Interaction of Styrylpyridinium Compound with Pathogenic Candida albicans Yeasts and Human Embryonic Kidney HEK-293 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemistry
2.2. Strains and Cell Lines
2.3. Adhesion Assay
2.4. Evaluation of Cytotoxicity
2.5. Fluorescence Measurement
2.6. Analysis of Gene Expression
2.7. Statistical Analysis
3. Results and Discussion
3.1. Adhesion of C. albicans to HEK-293 Cells
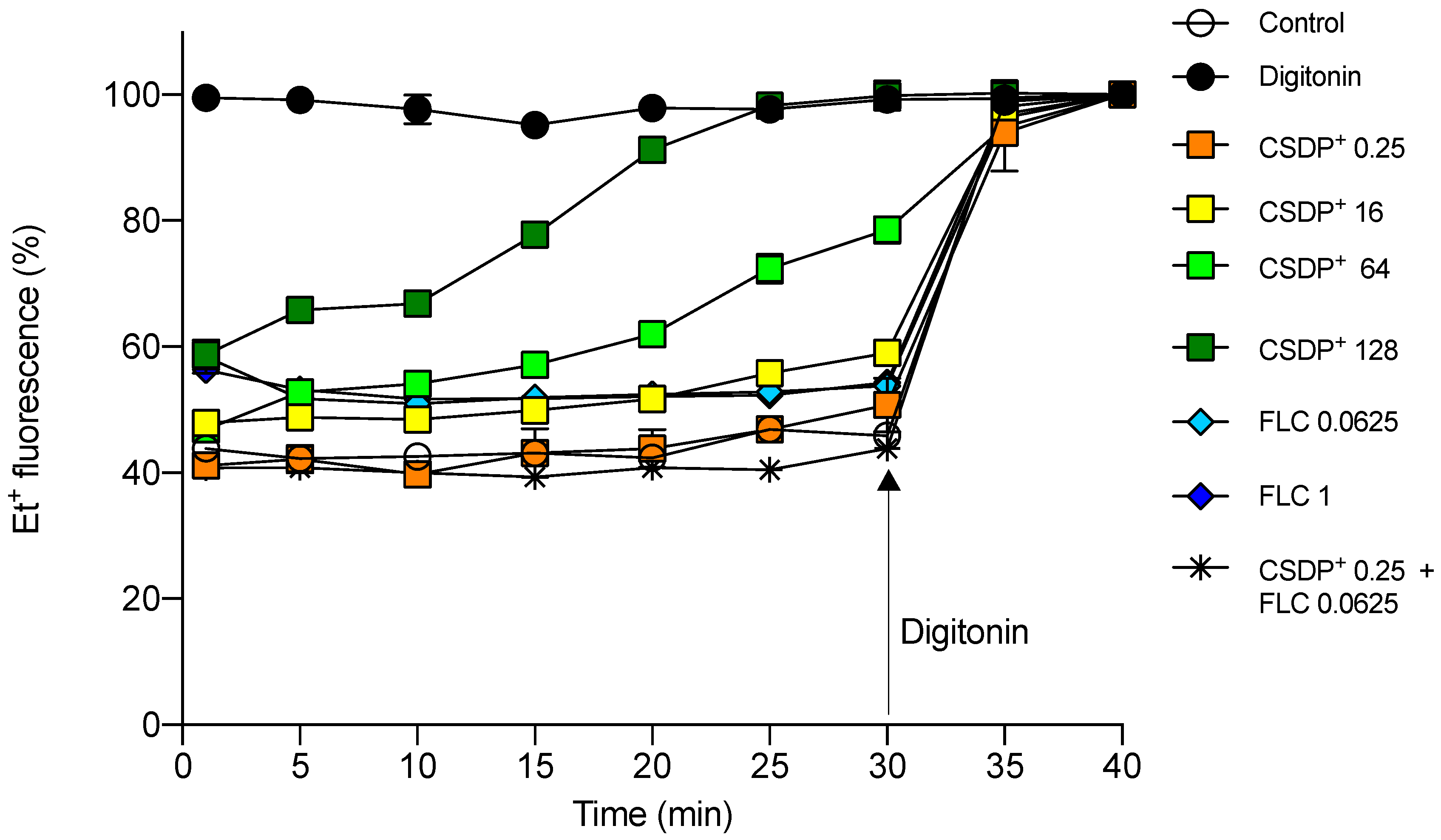
3.2. Cytotoxicity to HEK-293 Cells
3.3. Effect of CSDP+ and Fluconazole on Et+ Fluorescence in HEK-293 Cell Suspensions
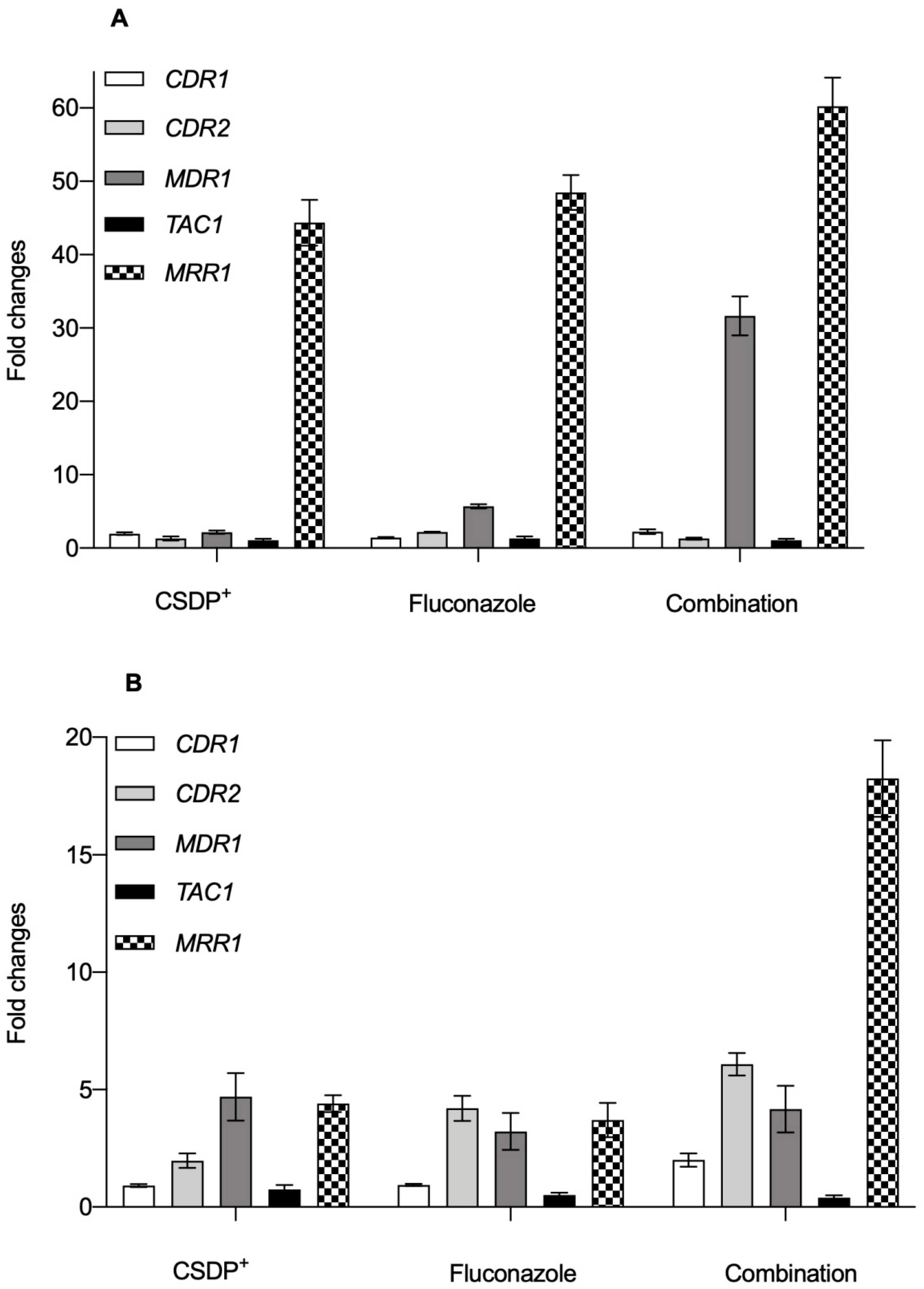
3.4. Expression of efflux pump genes in C. albicans
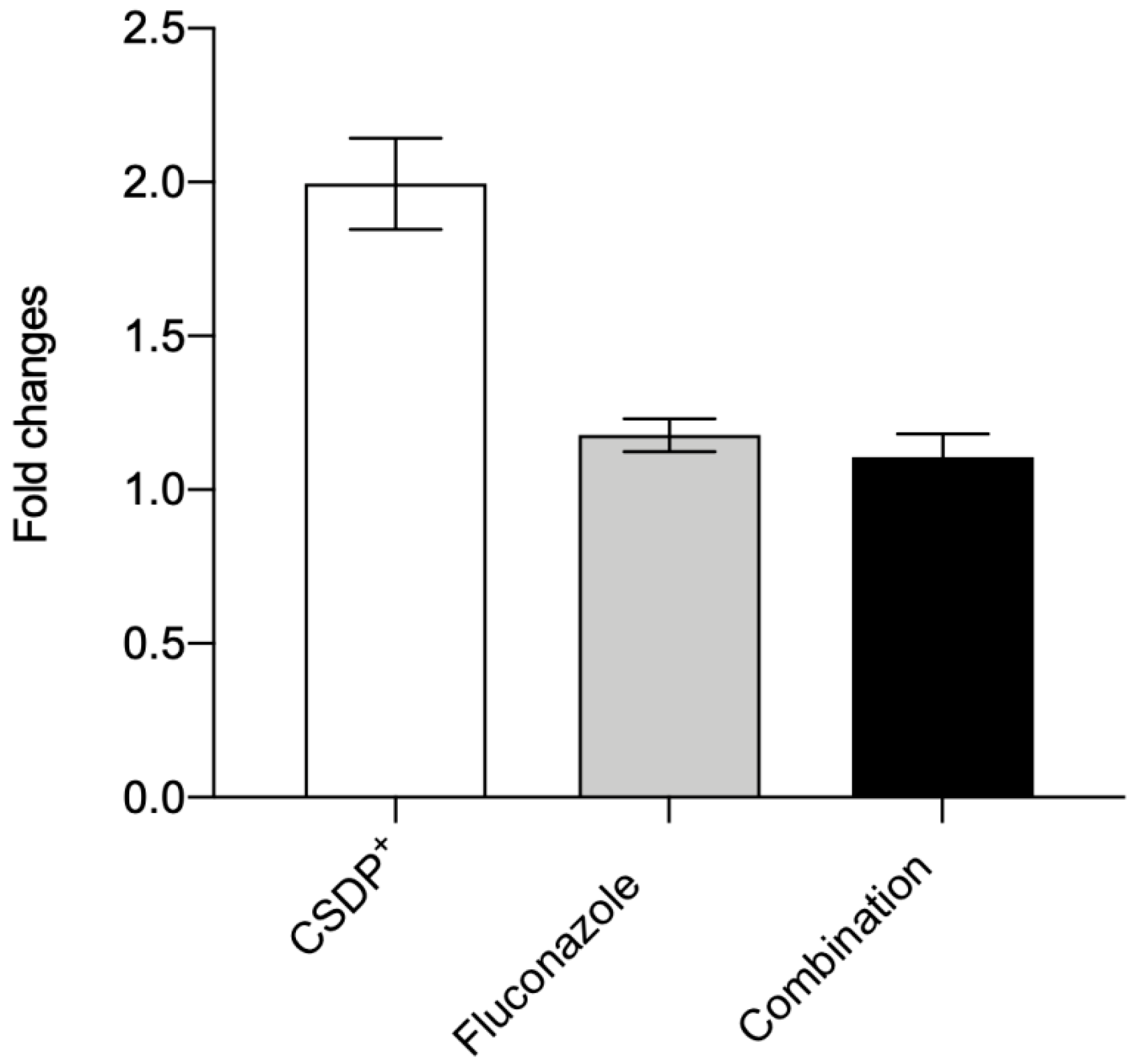
3.5. Expression of the efflux pump ABCB1 gene in HEK-293
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- White, T.C.; Holleman, S.; Dy, F.; Mirels, L.F.; Stevens, D.A. Resistance Mechanisms in Clinical Isolates of Candida albicans. Antimicrob. Agents Chemother. 2002, 46, 1704–1713. [Google Scholar] [CrossRef]
- Teo, J.Q.; Lee, S.J.-Y.; Tan, A.-L.; Lim, R.S.-M.; Cai, Y.; Lim, T.-P.; Kwa, A.L.H. Molecular mechanisms of azole resistance in Candida bloodstream isolates. BMC Infect. Dis. 2019, 19, 63. [Google Scholar] [CrossRef]
- Li, F.; Palecek, S.P. EAP1, a Candida albicans Gene Involved in Binding Human Epithelial Cells. Eukaryot. Cell 2003, 2, 1266–1273. [Google Scholar] [CrossRef]
- Mitchell, A.P. Dimorphism and virulence in Candida albicans. Curr. Opin. Microbiol. 1998, 1, 687–692. [Google Scholar] [CrossRef]
