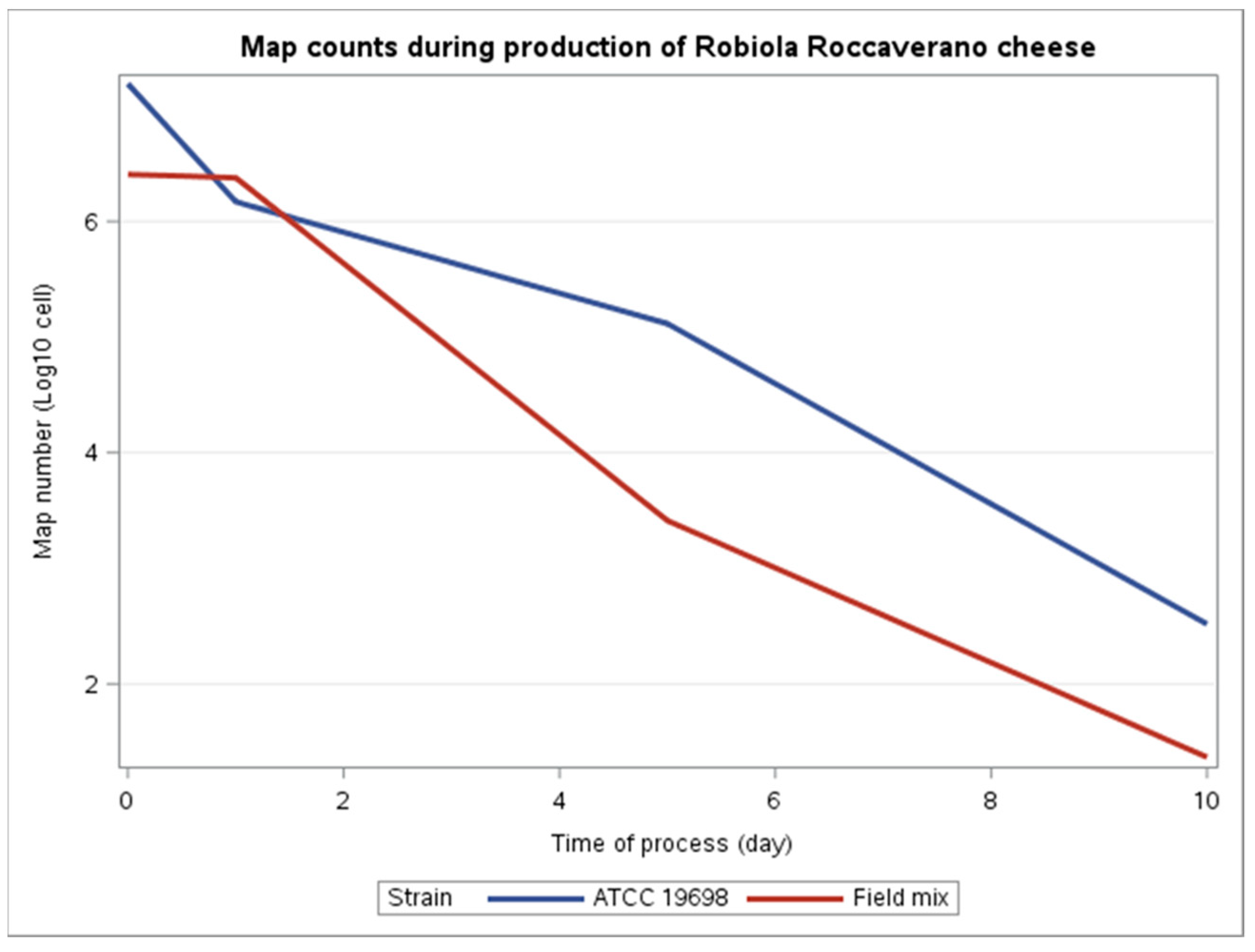
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fecteau, M.-E. Paratuberculosis in Cattle. Vet. Clin. N. Am. Food Anim. Pract. 2018, 34, 209–222. [Google Scholar] [CrossRef]
- Garvey, M. Mycobacterium Avium Paratuberculosis: A Disease Burden on the Dairy Industry. Animals 2020, 10, 1773. [Google Scholar] [CrossRef]
- Windsor, P.A. Paratuberculosis in Sheep and Goats. Vet. Microbiol. 2015, 181, 161–169. [Google Scholar] [CrossRef]
- Mercier, P.; Freret, S.; Laroucau, K.; Gautier, M.-P.; Brémaud, I.; Bertin, C.; Rossignol, C.; Souriau, A.; Guilloteau, L.A. A Longitudinal Study of the Mycobacterium Avium Subspecies Paratuberculosis Infection Status in Young Goats and Their Mothers. Vet. Microbiol. 2016, 195, 9–16. [Google Scholar] [CrossRef]
- Robbe-Austerman, S. Control of Paratuberculosis in Small Ruminants. Vet. Clin. N. Am. Food Anim. Pract. 2011, 27, 609–620. [Google Scholar] [CrossRef]
- Kuenstner, J.T.; Naser, S.; Chamberlin, W.; Borody, T.; Graham, D.Y.; McNees, A.; Hermon-Taylor, J.; Hermon-Taylor, A.; Dow, C.T.; Thayer, W.; et al. The Consensus from the Mycobacterium Avium Ssp. Paratuberculosis (MAP) Conference 2017. Front. Public Health 2017, 5, 208. [Google Scholar] [CrossRef]
- Atreya, R.; Bülte, M.; Gerlach, G.-F.; Goethe, R.; Hornef, M.W.; Köhler, H.; Meens, J.; Möbius, P.; Roeb, E.; Weiss, S. Facts, Myths and Hypotheses on the Zoonotic Nature of Mycobacterium Avium Subspecies Paratuberculosis. Int. J. Med. Microbiol. 2014, 304, 858–867. [Google Scholar] [CrossRef]
- Waddell, L.A.; Rajić, A.; Stärk, K.D.C.; McEwen, S.A. The Zoonotic Potential of Mycobacterium Avium Ssp. Paratuberculosis: A Systematic Review and Meta-Analyses of the Evidence. Epidemiol. Infect. 2015, 143, 3135–3157. [Google Scholar] [CrossRef]
- Grant, I.R. Zoonotic Potential of Mycobacterium Avium Ssp. Paratuberculosis: The Current Position. J. Appl. Microbiol. 2005, 98, 1282–1293. [Google Scholar] [CrossRef]
- Eltholth, M.M.; Marsh, V.R.; Van Winden, S.; Guitian, F.J. Contamination of Food Products with Mycobacterium Avium Paratuberculosis: A Systematic Review: MAP and Food Products. J. Appl. Microbiol. 2009, 107, 1061–1071. [Google Scholar] [CrossRef]
- Gill, C.O.; Saucier, L.; Meadus, W.J. Mycobacterium Avium Subsp. Paratuberculosis in Dairy Products, Meat, and Drinking Water. J. Food Prot. 2011, 74, 480–496. [Google Scholar] [CrossRef]
