Development and Validation of a Loop-Mediated Isothermal Amplification (LAMP) Assay for Rapid Detection of Glaesserella (Haemophilus) parasuis
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Cultivation Conditions, Genomic DNA Isolation, and Species Confirmation
2.3. Native Samples and Culture-Based Detection of G. parasuis
2.4. Development of the LAMP Assay
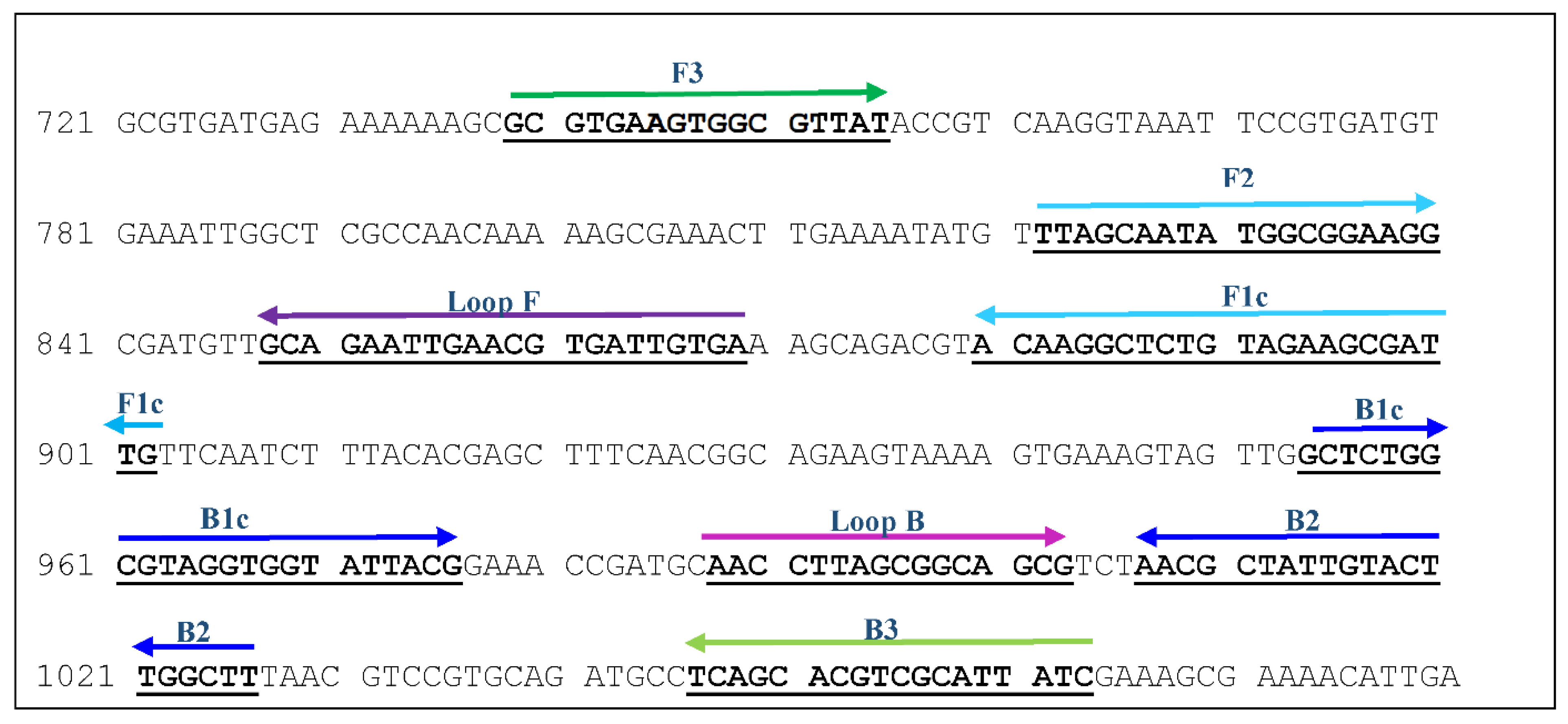
2.4.1. Target Gene and Primer Design
2.4.2. LAMP Reaction Mixture and Amplification
2.5. Alternative LAMP Assay with Colorimetric Detection and Gel Electrophoresis
2.6. Analytical Sensitivity of the LAMP Assay
2.7. Analytical Specificity of the LAMP Assay
2.8. Determination of the Limit of Detection
2.9. Investigation of Spiked Bronchoalveolar Lavage Fluid
3. Results
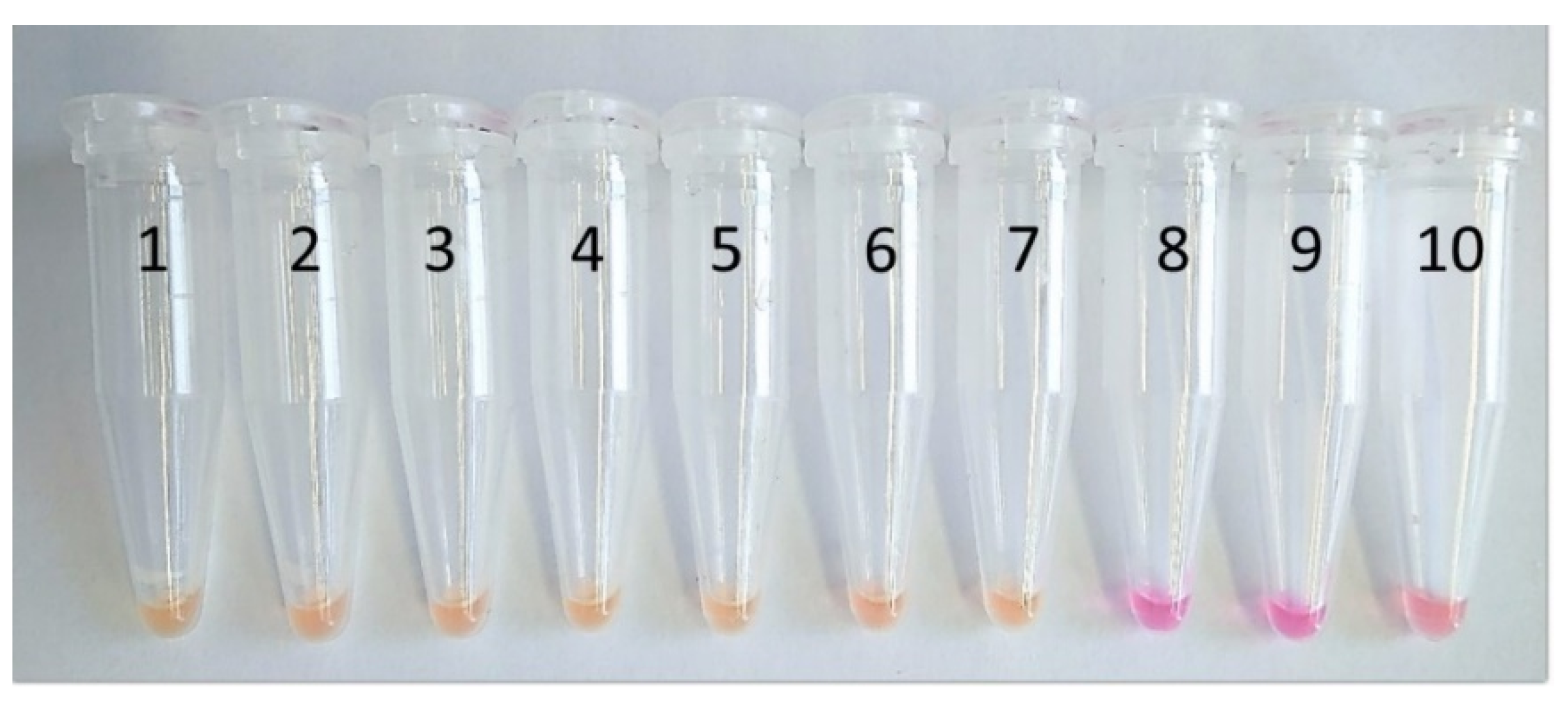
3.1. Assay Optimisation
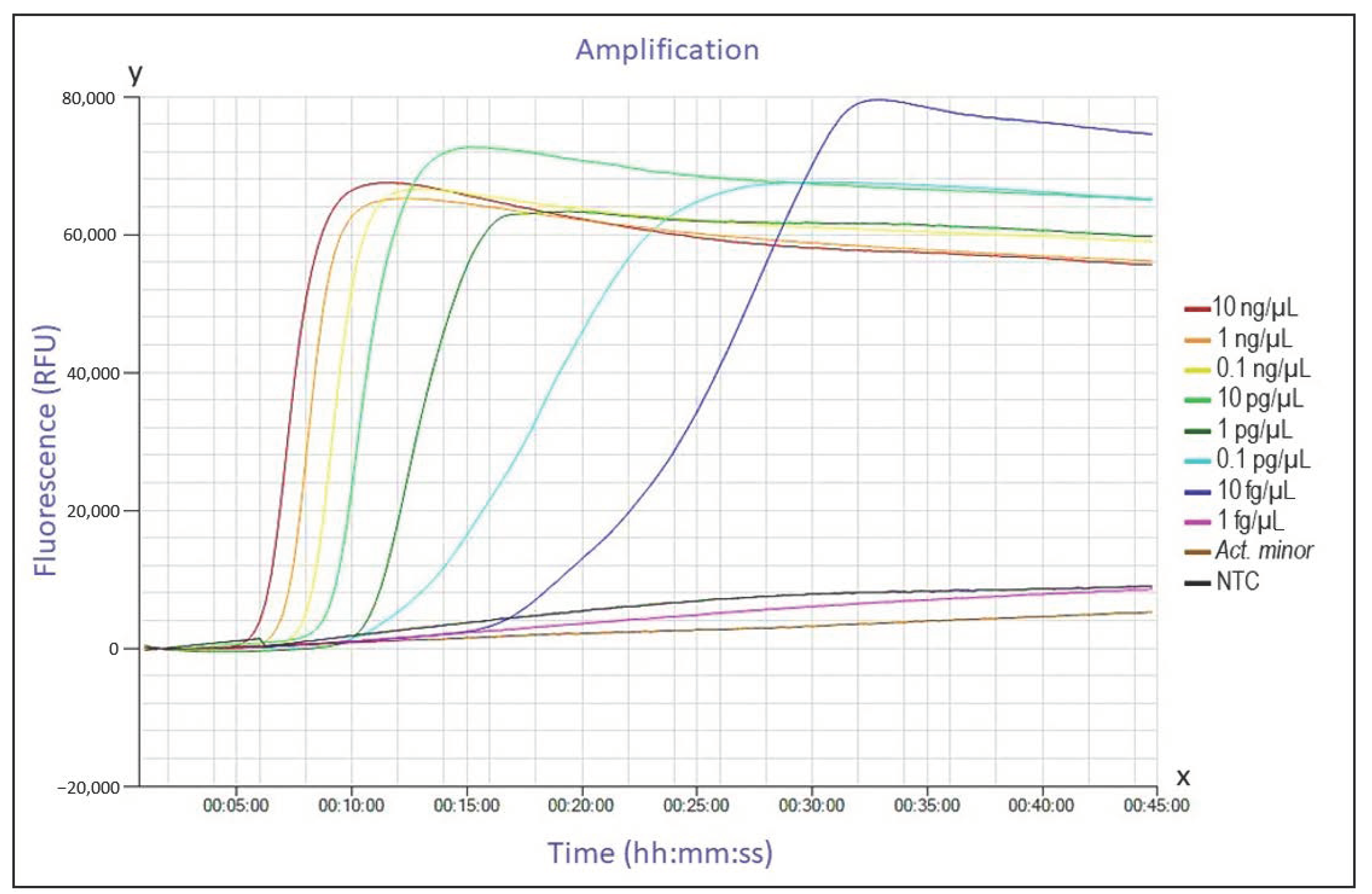
3.2. Analytical Sensitivity
3.3. Analytical Specificity
3.4. Detection of G. parasuis in Spiked Bronchoalveolar Lavage Fluids and Limit of Detection
3.5. Detection of G. parasuis in Clinical Field Samples
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dickerman, A.; Bandara, A.B.; Inzana, T.J. Phylogenomic analysis of Haemophilus parasuis and proposed reclassification to Glaesserella parasuis, gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 2020, 70, 180–186. [Google Scholar] [CrossRef]
- Kirkwood, R.; Rawluk, S.; Cegielski, A.; Otto, A.J. Effect of pig age and autogenous sow vaccination on nasal mucosal colonization of pigs by Haemophilus parasuis. Swine Health Prod. 2001, 9, 77–79. [Google Scholar]
- Liu, S.; Li, W.; Wang, Y.; Gu, C.-Q.; Liu, X.; Charreyre, C.; Fan, S.; He, Q. Coinfection with Haemophilus parasuis serovar 4 increases the virulence of porcine circovirus type 2 in piglets. Virol. J. 2017, 14, 227. [Google Scholar] [CrossRef]
- Nedbalcová, K.; Šatrán, P.; Jaglic, Z.; Ondriasova, R.; Kucerova, Z. Haemophilus parasuis and Glässer’s disease in pigs: A review. Vet. Med. 2006, 51, 168–179. [Google Scholar] [CrossRef]
