Exogenous Fungal Endophthalmitis: Clues to Aspergillus Aetiology with a Pharmacological Perspective
Abstract
1. Introduction
2. Materials and Methods
3. Epidemiology and Risk Factors
3.1. Endophthalmitis after Ocular Surgery or Invasive Procedures
3.1.1. Endophthalmitis Post-Cataract Surgery
3.1.2. Endophthalmitis Post-Vitrectomy
3.1.3. Endophthalmitis Post-Intravitreal Injection
3.1.4. Endophthalmitis Post-Keratoplasty
3.1.5. Epidemiology of Endophthalmitis after Keratomycosis
3.1.6. Epidemiology of Post-traumatic Endophthalmitis
4. Clinical Features
5. Diagnosis
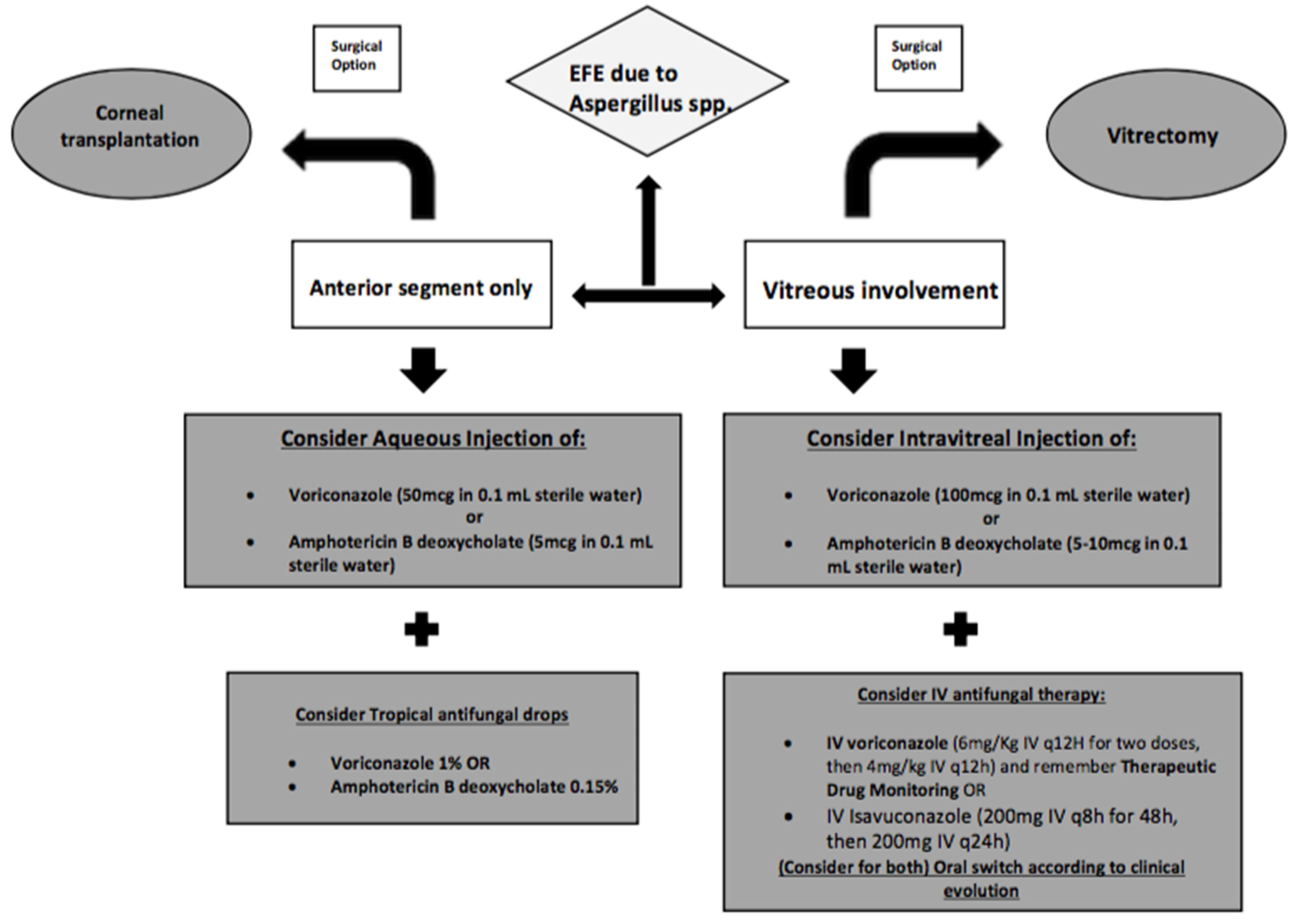
6. Pharmacokinetics and Pharmacodynamics of Antifungals in EFE
6.1. Intravitreal Antifungals
6.1.1. Intravitreal Polyenes in Exogenous Fungal Endophthalmitis (EXFE)
6.1.2. Intravitreal Azoles in EFE
6.1.3. Place in Therapy of Intravitreal Echinocandins
6.2. Systemic Antifungal Therapy
6.3. Surgical Treatment
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Durand, M.L. Bacterial and Fungal Endophthalmitis. Clin. Microbiol. Rev. 2017, 30, 597–613. [Google Scholar] [PubMed]
- Keynan, Y.; Finkelman, Y.; LagacÃ-Wiens, P. The microbiology of endophthalmitis: Global trends and a local perspective. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2879–2886. [Google Scholar]
- Vilela, R.C.; Vilela, L.; Vilela, P.; Vilela, R.; Motta, R.; Pôssa, A.P.; de Almeida, C.; Mendoza, L. Etiological agents of fungal endophthalmitis: Diagnosis and management. Int. Ophthalmol. 2014, 34, 707–721. [Google Scholar]
- Silva, R.A.; Sridhar, J.; Miller, D.; Wykoff, C.C.; Flynn, H.W., Jr. Exogenous fungal endophthalmitis: An analysis of isolates and susceptibilities to antifungal agents over a 20-year period (1990–2010). Am. J. Ophthalmol. 2015, 159, 257–264.e1. [Google Scholar] [PubMed]
- Rychener, R.O. Intra-ocular mycosis. Trans. Am. Ophthalmol. Soc. 1933, 31, 477–496. [Google Scholar] [PubMed]
- Sharma, S.; Padhi, T.R.; Basu, S.; Kar, S.; Roy, A.; Das, T. Endophthalmitis patients seen in a tertiary eye care centre in Odisha: A clinico-microbiological analysis. Indian J. Med. Res. 2014, 139, 91–98. [Google Scholar] [PubMed]
- Glass, R.B.J.; Hertzanu, Y.; Mendelsohn, D.B.; Posen, J. Paranasal sinus aspergillosis: A case report with computed tomogram findings. J. Laryngol. Otol. 1984, 98, 199–205. [Google Scholar]
- Denning, D.W. Invasive aspergillosis. Clin. Infect. Dis. 1998, 26, 781–805. [Google Scholar]
- Wykoff, C.C.; Flynn, H.W., Jr.; Miller, D.; Scott, I.U.; Alfonso, E.C. Exogenous fungal endophthalmitis: Microbiology and clinical outcomes. Ophthalmology 2008, 115, 1501–1507. [Google Scholar]
- Dave, V.P.; Pappuru, R.R.; Pathengay, A.; Gupta, R.; Joseph, J.; Sharma, S.; Das, T. Aspergillus Endophthalmitis: Clinical Presentations and Factors Determining Outcomes. Asia Pac. J. Ophthalmol. 2020, 9, 9–13. [Google Scholar]