- Liu, H. Transcriptional control of dimorphism in Candida albicans. Curr. Opin. Microbiol. 2001, 4, 728–735. [Google Scholar] [CrossRef]
- Vaitkienė, S.; Kuliešienė, N.; Sakalauskaitė, S.; Bekere, L.; Krasnova, L.; Vigante, B.; Duburs, G.; Daugelavičius, R. Antifungal activity of styrylpyridinium compounds against Candida albicans. Chem. Biol. Drug Des. 2020. [Google Scholar] [CrossRef] [PubMed]
- Dubur, G.Y.; Dobretsov, G.E.; Deme, A.K.; Dubure, R.R.; Lapshin, E.N.; Spirin, M.M. Fluorescent probes based on styrylpyridinium derivatives: Optical properties and membrane binding. J. Biochem. Biophys. Methods 1984, 10, 123–134. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Inoue, T.; Hirai, T. Local Viscosity Analysis of Triblock Copolymer Micelle with Cyanine Dyes as a Fluorescent Probe. Langmuir 2010, 26, 17505–17512. [Google Scholar] [CrossRef] [PubMed]
- Wyrzykiewicz, E.; Prukała, W.; Kedzia, B. Synthesis and antimicrobial properties of N-substituted derivatives of (E)-alpha-(or gamma)-azastilbenols. Farmaco 1994, 49, 127–131. [Google Scholar] [PubMed]
- Prasad, R.; Gaur, N.A.; Gaur, M.; Komath, S.S. Efflux Pumps in Drug Resistance of Candida. Infect. Disord. Drug Targets 2006, 6, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Franz, R.; Michel, S.; Morschhäuser, J. A fourth gene from the Candida albicans CDR family of ABC transporters. Gene 1998, 220, 91–98. [Google Scholar] [CrossRef]