- Solano-Aguilar, G.I.; Pijoan, C.; Rapp-Gabrielson, V.; Collins, J.; Carvalho, L.F.; Winkelman, N. Protective role of maternal antibodies against Haemophilus parasuis infection. Am. J. Vet. Res. 1999, 60, 81–87. [Google Scholar] [PubMed]
- Little, T.W. Haemophilus infection in pigs. Vet. Rec. 1970, 87, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Peet, R.L.; Fry, J.; Lloyd, J.; Henderson, J.; Curran, J.; Moir, D. Haemophilus parasuis septicaemia in pigs. Aust. Vet. J. 1983, 60, 187. [Google Scholar] [CrossRef] [PubMed]
- Olvera, À.; Cerdag-Cuellar, M.; Mentaberre, G.; Casas-Díaz, E.; Lavín, S.; Marco, I.; Aragon, V. First isolation of Haemophilus parasuis and other NAD-dependent Pasteurellaceae of swine from European wild boars. Vet. Microbiol. 2007, 125, 182–186. [Google Scholar] [CrossRef]
- Reiner, G.; Fresen, C.; Bronnert, S.; Haack, I.; Willems, H. Prevalence of Haemophilus parasuis infection in hunted wild boars (Sus scrofa) in Germany. Eur. J. Wildl. Res. 2010, 56, 815–818. [Google Scholar] [CrossRef]
- Kielstein, P.; Rapp-Gabrielson, V.J. Designation of 15 serovars of Haemophilus parasuis on the basis of immunodiffusion using heat-stable antigen extracts. J. Clin. Microbiol. 1992, 30, 862–865. [Google Scholar] [CrossRef]
- Ma, L.; Wang, L.; Chu, Y.; Li, X.; Cui, Y.; Chen, S.; Zhou, J.; Li, C.; Lu, Z.; Liu, J.; et al. Characterization of Chinese Haemophilus parasuis Isolates by Traditional Serotyping and Molecular Serotyping Methods. PLoS ONE 2016, 11, e0168903. [Google Scholar] [CrossRef] [PubMed]
- Pires Espíndola, J.; Balbinott, N.; Trevisan Gressler, L.; Machado, G.; Silene Klein, C.; Rebelatto, R.; Gutiérrez Martín, C.B.; Kreutz, L.C.; Schryvers, A.B.; Frandoloso, R. Molecular serotyping of clinical strains of Haemophilus (Glaesserella) parasuis brings new insights regarding Glässer’s disease outbreaks in Brazil. PeerJ 2019, 7, e6817. [Google Scholar] [CrossRef] [PubMed]
- Rapp-Gabrielson, V.J.; Gabrielson, D.A.A. Prevalence of Haemophilus parasuis serovars among isolates from swine. Am. J. Vet. Res. 1992, 53, 659–664. [Google Scholar] [PubMed]