- Narang, S.; Gupta, A.; Gupta, V.; Dogra, M.R.; Ram, J.; Pandav, S.S.; Chakrabarti, A. Fungal endophthalmitis following cataract surgery: Clinical presentation, microbiological spectrum, and outcome. Am. J. Ophthalmol. 2001, 132, 609–617. [Google Scholar] [PubMed]
- Smith, T.C.; Benefield, R.J.; Kim, J.H. Risk of Fungal Endophthalmitis Associated with Cataract Surgery: A Mini-Review. Mycopathologia 2015, 180, 291–297. [Google Scholar] [PubMed]
- Jindal, A.; Pathengay, A.; Mithal, K.; Jalali, S.; Mathai, A.; Pappuru, R.R.; Narayanan, R.; Chhablani, J.; Motukupally, S.R.; Sharma, S.; et al. Microbiologic spectrum and susceptibility of isolates in acute postcataract surgery endophthalmitis: Are they same as they were more than a decade ago? Br. J. Ophthalmol. 2014, 98, 414–416. [Google Scholar] [PubMed]
- Lalitha, P.; Sengupta, S.; Ravindran, R.D.; Sharma, S.; Joseph, J.; Ambiya, V.; Das, T. A literature review and update on the incidence and microbiology spectrum of postcataract surgery endophthalmitis over past two decades in India. Indian J. Ophthalmol. 2017, 65, 673–677. [Google Scholar] [PubMed]
- Recchia, F.M.; Busbee, B.G.; Pearlman, R.B.; Carvalho-Recchia, C.A.; Ho, A.C. Changing trends in the microbiologic aspects of postcataract endophthalmitis. Arch. Ophthalmol. 2005, 123, 341–346. [Google Scholar]
- Shirodkar, A.R.; Pathengay, A.; Flynn, H.W., Jr.; Albini, T.A.; Berrocal, A.M.; Davis, J.L.; Lalwani, G.A.; Murray, T.G.; Smiddy, W.E.; Miller, D. Delayed- versus acute-onset endophthalmitis after cataract surgery. Am. J. Ophthalmol. 2012, 153, 391–398. [Google Scholar] [PubMed]
- Sheng, Y.; Sun, W.; Gu, Y.; Lou, J.; Liu, W. Endophthalmitis after cataract surgery in China, 1995-2009. J. Cataract. Refract. Surg. 2011, 37, 1715–1722. [Google Scholar]
- Yannuzzi, N.A.; Si, N.; Relhan, N.; Kuriyan, A.E.; Albini, T.A.; Berrocal, A.M.; Davis, J.L.; Smiddy, W.E.; Townsend, J.; Miller, D.; et al. Endophthalmitis After Clear Corneal Cataract Surgery: Outcomes Over Two Decades. Am. J. Ophthalmol. 2017, 174, 155–159. [Google Scholar]
- Sen, S.; Lalitha, P.; Mishra, C.; Parida, H.; Rameshkumar, G.; Kannan, N.B.; Ramasamy, K. Post-cataract Surgery Fungal Endophthalmitis: Management Outcomes and Prognostic Factors. Ocul. Immunol. Inflamm. 2020, 10, 1–7. [Google Scholar]
- Chee, Y.E.; Eliott, D. The Role of Vitrectomy in the Management of Fungal Endophthalmitis Semin. Ophthalmology 2017, 32, 29–35. [Google Scholar]
- Chen, G.; Tzekov, R.; Li, W.; Jiang, F.; Mao, S.; Tong, Y. Incidence of endophthalmitis after vitectomy: A Systematic Review and Meta-analysis. Retina 2019, 39, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Bhende, M.; Raman, R.; Singh, N.; Jain, M.; Sharma, T.; Gopal, L.; Bhende, P.S.; Srinivasan, S.; Jambulingam, M.; Vitreoretinal Study Group; et al. Risk Factors for Endophthalmitis after Pars Plana Vitrectomies in a Tertiary Eye Institute in India. Ophthalmol. Retina. 2018, 2, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Dave, V.P.; Pathengay, A.; Schwartz, S.G.; Flynn, H.W., Jr. Endophthalmitis following pars plana vitrectomy: A literature review of incidence, causative organisms, and treatment outcomes. Clin. Ophthalmol. 2014, 8, 2183–2188. [Google Scholar] [PubMed]