- Dimareli-Malli, Z. Detection of Mycobacterium Avium Subsp. Paratuberculosis in Milk from Cclinically Affected Sheep and Goats. Int. J. Appl. Res. Vet. Med. 2010, 8, 44–50. [Google Scholar]
- Sweeney, R.W.; Whitlock, R.H.; Rosenberger, A.E. Mycobacterium Paratuberculosis Cultured from Milk and Supramammary Lymph Nodes of Infected Asymptomatic Cows. J. Clin. Microbiol. 1992, 30, 166–171. [Google Scholar] [CrossRef]
- Nebbia, P.; Robino, P.; Zoppi, S.; De Meneghi, D. Detection and Excretion Pattern of Mycobacterium Avium Subspecies Paratuberculosis in Milk of Asymptomatic Sheep and Goats by Nested-PCR. Small Rumin. Res. 2006, 66, 116–120. [Google Scholar] [CrossRef]
- Ricchi, M.; De Cicco, C.; Kralik, P.; Babak, V.; Boniotti, M.B.; Savi, R.; Cerutti, G.; Cammi, G.; Garbarino, C.; Arrigoni, N. Evaluation of Viable Mycobacterium Avium Subsp. Paratuberculosis in Milk Using Peptide-Mediated Separation and Propidium Monoazide QPCR. FEMS Microbiol. Lett. 2014, 356, 127–133. [Google Scholar] [CrossRef][Green Version]
- Hanifian, S. Survival of Mycobacterium Avium Subsp. Paratuberculosis in Ultra-Filtered White Cheese. Lett. Appl. Microbiol. 2014, 58, 466–471. [Google Scholar] [CrossRef]
- Ricchi, M.; Barbieri, G.; Taddei, R.; Belletti, G.L.; Carra, E.; Cammi, G.; Garbarino, C.A.; Arrigoni, N. Effectiveness of Combination of Mini-and Microsatellite Loci to Sub-Type Mycobacterium Avium Subsp. Paratuberculosis Italian Type C Isolates. BMC Vet. Res. 2011, 7, 54. [Google Scholar] [CrossRef]
- Ricchi, M.; Savi, R.; Bolzoni, L.; Pongolini, S.; Grant, I.R.; De Cicco, C.; Cerutti, G.; Cammi, G.; Garbarino, C.A.; Arrigoni, N. Estimation of Mycobacterium Avium Subsp. Paratuberculosis Load in Raw Bulk Tank Milk in Emilia-Romagna Region (Italy) by qPCR. Microbiologyopen 2016, 5, 551–559. [Google Scholar] [CrossRef]
- Kralik, P.; Ricchi, M. A Basic Guide to Real Time PCR in Microbial Diagnostics: Definitions, Parameters, and Everything. Front. Microbiol. 2017, 8, 108. [Google Scholar] [CrossRef]
- Ricchi, M.; Bertasio, C.; Boniotti, M.B.; Vicari, N.; Russo, S.; Tilola, M.; Bellotti, M.A.; Bertasi, B. Comparison among the Quantification of Bacterial Pathogens by QPCR, DPCR, and Cultural Methods. Front. Microbiol. 2017, 8, 1174. [Google Scholar] [CrossRef]