- Tadjine, M.; Mittal, K.R.; Bourdon, S.; Gottschalk, M. Development of a New Serological Test for Serotyping Haemophilus parasuis Isolates and Determination of Their Prevalence in North America. J. Clin. Microbiol. 2004, 42, 839–840. [Google Scholar] [CrossRef]
- Luppi, A.; Bonilauri, P.; Dottori, M.; Iodice, G.; Gherpelli, Y.; Merialdi, G.; Maioli, G.; Martelli, P. Haemophilus parasuis Serovars Isolated from Pathological Samples in Northern Italy. Transbound. Emerg. Dis. 2013, 60, 140–142. [Google Scholar] [CrossRef]
- Angen, Ø.; Svensmark, B.; Mittal, K.R. Serological characterization of Danish Haemophilus parasuis isolates. Vet. Microbiol. 2004, 103, 255–258. [Google Scholar] [CrossRef]
- Jia, A.; Zhou, R.; Fan, H.; Yang, K.; Zhang, J.; Xu, Y.; Wang, G.; Liao, M. Development of Serotype-Specific PCR Assays for Typing of Haemophilus parasuis Isolates Circulating in Southern China. J. Clin. Microbiol. 2017, 55, 3249–3257. [Google Scholar] [CrossRef]
- Cai, X.; Chen, H.; Blackall, P.J.; Yin, Z.; Wang, L.; Liu, Z.; Jin, M. Serological characterization of Haemophilus parasuis isolates from China. Vet. Microbiol. 2005, 111, 231–236. [Google Scholar] [CrossRef]
- Lin, W.-H.; Shih, H.-C.; Lin, C.-F.; Yang, C.-Y.; Chang, Y.-F.; Lin, C.-N.; Chiou, M.-T. Molecular serotyping of Haemophilus parasuis isolated from diseased pigs and the relationship between serovars and pathological patterns in Taiwan. PeerJ 2018, 6, e6017. [Google Scholar] [CrossRef]
- Amano, H.; Shibata, M.; Kajio, N.; Morozumi, T. Pathologic Observations of Pigs Intranasally Inoculated with Serovar 1, 4 and 5 of Haemophilus parasuis Using Immunoperoxidase Method. J. Vet. Med. Sci. 1994, 56, 639–644. [Google Scholar] [CrossRef]
- Segalés, J.; Domingo, M.; Solano, G.I.; Pijoan, C. Immunohistochemical Detection of Haemophilus Parasuis Serovar 5 in Formalin-Fixed, Paraffin-Embedded Tissues of Experimentally Infected Swine. J. Vet. Diagn. Investig. 1997, 9, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, M.; Blackall, P.J. Establishment, validation and use of the Kielstein-Rapp-Gabrielson serotyping scheme for Haemophilus parasuis. Aust. Vet. J. 2000, 78, 172–174. [Google Scholar] [CrossRef] [PubMed]