- Sanglard, D.; Kuchler, K.; Ischer, F.; Pagani, J.L.; Monod, M.; Bille, J. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob. Agents Chemother. 1995, 39, 2378–2386. [Google Scholar] [CrossRef] [PubMed]
- Lohberger, A.; Coste, A.T.; Sanglard, D. Distinct Roles of Candida albicans Drug Resistance Transcription FactorsTAC1, MRR1, andUPC2in Virulence. Eukaryot. Cell 2013, 13, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Coste, A.T.; Crittin, J.; Bauser, C.; Rohde, B.; Sanglard, D. Functional Analysis of cis- and trans-Acting Elements of the Candida albicans CDR2 Promoter with a Novel Promoter Reporter System. Eukaryot. Cell 2009, 8, 1250–1267. [Google Scholar] [CrossRef]
- Schubert, S.; Rogers, P.D.; Morschhäuser, J. Gain-of-Function Mutations in the Transcription Factor MRR1 Are Responsible for Overexpression of the MDR1 Efflux Pump in Fluconazole-Resistant Candida dubliniensis Strains. Antimicrob. Agents Chemother. 2008, 52, 4274–4280. [Google Scholar] [CrossRef]
- Huang, R.; Vider, J.; Serganova, I.; Blasberg, R. ATP-Binding Cassette Transporters Modulate Both Coelenterazine- and D-Luciferin-Based Bioluminescence Imaging. Mol. Imaging 2011, 10, 215–226. [Google Scholar] [CrossRef]
- Fukazawa, Y.; Kagaya, K. Molecular bases of adhesion of Candida albicans. J. Med. Vet. Mycol. 1997, 35, 87–99. [Google Scholar] [CrossRef]