- Charles, W.G.; Scott, E.I.U.; Flynn, H.W.; Smiddy, W.E.; Newton, J. Endophthalmitis after pars plana vitrectomy: Incidence, causative organisms, and visual acuity outcomes. Am. J. Ophthalmol. 2004, 138, 799–802. [Google Scholar]
- Merani, R.; Hunyor, A.P. Endophthalmitis following intravitreal anti-vascular endothelial growth factor (VEGF) injection: A comprehensive review. Int. J. Retina Vitreous 2015, 1, 1–19. [Google Scholar] [CrossRef]
- Baudin, F.; Benzenine, E.; Mariet, A.; Bron, A.M.; Vincent Daien, V.; Korobelnik, J.F.; Quantin, C.; Creuzot-Garcher, C. Association of Acute Endophthalmitis with Intravitreal Injections of Corticosteroids or Anti-Vascular Growth Factor Agents in a Nationwide Study in France. JAMA Ophthalmol. 2018, 136, 1352–1358. [Google Scholar] [CrossRef]
- Reibaldi, M.; Pulvirenti, A.; Avitabile, T.; Bonfiglio, V.; Russo, A.; Mariotti, C.; Bucolo, C.; Mastropasqua, R.; Parisi, G.; Longo, A. Pooled estimates of incidence of endophthalmitis after intravitreal inijection of antivascular endothelial growth factor agents with and without topical antibiotic prophylaxis. Retina 2018, 38, 1–11. [Google Scholar] [CrossRef]
- Bhavsar, A.R.; Glassman, A.R.; Stockdale, C.R.; Jampol, L.M. Diabetic Retinopathy Clinical Research Network. Elimination of Topical Antibiotics for Intravitreous Injections and the Importance of Using Povidone-Iodine: Update from the Diabetic Retinopathy Clinical Research Network. JAMA Ophthalmol. 2016, 134, 1181–1183. [Google Scholar] [CrossRef]
- Reibaldi, M.; Avitabile, T.; Bandello, F.; Longo, A.; Bonfiglio, V.; Russo, A.; Castellino, N.; Rejdak, R.; Nowomiejska, K.; Toro, M.; et al. The Effectiveness of 0.6% Povidone Iodine Eye Drops in Reducing the Conjunctival Bacterial Load and Needle Contamination in Patients Undergoing Anti-VEGF Intravitreal Injection: A Prospective, Randomized Study. J. Clin. Med. 2019, 13, 1031. [Google Scholar] [CrossRef]
- Durga, S.B.; Wibbelsman, T.D.; Buch, P.M.; Rapuano, S.B.; Obeid, A.; Ho, A.C.; Hsu, J.; Regillo, C.D.; Ayres, B.D.; Hammersmith, K.M.; et al. Endophthalmitis Rates and Clinical Outcomes Following Penetrating and Endothelial Keratoplasty. Am. J. Ophthalmol. 2019, 207, 426–427. [Google Scholar]