- Angen, Ø.; Oliveira, S.; Ahrens, P.; Svensmark, B.; Leser, T.D. Development of an improved species specific PCR test for detection of Haemophilus parasuis. Vet. Microbiol. 2007, 119, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.; Galina, L.; Pijoan, C. Development of a PCR test to diagnose Haemophilus parasuis infections. J. Vet. Diagn. Investig. 2001, 13, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Howell, K.J.; Weinert, L.A.; Langford, P.; Rycroft, A.N.; Wren, B.W.; Maskell, D.J.; Tucker, A.W.; Peters, S.E.; Wang, J.; Hernandez-Garcia, J.; et al. “Pathotyping” Multiplex PCR Assay for Haemophilus parasuis: A Tool for Prediction of Virulence. J. Clin. Microbiol. 2017, 55, 2617–2628. [Google Scholar] [CrossRef]
- Turni, C.; Pyke, M.; Blackall, P.J. Validation of a real-time PCR for Haemophilus parasuis. J. Appl. Microbiol. 2010, 108, 1323–1331. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchai, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification (LAMP) of DNA. Nucleic Acids Res. 2000, 28, E63. [Google Scholar] [CrossRef]
- Li, Y.; Fan, P.; Zhou, S.; Zhang, L. Loop-mediated isothermal amplification (LAMP): A novel rapid detection platform for pathogens. Microb. Pathog. 2017, 107, 54–61. [Google Scholar] [CrossRef]
- Domesle, K.J.; Yang, Q.; Hammack, T.S.; Ge, B. Validation of a Salmonella loop-mediated isothermal amplification assay in animal food. Int. J. Food Microbiol. 2018, 264, 63–76. [Google Scholar] [CrossRef]
- Da Silva, S.J.R.; Paiva, M.H.S.; Guedes, D.R.D.; Krokovsky, L.; De Melo, F.L.; Da Silva, M.A.L.; Da Silva, A.; Ayres, C.F.J.; Pena, L.J. Development and Validation of Reverse Transcription Loop-Mediated Isothermal Amplification (RT-LAMP) for Rapid Detection of ZIKV in Mosquito Samples from Brazil. Sci. Rep. 2019, 9, 4494. [Google Scholar] [CrossRef]
- Zhang, Y.; Odiwuor, N.; Xiong, J.; Sun, L.; Nyaruaba, R.O.; Wei, H.; Tanner, N.A. Rapid Molecular Detection of SARS-CoV-2 (COVID-19) Virus RNA Using Colorimetric LAMP. medRxiv 2020. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, Y.; Liu, Y.; Chen, P.; Li, W.; Liu, S.; Zou, H.; He, Q. Development and evaluation of loop-mediated isothermal amplification for rapid detection of Haemophilus parasuis. FEMS Microbiol. Lett. 2010, 313, 54–60. [Google Scholar] [CrossRef]