- Sardi, J.C.; Duque, C.; Mariano, F.S.; Marques, M.; Hofling, J.F.; Goncalves, R.B. Adhesion and invasion of Candida albicans from periodontal pockets of patients with chronic periodontitis and diabetes to gingival human fibroblasts. Med. Mycol. 2012, 50, 43–49. [Google Scholar] [CrossRef][Green Version]
- Rodrigues, L.; Ramos, J.; Couto, I.; Amaral, L.; Viveiros, M. Ethidium bromide transport across Mycobacterium smegmatis cell-wall: Correlation with antibiotic resistance. BMC Microbiol. 2011, 11, 35. [Google Scholar] [CrossRef]
- Spengler, G.; Molnár, J.; Viveiros, M.; Amaral, L. Thioridazine induces apoptosis of multidrug-resistant mouse lymphoma cells transfected with the human ABCB1 and inhibits the expression of P-glycoprotein. Anticancer. Res. 2011, 31, 4201–4205. [Google Scholar]
- Shukla, J.N.; Kalsi, M.; Sethi, A.; Narva, K.E.; Fishilevich, E.; Singh, S.; Mogilicherla, K.; Palli, S.R. Reduced stability and intracellular transport of dsRNA contribute to poor RNAi response in lepidopteran insects. RNA Biol. 2016, 13, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.M.A.; Piddock, L.J.V. How to Measure Export via Bacterial Multidrug Resistance Efflux Pumps. mBio 2016, 7, e00840-16. [Google Scholar] [CrossRef] [PubMed]
- Olmsted, J., 3rd; Kearns, D.R. Mechanism of ethidium bromide fluorescence enhancement on binding to nucleic acids. Biochemistry 1977, 16, 3647–3654. [Google Scholar] [CrossRef] [PubMed]
- Niklas, J.; Melnyk, A.; Yuan, Y.; Heinzle, E. Selective permeabilization for the high-throughput measurement of compartmented enzyme activities in mammalian cells. Anal. Biochem. 2011, 416, 218–227. [Google Scholar] [CrossRef]