- Spadea, L.; Abbouda, A.; Abicca, I.; Paroli, M.P. Aspergillus flavus endophthalmitis after penetrating keratoplasty combined with cataract phacoemulsification and IOL implantation. Int. Ophthalmol. 2015, 35, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, S.S.; Alrajhi, A.; Alkahtani, E. Endophthalmitis following Keratoplasty: Incidence, Microbial Profile, Visual and Structural Outcomes. Ocul. Immunol. Inflamm. 2014, 22, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Jastaneiah, S.S.; Al-Rajhi, A.A.; Abbott, D. Ocular mycosis at a referral center in Saudi Arabia: A 20-year study. Saudi J. Ophthalmol. 2011, 25, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Spadea, L.; Giannico, M.I. Diagnostic and Management Strategies of Aspergillus Endophthalmitis: Current Insights. Clin. Ophthalmol. 2019, 13, 2573–2582. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Y.; Zhang, L.; Yin, X.L.; Sun, S.Y. Endophthalmitis associated with fungal keratitis and penetrating injuries in North China. Eur. J. Ophthalmol. 2020, 30, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Chen, N.; Dong, X.G.; Yuan, G.Q.; Yu, B.; Xie, L.X. Surgical management of fungal endophthalmitis resulting from fungal keratitis. Int. J. Ophthalmol. 2016, 18, 848–853. [Google Scholar]
- Shen, Y.C.; Wang, C.Y.; Tsai, H.Y.; Lee, H.N. Intracameral voriconazole injection in the treatment of fungal endophthalmitis resulting from keratitis. Am. J. Ophthalmol. 2010, 149, 916–921. [Google Scholar] [CrossRef]
- Bhagat, N.; Nagori, S.; Zarbin, M. Post-traumatic Infectious Endophthalmitis. Surv. Ophthalmol. 2011, 56, 214–251. [Google Scholar] [CrossRef]
- Gupta, A.; Srinivasan, R.; Kaliaperumal, S.; Saha, I. Post-traumatic fungal endophthalmitis—A prospective study. Eye 2008, 22, 13–17. [Google Scholar] [CrossRef]
- Ramakrishnan, R.; Bharathi, M.J.; Shivkumar, C.; Mittal, S.; Meenakshi, R.; Khadeer, M.A.; Avasthi, A. Microbiological profile of culture-proven cases of exogenous and endogenous endophthalmitis: A 10-year retrospective study. Eye 2009, 23, 945–956. [Google Scholar] [CrossRef]
- Kalkanci, A.; Ozdek, S. Ocular fungal infections. Curr. Eye Res. 2011, 36, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, S.C.; Flynn, H.W., Jr.; Zwickey, T.A.; Forster, R.K.; Tsiligianni, A.; Culbertson, W.W.; Mandelbaum, S. Exogenous fungal endophthalmitis. Ophthalmology 1988, 95, 19–30. [Google Scholar] [CrossRef]
- Ogawa, M.; Sugita, S.; Watanabe, K.; Shimizu, N.; Mochizuki, M. Novel diagnosis of fungal endophthalmitis by broad-range real-time PCR detection of fungal 28S ribosomal DNA. Graefe’s Arch. Clin. Exp. Ophthalmol. 2012, 250, 1877–1883. [Google Scholar] [CrossRef] [PubMed]
- Radhika, M.; Mithal, K.; Bawdekar, A.; Dave, V.; Jindal, A.; Relhan, N.; Flynn, H.W. Pharmacokinetics of intravitreal antibiotics in endophthalmitis. J. Ophthalmic. Inflamm. Infect. 2014, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sallam, A.B.; Kirkland, K.A.; Barry, R.; Soliman, M.K.; Ali, T.K.; Lightman, S. A Review of Antimicrobial Therapy for Infectious Uveitis of the Posterior Segment. Medical Hypothesis. Discov. Innov. Ophthalmol. J. 2018, 7, 140–155. [Google Scholar]
