Abstract
The second largest sink for atmospheric methane (CH4) is atmospheric methane oxidizing-bacteria (atmMOB). How atmMOB are able to sustain life on the low CH4 concentrations in air is unknown. Here, we show that during growth, with air as its only source for energy and carbon, the recently isolated atmospheric methane-oxidizer Methylocapsa gorgona MG08 (USCα) oxidizes three atmospheric energy sources: CH4, carbon monoxide (CO), and hydrogen (H2) to support growth. The cell-specific CH4 oxidation rate of M. gorgona MG08 was estimated at ~0.7 × 10−18 mol cell−1 h−1, which, together with the oxidation of CO and H2, supplies 0.38 kJ Cmol−1 h−1 during growth in air. This is seven times lower than previously assumed necessary to support bacterial maintenance. We conclude that atmospheric methane-oxidation is supported by a metabolic flexibility that enables the simultaneous harvest of CH4, H2 and CO from air, but the key characteristic of atmospheric CH4 oxidizing bacteria might be very low energy requirements.
1. Introduction
Atmospheric methane-oxidizing bacteria (atmMOB) remove 9–47 Tg methane (CH4) from the atmosphere annually [1]. The most common hypothesis for explaining how these bacteria can sustain life by oxidizing atmospheric CH4 is the “high affinity” model, in which a special form of particulate CH4 monooxygenase allows atmMOB to oxidize the low atmospheric CH4 concentrations at a high rate [2]. Other hypotheses include the utilization of alternative energy sources or a high specific affinity (Vmax(app)/Km(app)) [3,4]. AtmMOB are found globally in soils [4] and belong to the two phylogenetic clusters USCα (Alphaproteobacteria) and USCγ (Gammaproteobacteria) [5]. The lack of pure cultures from USCα and USCγ has prevented studies of the energy metabolism of atmMOB [3]. Conventional methane oxidizing bacteria (MOB) are expected to oxidize around 0.2–17 × 10−18 mol CH4 cell−1 h−1 at atmospheric CH4 concentrations at 25 °C, but such rates have been considered too low to provide sufficient energy for growth [6]. Thus, atmMOB were expected to either utilize alternative energy sources or have high affinities for CH4 [3]. The latter is supported by observations reporting apparent high affinity CH4 oxidation by microbial communities in oxic soils [7]. Additionally, oxidation rates of 800 × 10−18 mol CH4 cell−1 h−1 were measured in forest soils, far surpassing the cell-specific activity of 40 × 10−18 mol CH4 cell−1 h−1 assumed to be necessary to support minimum cell maintenance requirements [6,8]. At 20 °C, these minimum requirements would demand an energy supply of 2.8 kJ Cmol h−1. It is assumed that the high forest soil rates at atmospheric CH4 concentrations are achieved by the combination of methane monooxygenases (MMO) with very low half-saturation constants (Km) and high cellular abundances of these enzymes.
The recently described USCα atmMOB species Methylocapsa gorgona MG08 has the highest specific affinity (Vmax(app)/Km(app)) for CH4 of all tested MOB at 195 × 10−12 L⋅cell−1⋅h−1. However, this translates into a cell-specific CH4 oxidation rate of only 10 × 10−18 mol CH4 cell−1 h−1 at atmospheric CH4 concentration [4], seemingly too low to support cellular maintenance [6]. Nevertheless, M. gorgona MG08 is able to grow at atmospheric (1.87 p.p.m.v.) CH4 concentrations [4]. It also carries a [NiFe] group 1 h high-affinity respiratory hydrogenase (hhyL and hhyS) and a [MoCu] class I respiratory carbon monoxide dehydrogenase, similar to those identified in atmospheric carbon monoxide (CO) and hydrogen (H2)-oxidizing microorganisms [4,9,10,11,12]. Recent studies have identified the utilization of atmospheric CO and H2 as energy sources for growth and survival in bacteria [9,11,12,13], and as support for bacterial primary production in Antarctic and Arctic environments [10,14]. Atmospheric CH4 concentrations are currently 1.87 p.p.m.v. [15], while the concentrations of atmospheric CO and H2 are lower. CO concentrations vary, with estimates for uninhabited areas around 0.1 p.p.m.v., while urban areas contain higher concentrations, often above 0.2 p.p.m.v. [16,17,18,19]. Atmospheric H2 concentrations are stable at approximately 0.53 p.p.m.v. [20]. Many MOB carry genes for carbon monoxide and hydrogen oxidation [4], and recently it has been shown that the thermoacidophilic Methylacidiphilum fumariolicum SolV can oxidize sub-atmospheric H2 with a high-affinity, membrane-associated [NiFe] hydrogenase [21], while the strain Methylocystis sp. SC2 oxidizes hydrogen at higher concentrations [22]. However, the oxidation of atmospheric CO and H2 to support growth has never been demonstrated for M. gorgona MG08 or any other MOB. We have studied how the atmospheric CH4 oxidizer M. gorgona MG08, in pure culture, harvests energy from the atmosphere for growth.