- Dunkel, N.; Blaß, J.; Rogers, P.D.; Morschhäuser, J. Mutations in the multi-drug resistance regulator MRR1, followed by loss of heterozygosity, are the main cause of MDR1 overexpression in fluconazole-resistant Candida albicans strains. Mol. Microbiol. 2008, 69, 827–840. [Google Scholar] [CrossRef]
- Lyons, C.N.; White, T.C. Transcriptional Analyses of Antifungal Drug Resistance in Candida albicans. Antimicrob. Agents Chemother. 2000, 44, 2296–2303. [Google Scholar] [CrossRef]
- Chen, L.M.; Xu, Y.H.; Zhou, C.L.; Zhao, J.; Li, C.Y.; Wang, R. Overexpression of CDR1 and CDR2 genes plays an important role in fluconazole resistance in Candida albicans with G487T and T916C mutations. J. Int. Med. Res. 2010, 38, 536–545. [Google Scholar] [CrossRef]
- Wang, J.; Yang, D.-H.; Yang, Y.; Wang, J.-Q.; Cai, C.-Y.; Lei, Z.-N.; Teng, Q.-X.; Wu, Z.-X.; Zhao, L.-G.; Chen, Z.-S. Overexpression of ABCB1 Transporter Confers Resistance to mTOR Inhibitor WYE-354 in Cancer Cells. Int. J. Mol. Sci. 2020, 21, 1387. [Google Scholar] [CrossRef]


| CDR1 | 5′-GTACTATCCATCAACCATCAGCACTT-3′ (forward) |
| 5′-GCCGTTCTTCCACCTTTTTGTA-3′ (reverse) | |
| CDR2 | 5′-TGCTGAACCGACAGACTCAGTT-3′ (forward) |
| 5′-AAGAGATTGCCAATTGTCCCATA-3′ (reverse) | |
| MDR1 | 5′-TCAGTCCGATGTCAGAAAATGC-3′ (forward) |
| 5′-GCAGTGGGAATTTGTAGTATGACAA-3′ (reverse) | |
| TAC1 | 5′-GAAATTGTTAATGACGGTTCTACCTTC-3′ (forward) |
| 5′-TATTCATATACCCAACCGGAAATTGG-3′ (reverse) | |