- Chen, H.-T.; Chu, Y.-F.; Liu, Y.-S.; Zhang, J.; Lu, Z. Loop-mediated isothermal amplification for the rapid detection of Haemophilus parasuis. FEMS Immunol. Med. Microbiol. 2010, 60, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-M.; Shen, H.-Y.; Liao, M.; Ren, T.; Guo, L.-L.; Xu, C.-G.; Feng, S.-X.; Fan, H.-Y.; Li, J.-Y.; Chen, J.-D.; et al. Detection of Haemophilus parasuis isolates from South China by loop-mediated isothermal amplification and isolate characterisation. Onderstepoort J. Vet. Res. 2012, 79, 6. [Google Scholar] [CrossRef]
- Prüller, S.; Turni, C.; Blackall, P.J.; Beyerbach, M.; Klein, G.; Kreienbrock, L.; Strutzberg-Minder, K.; Kaspar, H.; Meemken, D.; Kehrenberg, C. Towards a Standardized Method for Broth Microdilution Susceptibility Testing of Haemophilus parasuis. J. Clin. Microbiol. 2017, 55, 264–273. [Google Scholar] [CrossRef]
- Brogden, S.; Pavlović, A.; Tegeler, R.; Kaspar, H.; De Vaan, N.; Kehrenberg, C. Antimicrobial susceptibility of Haemophilus parasuis isolates from Germany by use of a proposed standard method for harmonized testing. Vet. Microbiol. 2018, 217, 32–35. [Google Scholar] [CrossRef]
- Bisping, W.; Amtsberg, G. Colour Atlas for the Diagnosis of Bacterial Pathogens in Animals; Paul Parey Scientific Publishers: Berlin, Germany, 1988; p. 339. [Google Scholar]
- Sange, M.D.; Becker, A.; Hassan, A.A.; Bülte, M.; Ganter, M.; Siebert, U.; Abdulmawjood, A. Development and validation of a loop-mediated isothermal amplification assay—A rapid and sensitive detection tool for Mycobacterium avium subsp. paratuberculosis in small ruminants. J. Appl. Microbiol. 2019, 127, 47–58. [Google Scholar] [CrossRef]
- AOAC. Guidelines for Standard Method Performance Requirements, 20th ed.; AOAC INTERNATIONAL: Rockville, MD, USA, 2016; Appendix F; p. 7. [Google Scholar]
- SAS® Institute Inc. User’s Guide (Release 9.4); SAS: Cary, NC, USA, 2013; Available online: https://support.sas.com (accessed on 13 October 2020).
