Identification of a blaVIM-1-Carrying IncA/C2 Multiresistance Plasmid in an Escherichia coli Isolate Recovered from the German Food Chain
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolate Origin and Antimicrobial Susceptibility Testing
2.2. Phenotypic and Genotypic Characterization
2.3. Farm Investigation
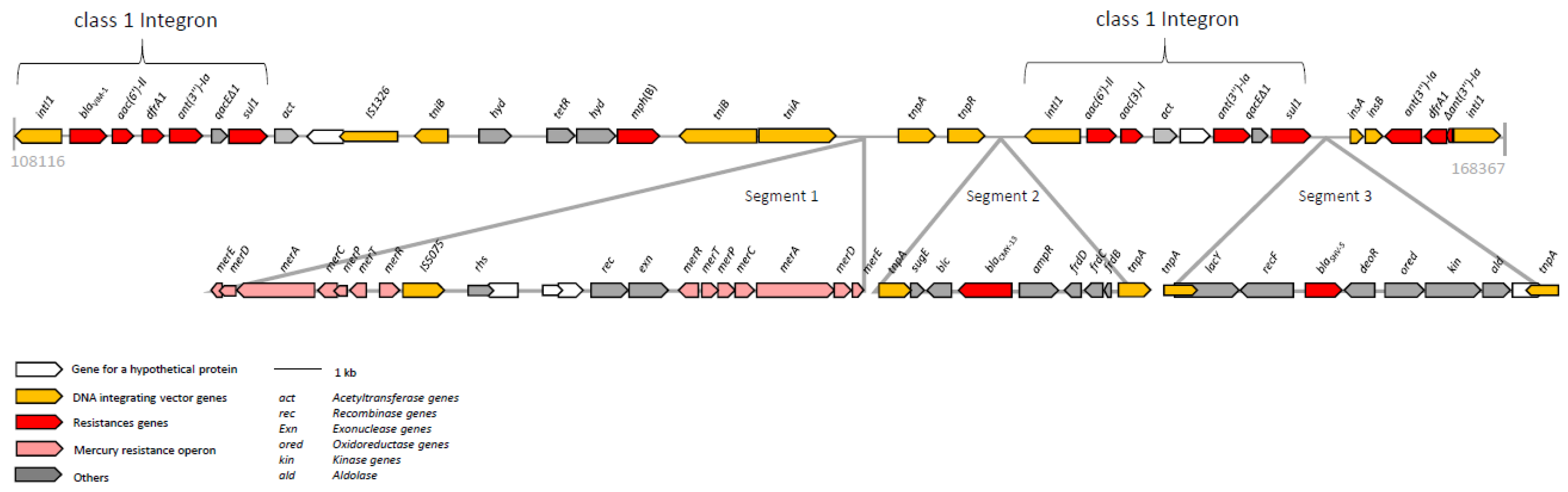
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO; Advisory Group on Integrated Surveillance of Antimicrobial Resistance (AGISAR). Critically Important Antimicrobials for Human Medicine: Ranking of Antimicrobial Agents for Risk Management of Antimicrobial Resistance due to Non-Human Use, 5th ed.; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Köck, R.; Daniels-Haardt, I.; Becker, K.; Mellmann, A.; Friedrich, A.W.; Mevius, D.; Schwarz, S.; Jurke, A. carbapenem-resistant enterobacteriaceae in wildlife, food-producing, and companion animals: A systematic review. Clin. Microbiol. Infect. 2018, 24, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Salahuddin, P.; Kumar, A.; Kha, A.U. Structure, function of serine and Metallo-β-lactamases and their Inhibitors. Curr. Protein. Pept. Sci. 2018, 19, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.; Fisher, J.F. Epidemiological expansion, structural studies, and clinical challenges of new β-lactamases from gram-negative bacteria. Annu. Rev. Microbiol. 2011, 65, 455–478. [Google Scholar] [CrossRef]
- Partridge, S.R.; Kwong, S.M.; Firth, N.; Jensen, S.O. Mobile genetic elements associated with antimicrobial resistance. Clin. Microbial. Rev. 2018, 31, e00088-17. [Google Scholar] [CrossRef] [PubMed]
- Potter, R.F.; D′Souza, A.W.; Dantas, G. The rapid spread of carbapenem-resistant enterobacteriaceae. Drug Resist. Updat. 2016, 29, 30–46. [Google Scholar] [CrossRef]
- Irrgang, A.; Fischer, J.; Grobbel, M.; Schmoger, S.; Skladnikiewicz-Ziemer, T.; Thomas, K.; Hensel, A.; Tenhagen, B.-A.; Kasbohrer, A.; Käsbohrer, A. Recurrent detection of VIM-1-producing Escherichia coli clone in German pig production. J. Antimicrob. Chemother. 2017, 72, 944–946. [Google Scholar]
- Borowiak, M.; Szabo, I.; Baumann, B.; Junker, E.; Hammerl, J.A.; Kaesbohrer, A.; Malorny, B.; Fischer, J. VIM-1-producing Salmonella infantis isolated from swine and minced pork meat in Germany. J. Antimicrob. Chemother. 2017, 72, 2131–2133. [Google Scholar] [CrossRef]
- Fischer, J.; Rodriguez, I.; Schmoger, S.; Friese, A.; Roesler, U.; Helmuth, R.; Guerra, B. Salmonella enterica subsp. enterica producing VIM-1 carbapenemase isolated from livestock farms. J. Antimicrob. Chemother. 2013, 68, 478–480. [Google Scholar] [CrossRef]
- Fischer, J.; San Jose, M.; Roschanski, N.; Schmoger, S.; Baumann, B.; Irrgang, A.; Friese, A.; Roesler, U.; Helmuth, R.; Guerra, B. Spread and persistence of VIM-1 carbapenemase-producing enterobacteriaceae in three German swine farms in 2011 and 2012. Vet. Microbiol. 2017, 200, 118–123. [Google Scholar] [CrossRef]
- Irrgang, A.; Tenhagen, B.-A.; Pauly, N.; Schmoger, S.; Kaesbohrer, A.; Hammerl, J.A. Characterization of VIM-1-producing, E. coli isolated from a German fattening pig farm by an improved isolation procedure. Front. Microbiol. 2019, 10, 2256. [Google Scholar] [CrossRef]