- Elguezabal, N.; Bastida, F.; Sevilla, I.A.; González, N.; Molina, E.; Garrido, J.M.; Juste, R.A. Estimation of Mycobacterium Avium Subsp. Paratuberculosis Growth Parameters: Strain Characterization and Comparison of Methods. Appl. Environ. Microbiol. 2011, 77, 8615–8624. [Google Scholar] [CrossRef]
- Galiero, A.; Fratini, F.; Turchi, B.; Colombani, G.; Nuvoloni, R.; Cerri, D. Detection of Mycobacterium Avium Subsp. Paratuberculosis in a Sheep Flock in Tuscany. Trop. Anim. Health Prod. 2015, 47, 1567–1571. [Google Scholar] [CrossRef] [PubMed]
- Hanifian, S.; Khani, S. Fate of Yersinia Enterocolitica during Manufacture, Ripening and Storage of Lighvan Cheese. Int. J. Food Microbiol. 2012, 156, 141–146. [Google Scholar] [CrossRef]
- Alemdar, S.; Ağaoğlu, S. Survival of Salmonella Typhimurium During the Ripening of Herby Cheese (Otlu Peynir). J. Food Saf. 2010, 30, 526–536. [Google Scholar] [CrossRef]
- Mohammadi, K.; Karim, G.; Razavilar, V.; Hanifian, S. Study on the Growth and Survival of Escherichia Coli O157:H7 during the Manufacture and Storage of Iranian White Cheese in Brine. Iran. J. Vet. Res. 2009, 10, 346–351. [Google Scholar]
- Sung, N.; Collins, M.T. Thermal Tolerance of Mycobacterium Paratuberculosis. Appl. Environ. Microbiol. 1998, 64, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Spahr, U.; Schafroth, K. Fate of Mycobacterium Avium Subsp.Paratuberculosis in Swiss Hard and Semihard Cheese Manufactured from Raw Milk. Appl. Environ. Microbiol. 2001, 67, 4199–4205. [Google Scholar] [CrossRef]
- Cammi, G.; Ricchi, M.; Galiero, A.; Daminelli, P.; Cosciani-Cunico, E.; Dalzini, E.; Losio, M.N.; Savi, R.; Cerutti, G.; Garbarino, C.; et al. Evaluation of Mycobacterium Avium Subsp. Paratuberculosis Survival during the Manufacturing Process of Italian Raw Milk Hard Cheeses (Parmigiano Reggiano and Grana Padano). Int. J. Food Microbiol. 2019, 305, 108247. [Google Scholar] [CrossRef]
- Gaggìa, F.; Nielsen, D.S.; Biavati, B.; Siegumfeldt, H. Intracellular PH of Mycobacterium Avium Subsp. Paratuberculosis Following Exposure to Antimicrobial Compounds Monitored at the Single Cell Level. Int. J. Food Microbiol. 2010, 141, S188–S192. [Google Scholar] [CrossRef]
- Kralik, P.; Babak, V.; Dziedzinska, R. The Impact of the Antimicrobial Compounds Produced by Lactic Acid Bacteria on the Growth Performance of Mycobacterium Avium Subsp. Paratuberculosis. Front. Microbiol. 2018, 9, 638. [Google Scholar] [CrossRef]