- Koukos, G.; Papadopoulos, C.; Tsalikis, L.; Sakellari, D.; Arsenakis, M.; Konstantinidis, A. Prevalence of Antibiotic Resistance Genes in Subjects with Successful and Failing Dental Implants. A Pilot Study. Open Dent. J. 2015, 8, 257–263. [Google Scholar] [CrossRef][Green Version]
- Bath, C.; Scott, M.; Sharma, P.M.; Gurung, R.B.; Phuentshok, Y.; Pefanis, S.; Colling, A.; Balasubramanian, N.S.; Firestone, S.M.; Ungvanijban, S.; et al. Further development of a reverse-transcription loop-mediated isothermal amplification (RT-LAMP) assay for the detection of foot-and-mouth disease virus and validation in the field with use of an internal positive control. Transbound. Emerg. Dis. 2020, 67, 2494–2506. [Google Scholar] [CrossRef]
- Best, N.; Rawlin, G.; Suter, R.; Rodoni, B.; Beddoe, T. Optimization of a Loop Mediated Isothermal Amplification (LAMP) Assay for In-Field Detection of Dichelobacter nodosus with aprV2 (VDN LAMP) in Victorian Sheep Flocks. Front. Vet. Sci. 2019, 6, 67. [Google Scholar] [CrossRef]
- De Kok, J.B.; Hendriks, J.C.M.; Van Solinge, W.W.; Willems, H.L.; Mensink, E.J.; Swinkels, D.W. Use of Real-Time Quantitative PCR to Compare DNA Isolation Methods. Clin. Chem. 1998, 44, 2201–2204. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.-Y.; Ooi, C.-H.; Jaimin, J.J.; Lau, Y.-L. Evaluation of WarmStart Colorimetric Loop-Mediated Isothermal Amplification Assay for Diagnosis of Malaria. Am. J. Trop. Med. Hyg. 2020, 102, 1370–1372. [Google Scholar] [CrossRef] [PubMed]
- Verma, G.; Sharma, S.; Raigond, B.; Pathania, S.; Naga, K.; Chakrabarti, S.K. Development and application of fluorescent loop mediated isothermal amplification technique to detect Phytophthora infestans from potato tubers targeting ITS-1 region. 3 Biotech 2019, 9, 345. [Google Scholar] [CrossRef] [PubMed]
- Seinige, D.; Von Köckritz-Blickwede, M.; Krischek, C.; Klein, G.; Kehrenberg, C. Influencing Factors and Applicability of the Viability EMA-qPCR for a Detection and Quantification of Campylobacter Cells from Water Samples. PLoS ONE 2014, 9, e113812. [Google Scholar] [CrossRef]
- Lund, V.; Fonahn, W.; Pettersen, J.E.; Caugant, D.A.; Ask, E.; Nysaeter, Å. Detection of Legionella by cultivation and quantitative real-time polymerase chain reaction in biological waste water treatment plants in Norway. J. Water Health 2014, 12, 543–554. [Google Scholar] [CrossRef]
- Grønbaek, L.M.; Angen, Ø.; Vigre, H.; Olsen, S.N. Evaluation of a nested PCR test and bacterial culture of swabs from the nasal passages and from abscesses in relation to diagnosis of Streptococcus equi infection (strangles). Equine Vet. J. 2006, 38, 59–63. [Google Scholar] [CrossRef]
- Lee, S.; Khoo, V.S.L.; Medriano, C.A.D.; Lee, T.; Park, S.-Y.; Bae, S. Rapid and in-situ detection of fecal indicator bacteria in water using simple DNA extraction and portable loop-mediated isothermal amplification (LAMP) PCR methods. Water Res. 2019, 160, 371–379. [Google Scholar] [CrossRef]

| Species | n | Serovars | Source/Reference |
|---|---|---|---|
| Glaesserella parasuis DSM 21448T type strain | 1 | ND | Leibniz-Institute DSMZ |
| Glaesserella parasuis field isolates | 29 | 1,2,3,4,5,6,8,9,10,11,12,13,14,15 | IVD GmbH; Institute collection [35,36] |
| Glaesserella parasuis field isolates | 30 | ND | |
| Actinobacillus minor CCUG 38923T type strain | 1 | Culture Collection University of Gothenburg | |
| Actinobacillus indolicus CCUG 39029T type strain | 1 | Culture Collection University of Gothenburg | |