- Pauly, N.; Hammerl, J.A.; Schwarz, S.; Grobbel, M.; Meemken, D.; Malorny, B.; Tenhagen, B.-A.; Käsbohrer, A.; Irrgang, A. Co-occurrence of the blaVIM-1 and blaSHV-12 genes on an IncHI2 plasmid of an Escherichia coli isolate recovered from German livestock. J. Antimicrob. Chemother. 2020. [Google Scholar] [CrossRef] [PubMed]
- Roschanski, N.; Guenther, S.; Vu, T.T.T.; Fischer, J.; Semmler, T.; Huehn, S.; Alter, T.; Roesler, U. VIM-1 carbapenemase-producing Escherichia coli isolated from retail seafood, Germany 2016. Eurosurveillance 2017, 22, 17-00032. [Google Scholar] [CrossRef] [PubMed]
- Irrgang, A.; Pauly, N.; Tenhagen, B.-A.; Grobbel, M.; Kaesbohrer, A.; Hammerl, A.J. A spill-over from public health? First detection of an OXA-48-producing Escherichia coli in a German pig farm. Microorganisms 2020, 8, 855. [Google Scholar] [CrossRef] [PubMed]
- Irrgang, A.; Tausch, S.H.; Pauly, N.; Grobbel, M.; Kaesbohrer, A.; Hammerl, J.A. First detection of GES-5-producing escherichia coli from livestock-an increasing diversity of carbapenemases recognized from german pig production. Microorganisms 2020, 8, 1593. [Google Scholar] [CrossRef]
- Garcia-Graells, C.; Berbers, B.; Verhaegen, B.; Vanneste, K.; Marchal, K.; Roosens, N.H.C.; Botteldoorn, N.; De Keersmaecker, S.C.J. First detection of a plasmid located carbapenem resistant bla(VIM-1) gene in E. coli isolated from meat products at retail in Belgium in 2015. Int. J. Food Microbiol. 2020, 324, 108624. [Google Scholar] [CrossRef]
- Guerra, B.; Junker, E.; Miko, A.; Helmuth, R.; Mendoza, M.C. Characterization and localization of drug resistance determinants in multidrug-resistant, integron-carrying Salmonella enterica serotype typhimurium strains. Microb. Drug Resist. 2004, 10, 83–91. [Google Scholar] [CrossRef]
- Rodriguez, I.; Barownick, W.; Helmuth, R.; Mendoza, M.C.; Rodicio, M.R.; Schroeter, A.; Guerra, B. Extended-spectrum {beta}-lactamases and AmpC {beta}-lactamases in ceftiofur-resistant Salmonella enterica isolates from food and livestock obtained in Germany during 2003-07. J. Antimicrob. Chemother. 2009, 64, 301–309. [Google Scholar] [CrossRef]
- Hadziabdic, S.; Fischer, J.; Borowiak, M.; Malorny, B.; Juraschek, K.; Kaesbohrer, A.; Guerra, B.; Deneke, C.; Gonzalez-Zorn, B.; Szabo, I. The blaNDM-1-carrying IncA/C2 plasmid underlies structural alterations and cointegrate formation in vivo. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef]
- Harmer, C.J.; Hall, R.M. The A to Z of A/C plasmids. Plasmid 2015, 80, 63–82. [Google Scholar] [CrossRef]
- Johnson, J.R.; Kuskowski, M.A.; Owens, K.; Gajewski, A.; Winokur, P.L. Phylogenetic origin and virulence genotype in relation to resistance to fluoroquinolones and/or extended-spectrum cephalosporins and cephamycins among Escherichia coli isolates from animals and humans. J. Infect. Dis. 2003, 188, 759–768. [Google Scholar] [CrossRef]
- Antonelli, A.; D’Andrea, M.M.; Montagnani, C.; Bartolesi, A.M.; Di Pilato, V.; Fiorini, P.; Torricelli, F.; Galli, L.; Rossolini, G.M. Newborn bacteraemia caused by an aeromonas caviae producing the VIM-1 and SHV-12 beta-lactamases, encoded by a transferable plasmid. J. Antimicrob. Chemother. 2016, 71, 272–274. [Google Scholar] [CrossRef] [PubMed]