| MRR1 | 5′-AACGCTGGTTATGGGTGA-3′ (forward) |
| 5′-TTTGCTGTTGGGCTTCTT-3′ (reverse) | |
| 18S | 5′-GGATTTACTGAAGACTAACTACTG-3′ (forward) |
| 5′-GAACAACAACCGATCCCTAGT-3′ (reverse) | |
| GAPDH | 5′-GTCTCCTCTGACTTCAACAGCG-3′ (forward) |
| 5′-ACCACCCTGTTGCTGTAGCCAA-3′ (reverse) | |
| ABCB1 | 5′-GTCCCAGGAGCCCATCCT-3′ (forward) |
| 5′-CCCGGCTGTTGTCTCCATA-3′ (reverse) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaitkienė, S.; Bekere, L.; Duburs, G.; Daugelavičius, R. Interaction of Styrylpyridinium Compound with Pathogenic Candida albicans Yeasts and Human Embryonic Kidney HEK-293 Cells. Microorganisms 2021, 9, 48. https://doi.org/10.3390/microorganisms9010048
Vaitkienė S, Bekere L, Duburs G, Daugelavičius R. Interaction of Styrylpyridinium Compound with Pathogenic Candida albicans Yeasts and Human Embryonic Kidney HEK-293 Cells. Microorganisms. 2021; 9(1):48. https://doi.org/10.3390/microorganisms9010048
Chicago/Turabian StyleVaitkienė, Simona, Laura Bekere, Gunars Duburs, and Rimantas Daugelavičius. 2021. "Interaction of Styrylpyridinium Compound with Pathogenic Candida albicans Yeasts and Human Embryonic Kidney HEK-293 Cells" Microorganisms 9, no. 1: 48. https://doi.org/10.3390/microorganisms9010048
APA StyleVaitkienė, S., Bekere, L., Duburs, G., & Daugelavičius, R. (2021). Interaction of Styrylpyridinium Compound with Pathogenic Candida albicans Yeasts and Human Embryonic Kidney HEK-293 Cells. Microorganisms, 9(1), 48. https://doi.org/10.3390/microorganisms9010048