| Actinobacillus porcinus CCUG 38924T type strain | 1 | Culture Collection University of Gothenburg | |
| Actinobacillus arthritis CCUG 24862T type strain | 1 | Culture Collection University of Gothenburg | |
| Actinobacillus pleuropneumoniae field isolates | 26 | Institute collection | |
| Mannheimia haemolytica field isolates | 11 | Institute collection | |
| Pasteurella multocida field isolates | 20 | Institute collection | |
| Bordetella bronchiseptica field isolates | 2 | Institute collection |
| Primer | Sequence | Positions in infB (Accession No. DQ410886) | Length (bp) |
|---|---|---|---|
| F3 | GCGTGAAGTGGCGTTAT | 739 | 17 |
| B3 | GATAATGCGACGTGCTGA | 1063 | 18 |
| FIP (F1c+F2) | CAATCGCTTCTACAGAGCCTTGT- TTAGCAATATGGCGGAAGG | 902, 822 | 23, 19 |
| BIP (B1c+B2) | GCTCTGGCGTAGGTGGTATTAC- AAGGCAAGTACAATAGCGTT | 954, 1026 | 22, 20 |
| Loop F | TCACAATCACGTTCAATTCTGC | 869 | 22 |
| Loop B | AACCTTAGCGGCAGCG | 988 | 16 |
| Primers | Standard Concentration of the Primer Mix | Concentrated Primer Mix |
|---|---|---|
| F3, B3 | 0.2 µM | 0.2 µM |
| FIP, BIP | 0.8 µM | 2 µM |
| Loop F, loop B | 0.4 µM | 1 µM |
| c (std. PM) | 10 ng/µL | 1 ng/µL | 0.1 ng/µL | 10 pg/µL | 1 pg/µL | 0.1 pg/µL | 10 fg/µL | 1 fg/µL | NTC |
| M Dt (mm:ss) | 10:25 | 11:30 | 12:55 | 14:40 | 16:55 | 19:50 | - | - | - |
| SD Dt (mm:ss) | 00:09 | 00:26 | 00:43 | 00:57 | 00:57 | 02:51 | - | - | - |
| M At (°C) | 85.83 | 85.83 | 85.97 | 85.93 | 85.90 | 85.97 | - | - | - |
| c (conc. PM) | 10 ng/µL | 1 ng/µL | 0.1 ng/µL | 10 pg/µL | 1 pg/µL | 0.1 pg/µL | 10 fg/µL | 1 fg/µL | NTC |
| M Dt (mm:ss) | 06:50 | 07:50 | 08:50 | 10:05 | 12:05 | 16:25 | 27:50 | - | - |
| SD Dt (mm:ss) | 00:09 | 00:09 | 00:09 | 00:09 | 00:09 | 01:40 | 11:25 | - | - |
| M At (°C) | 85.47 | 85.47 | 85.53 | 85.57 | 85.47 | 85.50 | 85.60 | - | - |
| DNA Isolation with KIT | HEAT-Extracted DNA | Results Summarized | ||||||
|---|---|---|---|---|---|---|---|---|
| Undiluted | Diluted 1:10 | Undiluted | Diluted 1:10 | |||||
| No. | Organ | MI | Dt (mm:ss) | Dt (mm:ss) | Dt (mm:ss) | Dt (mm:ss) | LAMP | PCR |
| 1 | pericardium | - | - | - | - | - | - | - |
| 2 | tonsils | - | - | - | - | - | - | - |
| 3 | tonsils | - | - | - | - | - | - | - |
| 4 | tonsils | - | - | - | - | - | - | - |
| 5 | tonsils | - | - | - | - | - | - | - |
| 6 | pericardium | - | 23:15 | - | - | - | +/- | - |
| 7 | pericardium | - | 27:00 | - | 38:00 | 28:15 | + | + |
| 8 | pericardium | - | - | - | - | - | - | - |
| 9 | pericardium | - | - | - | - | - | - | - |
| 10 | pericardium | - | - | - | - | - | - | - |
| 11 | pericardium | - | 13:15 | 12:15 | 42:45 | 27:15 | + | + |
| 12 | bronchi | + | 14:30 | 15:15 | 35:45 | 13:30 | + | + |
| 13 | bronchi | + | 18:15 | 12:30 | 41:30 | 16:45 | + | + |
| 14 | bronchi | + | 13:30 | 13:15 | 35:45 | 12:15 | + | + |
| 15 | bronchi | + | 18:15 | - | 19:45 | 20:45 | + | + |
| 16 | joint | + | 14:15 | 10:45 | 41:45 | 23:00 | + | + |
| 17 | serous membrane | - | 14:15 | 14:30 | 44:00 | 28:00 | + | + |
| 18 | brain | - | - | - | - | - | - | - |
| 19 | pericardium | + | 19:45 | 14:00 | 24:45 | 14:45 | + | + |
| 21 | bronchi | + | - | - | - | - | - | - |
| 22 | serous membrane | + | 11:00 | 12:00 | 13:00 | 14:30 | + | + |
| 23 | lung | + | - | - | - | - | - | - |
| 24 | pericardium | + | 11:30 | 12:45 | 21:15 | 27:30 | + | + |
| 25 | pericardium | + | 14:00 | 15:45 | - | - | +/- | + |
| 26 | pericardium | - | - | ND | ND | ND | - | - |