- Drieux, L.; Decré, D.; Frangeul, L.; Arlet, G.; Jarlier, V.; Sougakoff, W. Complete nucleotide sequence of the large conjugative pTC2 multireplicon plasmid encoding the VIM-1 metallo-β-lactamase. J. Antimicrob. Chemother. 2013, 68, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Kohler, P.; Tijet, N.; Kim, H.C.; Johnstone, J.; Edge, T.; Patel, S.N.; Seah, C.; Willey, B.; Coleman, B.; Green, K.; et al. The Toronto invasive bacterial diseases network. Dissemination of Verona integron-encoded Metallo-β-lactamase among clinical and environmental enterobacteriaceae isolates in Ontario, Canada. Sci. Rep. 2020, 10, 18580. [Google Scholar] [CrossRef] [PubMed]
- Osborn, A.M.; Bruce, K.D.; Strike, P.; Ritchie, D.A. Distribution, diversity and evolution of the bacterial mercury resistance (mer) operon. FEMS Microbiol. Rev. 1997, 19, 239–262. [Google Scholar] [CrossRef]
- Miriagou, V.; Tzouvelekis, L.S.; Villa, L.; Lebessi, E.; Vatopoulos, A.C.; Carattoli, A.; Tzelepi, E. CMY-13, a novel inducible cephalosporinase encoded by an Escherichia coli plasmid. Antimicrob. Agents Chemother. 2004, 48, 3172–3174. [Google Scholar] [CrossRef]
- Liakopoulos, A.; Mevius, D.; Ceccarelli, D. A review of SHV extended-spectrum β-lactamases: Neglected yet ubiquitous. Front. Microbial. 2016, 7, 1374. [Google Scholar] [CrossRef]
| Isolate | AMP | AZI | CHL | CIP | COL | ERP | FEP | FOT | FOX | GEN | IMI | MERO | NAL | SMX | TAZ | TET | TGC | TMP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 19-AB01133 | >64 | 16 | 16 | >8 | ≤1 | >2 | >32 | >64 | >64 | 32 | 8 | 8 | >128 | >1024 | >8 | 4 | ≤0.25 | >32 |
| TK_19-AB01133 | >64 | 8 | 8 | ≤0.015 | ≤1 | >2 | >32 | >64 | >64 | 4 | 8 | 4 | ≤4 | >1024 | >8 | 4 | ≤0.25 | >32 |
| K12 J53 | 4 | 8 | ≤8 | ≤0.015 | ≤1 | ≤0.015 | ≤0.06 | ≤0.25 | 4 | 2 | 0.25 | ≤0.03 | ≤4 | ≤8 | ≤0.5 | 4 | 0.5 | ≤0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pauly, N.; Hammerl, J.A.; Grobbel, M.; Käsbohrer, A.; Tenhagen, B.-A.; Malorny, B.; Schwarz, S.; Meemken, D.; Irrgang, A. Identification of a blaVIM-1-Carrying IncA/C2 Multiresistance Plasmid in an Escherichia coli Isolate Recovered from the German Food Chain. Microorganisms 2021, 9, 29. https://doi.org/10.3390/microorganisms9010029
Pauly N, Hammerl JA, Grobbel M, Käsbohrer A, Tenhagen B-A, Malorny B, Schwarz S, Meemken D, Irrgang A. Identification of a blaVIM-1-Carrying IncA/C2 Multiresistance Plasmid in an Escherichia coli Isolate Recovered from the German Food Chain. Microorganisms. 2021; 9(1):29. https://doi.org/10.3390/microorganisms9010029
Chicago/Turabian StylePauly, Natalie, Jens Andre Hammerl, Mirjam Grobbel, Annemarie Käsbohrer, Bernd-Alois Tenhagen, Burkhard Malorny, Stefan Schwarz, Diana Meemken, and Alexandra Irrgang. 2021. "Identification of a blaVIM-1-Carrying IncA/C2 Multiresistance Plasmid in an Escherichia coli Isolate Recovered from the German Food Chain" Microorganisms 9, no. 1: 29. https://doi.org/10.3390/microorganisms9010029
APA StylePauly, N., Hammerl, J. A., Grobbel, M., Käsbohrer, A., Tenhagen, B.-A., Malorny, B., Schwarz, S., Meemken, D., & Irrgang, A. (2021). Identification of a blaVIM-1-Carrying IncA/C2 Multiresistance Plasmid in an Escherichia coli Isolate Recovered from the German Food Chain. Microorganisms, 9(1), 29. https://doi.org/10.3390/microorganisms9010029