- Ali, Z.I.; Saudi, A.M.; Albrecht, R.; Talaat, A.M. The Inhibitory Effect of Nisin on Mycobacterium Avium Ssp. Paratuberculosis and Its Effect on Mycobacterial Cell Wall. J. Dairy Sci. 2019, 102, 4935–4944. [Google Scholar] [CrossRef] [PubMed]
- Chiodini, R.J.; Hermon-Taylor, J. The Thermal Resistance of Mycobacterium Paratuberculosis in Raw Milk under Conditions Simulating Pasteurization. J. Vet. Diagn. Investig. 1993, 5, 629–631. [Google Scholar] [CrossRef] [PubMed]
- Grant, I.R.; Hitchings, E.I.; McCartney, A.; Ferguson, F.; Rowe, M.T. Effect of Commercial-Scale High-Temperature, Short-Time Pasteurization on the Viability of Mycobacterium Paratuberculosis in Naturally Infected Cows’ Milk. Appl. Environ. Microbiol. 2002, 68, 602–607. [Google Scholar] [CrossRef]
- Grant, I.R.; Williams, A.G.; Rowe, M.T.; Muir, D.D. Efficacy of Various Pasteurization Time-Temperature Conditions in Combination with Homogenization on Inactivation of Mycobacterium Avium Subsp. Paratuberculosis in Milk. Appl. Environ. Microbiol. 2005, 71, 2853–2861. [Google Scholar] [CrossRef] [PubMed]
- Stephan, R.; Schumacher, S.; Tasara, T.; Grant, I.R. Prevalence of Mycobacterium Avium Subspecies Paratuberculosis in Swiss Raw Milk Cheeses Collected at the Retail Level. J. Dairy Sci. 2007, 90, 3590–3595. [Google Scholar] [CrossRef] [PubMed]
- Ikonomopoulos, J.; Pavlik, I.; Bartos, M.; Svastova, P.; Ayele, W.Y.; Roubal, P.; Lukas, J.; Cook, N.; Gazouli, M. Detection of Mycobacterium Avium Subsp. Paratuberculosis in Retail Cheeses from Greece and the Czech Republic. Appl. Environ. Microbiol. 2005, 71, 8934–8936. [Google Scholar] [CrossRef]
- Clark, D.L., Jr.; Anderson, J.L.; Koziczkowski, J.J.; Ellingson, J.L.E. Detection of Mycobacterium Avium Subspecies Paratuberculosis Genetic Components in Retail Cheese Curds Purchased in Wisconsin and Minnesota by PCR. Mol. Cell. Probes 2006, 20, 197–202. [Google Scholar] [CrossRef]
- Giese, S. Detection of Mycobacterium Avium Subsp. Paratuberculosis in Milk from Clinically Affected Cows by PCR and Culture. Vet. Microbiol. 2000, 77, 291–297. [Google Scholar] [CrossRef]
- Martucciello, A.; Galletti, G.; Pesce, A.; Russo, M.; Sannino, E.; Arrigoni, N.; Ricchi, M.; Tamba, M.; Brunetti, R.; Ottaiano, M.; et al. Short Communication: Seroprevalence of Paratuberculosis in Italian Water Buffaloes (Bubalus Bubalis) in the Region of Campania. J. Dairy Sci. 2021, 104, 6194–6199. [Google Scholar] [CrossRef] [PubMed]