| 27 | bronchi | - | - | ND | ND | ND | - | - |
| 28 | serous membrane | - | - | ND | ND | ND | - | - |
| 29 | pericardium | - | - | ND | ND | ND | - | - |
| 30 | pericardium | - | - | ND | ND | ND | - | - |
| 31 | pericardium | - | 18:15 | ND | ND | ND | - | - |
| 32 | pericardium | - | - | ND | ND | ND | - | - |
| 33 | serous membrane | - | - | ND | ND | ND | - | - |
| 34 | pericardium | - | 38:15 | ND | ND | ND | - | - |
| 35 | pericardium | - | - | ND | ND | ND | - | - |
| 36 | pericardium | - | - | ND | ND | ND | - | - |
| Detection Method Used | Agreement of Results in % | 95% Confidence Intervals | ||
|---|---|---|---|---|
| Variable 1 | Variable 2 | Lower | Upper | |
| Culture-based detection | LAMP kit-extracted DNA, undiluted | 77.1 | 59.9 | 89.6 |
| Culture-based detection | LAMP, heat-extracted DNA, undiluted | 75.0 | 53.3 | 90.2 |
| Conventional PCR | LAMP kit-extracted DNA, undiluted | 91.4 | 75.9 | 98.2 |
| Conventional PCR | LAMP, heat-extracted DNA, undiluted | 95.8 | 78.9 | 99.9 |
| LAMP kit-extracted DNA, undiluted | LAMP kit-extracted DNA, diluted 1:10 | 87.5 | 67.6 | 97.3 |
| LAMP, heat-extracted DNA, undiluted | LAMP kit-extracted DNA, diluted 1:10 | 100 | 85.8 | 100 |
| LAMP kit-extracted DNA, undiluted | LAMP, heat-extracted DNA, undiluted | 91.7 | 73.0 | 99.0 |
| Culture-based detection | Conventional PCR | 85.7 | 69.7 | 95.2 |
| Parameter | Availability for This Assay |
|---|---|
| Primer concentration | Yes |
| Amplification temperature | Yes |
| Inclusion of the fifth and sixth (loop) primers | Yes |
| Threshold | Yes |
| Real-time | Yes |
| Inclusion of closely related species in the determination of analytical specificity | Actinobacillus minor, Actinobacillus indolicus, Actinobacillus porcinus, Actinobacillus arthritis (among others) |
| Analytical sensitivity | 10 fg/µL DNA |
| Analytical specificity | Inclusivity and exclusivity of 100% |
| Comparison of target genes | Yes |
| Comparison of DNA extraction methods | Yes |
| Detection in clinical field samples | Yes |
| Transportability to field | Yes |
| Reproducibility of the assay | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pilchová, V.; Seinige, D.; Hennig-Pauka, I.; Büttner, K.; Abdulmawjood, A.; Kehrenberg, C. Development and Validation of a Loop-Mediated Isothermal Amplification (LAMP) Assay for Rapid Detection of Glaesserella (Haemophilus) parasuis. Microorganisms 2021, 9, 41. https://doi.org/10.3390/microorganisms9010041
Pilchová V, Seinige D, Hennig-Pauka I, Büttner K, Abdulmawjood A, Kehrenberg C. Development and Validation of a Loop-Mediated Isothermal Amplification (LAMP) Assay for Rapid Detection of Glaesserella (Haemophilus) parasuis. Microorganisms. 2021; 9(1):41. https://doi.org/10.3390/microorganisms9010041
Chicago/Turabian StylePilchová, Veronika, Diana Seinige, Isabel Hennig-Pauka, Kathrin Büttner, Amir Abdulmawjood, and Corinna Kehrenberg. 2021. "Development and Validation of a Loop-Mediated Isothermal Amplification (LAMP) Assay for Rapid Detection of Glaesserella (Haemophilus) parasuis" Microorganisms 9, no. 1: 41. https://doi.org/10.3390/microorganisms9010041
APA StylePilchová, V., Seinige, D., Hennig-Pauka, I., Büttner, K., Abdulmawjood, A., & Kehrenberg, C. (2021). Development and Validation of a Loop-Mediated Isothermal Amplification (LAMP) Assay for Rapid Detection of Glaesserella (Haemophilus) parasuis. Microorganisms, 9(1), 41. https://doi.org/10.3390/microorganisms9010041