- Sharma, B.; Kataria, P.; Anand, R.; Gupta, R.; Kumar, K.; Kumar, S.; Gupta, R. Efficacy profile of intracameral amphotericin B the often forgotten step. Asia Pac. J. Ophthalmol. 2015, 4, 360–366. [Google Scholar] [CrossRef]
- Luaces-Rodríguez, A.; González-Barcia, M.; Blanco-Teijeiro, M.J.; Gil-Martínez, M.; Gonzalez, F.; Gómez-Ulla, F.; Lamas, M.J.; Otero-Espinar, F.J.; Fernández-Ferreiro, A. Review of intraocular pharmacokinetics of anti-infectives commonly used in the treatment of infectious endophthalmitis. Pharmaceutics 2018, 10, 66. [Google Scholar] [CrossRef]
- Wingard, L.B.; Zuravleff, J.J.; Doft, B.H.; Berk, L.; Rinkoff, J. Intraocular distribution of intravitreally administered amphotericin B in normal and vitrectomized eyes. Investigative Ophthalmol. Vis. Sci. 1989, 30, 2184–2189. [Google Scholar]
- Sen, P.; Gopal, L.; Sen, P.R. Intravitreal voriconazole for drug-resistant fungal endophthalmitis. Retina 2006, 26, 935–939. [Google Scholar] [CrossRef]
- Wu, X.-G.; Yany, L.-N.; Xin, M.; Jiang, H.R. Anti-infectious activity of intravitreal injectable voriconazole microspheres on experimental rabbit fungal endophthalmitis caused by Aspergillus fumigatus. J. Pharm. Sci. 2011, 100, 1745–1759. [Google Scholar]
- Goyal, J.; Fernandes, M.; Shah, S.G. Intracameral voriconazole injection in the treatment of fungal endophthalmitis resulting from keratitis. Am. J. Ophthalmol. 2010, 150, 939–940. [Google Scholar] [CrossRef] [PubMed]
- Guest, J.M.; Singh, P.K.; Revankar, S.G.; Chandrasekar, P.H.; Kumar, A. Isavuconazole for Treatment of Experimental Fungal Endophthalmitis Caused by Aspergillus fumigatus. Antimicrob. Agents Chemother. 2018, 62, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kusbeci, T.; Avci, B.; Cetinkaya, Z.; Ozturk, F.; Yavas, G.; Ermis, S.S.; Inan, U.U. The effects of caspofungin and voriconazole in experimental Candida endophthalmitis. Curr. Eye Res. 2007, 32, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.C.; Liang, C.Y.; Wang, C.Y.; Lin, K.H.; Hsu, M.Y.; Yuen, H.L.; Wei, L.C. Pharmacokinetics and safety of intravitreal caspofungin. Antimicrob. Agents Chemother. 2014, 58, 7234–7239. [Google Scholar] [CrossRef]
- Durand, M.L.; Kim, I.K.; D’Amico, D.J.; Loewenstein, J.I.; Tobin, E.H.; Kieval, S.J.; Martin, S.S.; Azar, D.T.; Miller, F.S.; Lujan, B.J.; et al. Successful treatment of Fusarium endophthalmitis with voriconazole and Aspergillus endophthalmitis with voriconazole plus caspofungin. Am. J. Ophthalmol. 2005, 140, 552–554. [Google Scholar] [CrossRef]
- Riddell, J.; Comer, G.M.; Kauffman, C.A. Treatment of endogenous fungal endophthalmitis: Focus on new antifungal agents. Clin. Infect. Dis. 2011, 52, 648–653. [Google Scholar] [CrossRef]
- Behera, U.C.; Budhwani, M.; Das, T.; Basu, S.; Padhi, T.R.; Barik, M.R.; Sharma, S. Role of early vitrectomy in the treatment of fungal endophthalmitis. Retina 2018, 38, 1385–1392. [Google Scholar] [CrossRef]