| Time of Sampling | Product | PCR Assay(MAP Cells/mL for Milk or Map Cells/g for Curd and Cheese) | Cultural Assay(CFU/g for Curd and Cheese) | ||
|---|---|---|---|---|---|
| Batch A | Batch B | Batch A | Batch B | ||
| T0 | Spiked Milk | 1.99 × 107 1.21 × 107 | 2.38 × 106 2.73 × 106 | n.d.a | 1 × 105 n.d. a |
| T1 | Curd | 1.82 × 106 1.20 × 106 | 2.34 × 106 2.43 × 106 | 6 × 105 3 × 105 | 6 × 105 1 × 105 |
| T2 | Cheese (5 days) | 2.62 × 104 6.50 × 105 | 1.19 × 103 5.61 × 103 | 5.5 × 104 3.5 × 104 | n.d.a |
| T3 | Cheese (10 days) | 1.97 × 102 5.5 × 102 | 1.10 × 101 4.9 × 101 | n.d. a | n.d. a |
| Time of Sampling | Product
(Batch A) | pH | aW | LAB b | Mesophilic Lactococci b |
|---|---|---|---|---|---|
| T0 | Spiked Milk | 6.21 | n.d.a | 4.89 | 3.2 |
| T1 | Curd | 4.25 | 0.986 | 9.23 | 4.9 |
| T2 | Cheese (5 days) | 4.25 | 0.983 | 7.78 | 5.2 |
| T3 | Cheese (10 days) | 4.11 | 0.981 | 6.81 | 8.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagliasso, G.; Di Blasio, A.; Vitale, N.; Romano, A.; Decastelli, L.; Quasso, A.; Ricchi, M.; Dondo, A.; Pastorino, P.; Gennero, M.S.; et al. Goat Paratuberculosis: Experimental Model for the Evaluation of Mycobacterium Persistence in Raw Milk Cheese. Microorganisms 2021, 9, 2032. https://doi.org/10.3390/microorganisms9102032
Pagliasso G, Di Blasio A, Vitale N, Romano A, Decastelli L, Quasso A, Ricchi M, Dondo A, Pastorino P, Gennero MS, et al. Goat Paratuberculosis: Experimental Model for the Evaluation of Mycobacterium Persistence in Raw Milk Cheese. Microorganisms. 2021; 9(10):2032. https://doi.org/10.3390/microorganisms9102032
Chicago/Turabian StylePagliasso, Giulia, Alessia Di Blasio, Nicoletta Vitale, Angelo Romano, Lucia Decastelli, Antonio Quasso, Matteo Ricchi, Alessandro Dondo, Paolo Pastorino, Maria Silvia Gennero, and et al. 2021. "Goat Paratuberculosis: Experimental Model for the Evaluation of Mycobacterium Persistence in Raw Milk Cheese" Microorganisms 9, no. 10: 2032. https://doi.org/10.3390/microorganisms9102032
APA StylePagliasso, G., Di Blasio, A., Vitale, N., Romano, A., Decastelli, L., Quasso, A., Ricchi, M., Dondo, A., Pastorino, P., Gennero, M. S., & Bergagna, S. (2021). Goat Paratuberculosis: Experimental Model for the Evaluation of Mycobacterium Persistence in Raw Milk Cheese. Microorganisms, 9(10), 2032. https://doi.org/10.3390/microorganisms9102032