- Celiker, H.; Kazokoglu, H. The role of pars plana vitrectomy in the management of fungal endogenous endophthalmitis. Eur. J. Ophthalmol. 2020, 30, 88–93. [Google Scholar] [CrossRef]
| Route of Administration | Class of Antifungal | Drug | Spectrum | Rate of Antifungal Resistance | Diffusion | Half-Life | Toxicity |
|---|---|---|---|---|---|---|---|
| Intravitreal | Polyenes | Amphotericin B | Very wide for Moulds and Candida spp. | Very Low | Low-moderate (High molecular weight, negative charge) | 1.4–15.1 days | Ocular, Dose related (<25 ug) |
| Azoles | Voriconazole | Wide for Aspergillus and Candida spp. | Low for Aspergillus, Low-moderate for Candida spp. | High | 2.5 h | Ocular, Dose related | |
| Isavuconazole | Wide for Aspergillus, Mucor and Candida spp. | Low (limited data) | High (Limited data) | NA | NA | ||
| Echinocandins | Caspofungin | Wide for Candida spp., Less for Aspergillus spp. | Low for Candida spp. | Low-moderate | 6.2 h | NA | |
| Systemic | Polyenes | Amphotericin B | Very wide for Moulds and Candida spp. | Very Low | Low | 153 h | Kidney, infusion-related, Na, K, Mg |
| Azoles | Voriconazole | Wide for Aspergillus and Candida spp. | Low for Aspergillus, Low-moderate for Candida spp. | High | 6 h | Visual, Kidney, GI, Skin, Na, K | |
| Isavuconazole | Wide for Aspergillus, Mucor and Candida spp. | Low (limited data) | High | 4–7 h | GI, Kidney, Na, K |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lupia, T.; Corcione, S.; Fea, A.M.; Reibaldi, M.; Fallico, M.; Petrillo, F.; Galdiero, M.; Scabini, S.; Polito, M.S.; Ciabatti, U.; et al. Exogenous Fungal Endophthalmitis: Clues to Aspergillus Aetiology with a Pharmacological Perspective. Microorganisms 2021, 9, 74. https://doi.org/10.3390/microorganisms9010074
Lupia T, Corcione S, Fea AM, Reibaldi M, Fallico M, Petrillo F, Galdiero M, Scabini S, Polito MS, Ciabatti U, et al. Exogenous Fungal Endophthalmitis: Clues to Aspergillus Aetiology with a Pharmacological Perspective. Microorganisms. 2021; 9(1):74. https://doi.org/10.3390/microorganisms9010074
Chicago/Turabian StyleLupia, Tommaso, Silvia Corcione, Antonio Maria Fea, Michele Reibaldi, Matteo Fallico, Francesco Petrillo, Marilena Galdiero, Silvia Scabini, Maria Sole Polito, Umberto Ciabatti, and et al. 2021. "Exogenous Fungal Endophthalmitis: Clues to Aspergillus Aetiology with a Pharmacological Perspective" Microorganisms 9, no. 1: 74. https://doi.org/10.3390/microorganisms9010074
APA StyleLupia, T., Corcione, S., Fea, A. M., Reibaldi, M., Fallico, M., Petrillo, F., Galdiero, M., Scabini, S., Polito, M. S., Ciabatti, U., & De Rosa, F. G. (2021). Exogenous Fungal Endophthalmitis: Clues to Aspergillus Aetiology with a Pharmacological Perspective. Microorganisms, 9(1), 74. https://doi.org/10.3390/microorganisms9010074