2. Materials and Methods
2.1. Cultivation
A young stationary culture of M. gorgona MG08 was prepared as follows. A 20 mL culture (1:10 (NMS:MilliQ) in diluted liquid nitrate mineral salts (NMS) medium (pH 6.8) (DSMZ medium 921) without EDTA) with a headspace of 20% CH4 in air (20 mL 100% CH4 mixed with 80 mL of air) was incubated for 12 days, reaching several days (2–4) into the stationary phase. Inoculum from this culture was precultured in 1:10 (NMS:MilliQ) diluted liquid nitrate mineral salts (NMS) medium (pH 6.8) (DSMZ medium 921) without EDTA under a headspace atmosphere of ~20 p.p.m.v. CH4 in air for two weeks. The headspace was created by injecting 20 µL of 100% CH4 into the 100 mL ambient air headspace of 120 mL serum bottles containing 20 mL medium, sealed with a butyl rubber stopper and crimp cap. This was used as a start culture for colony growth on filters. The cell concentration in the suspension was determined by fluorescence microscopy after filtration on Anodisc filters (Whatman 6809-6022, Merck, Darmstadt, Germany) and 1000× SYBRgreen I (Molecular probes S-7567, ThermoFisher, Waltham, MA, USA) staining as previously described [4]. For staining, filters were transferred (bacteria side up) on top of 200 µL droplets of 1000× SYBRgreen and incubated for 10 minutes. In the next step, filters were washed twice by transferring them onto 1 mL milliQ water and then air-dried. The whole procedure was performed at room temperature and in the dark. A fresh-made anti-fading solution consisting of 0.1% p-phenylenediamine dihydrochloride in 1:1 glycerol and PBS (phosphate buffered saline pH 7.2) was used for mounting the filters on slides with cover slips.
From the start cultures, the required volume and cell density needed to achieve a cell density of ~20 cells per 63× photo area, for a total of ~1 × 106 cells per filter, was selected for filter cultivation. For the transfer of cells onto the 47 mm polycarbonate filter, an autoclaved Millipore vacuum filter holder system (cat no XX1004700, Merck, Darmstadt, Germany) with 300 mL glass funnels was used. Firstly, a GF/C filter (Whatman 1822-047, Merck, Darmstadt, Germany) was added as support filter. A polycarbonate filter (Whatman, Nucleopore 111106, Merck, Darmstadt, Germany) was then added and the funnel was clamped on top. Even distributions of cells on the filters were obtained by pouring 100 mL of autoclaved milliQ-water into the funnel before mixing in 0.1 mL of the start culture cell suspension and applying vacuum. The funnel walls were rinsed twice with 15 mL sterile water during suction. The polycarbonate filters were then transferred into 250 mL bottles (DURAN, Merck, Darmstadt, Germany) in a Holten LaminAir bench and left floating on 50 mL of 1:10 (NMS:MilliQ) diluted liquid NMS medium (pH 6.8) without EDTA. Finally, the bottles were covered with gas permeable parafilm (Merck, Darmstadt, Germany). These bottles were incubated in a ventilated room at 20 °C in darkness for nine months (experiment 1) or 4.5 months (experiment 2), after which the gas uptake experiments were initiated. In Experiment 1, an additional inspection of a filter was performed after eight months. This included a visual inspection and counting of 60 colonies on two different filters to show that the number of generations in almost all growing colonies had surpassed seven (64 cells), indicating that the filters had sufficient biomass to initiate gas uptake experiments.
2.2. Temperature Selection
All cultivations and experiments were carried out at 20 °C. This temperature was chosen as it is in the middle of the optimum temperature range for growth (~15–~27 °C) of M. gorgona MG08 and was the temperature at which previous experiments were carried out [4].
2.3. Gas Uptake and Leakage Experiments
Three experiments were carried out: two gas uptake experiments that included controls and one control experiment. Experiment 1 consisted of 53 × 120 mL bottles, some containing cultures of M. gorgona MG08 on filters floating on liquid media. Experiment 2 consisted of 15 bottles. The control experiment consisted of 18 bottles.
In all experiments, the bottles contained 50 mL of 1/10 diluted NMS medium without EDTA. All gases used were of the highest available quality (6.0) (all gases were supplied by AGA). For the first gas uptake experiment, a total of 7 different setups (A–G) of five replicates each were created: A–E contained filters with M. gorgona MG08 cells, and F and G did not contain M. gorgona MG08 cells. (A) five bottles with 2–4 p.p.m.v. CH4, 2–4 p.p.m.v. H2 and 2–4 p.p.m.v. CO in synthetic air. (B) five bottles with 2–4 p.p.m.v. H2 and 2–4 p.p.m.v. CO in synthetic air; (C) five bottles with 2–4 p.p.m.v. CH4 in synthetic air. (D) five bottles with an atmosphere of ambient air (compressed outdoor air); (E) five bottles with synthetic air. (F) five bottles with 2–4 p.p.m.v. CH4, 2–4 p.p.m.v. H2 and 2–4 p.p.m.v. CO in synthetic air. (G) five bottles with ambient air atmosphere.
For the second gas uptake experiment, one condition included cells; five bottles with ~4 p.p.m.v. CH4, ~4 p.p.m.v. H2 and ~4 p.p.m.v. CO in synthetic air. Two conditions included sterile filters; five bottles with ~4 p.p.m.v. CH4, ~4 p.p.m.v. H2 and ~4 p.p.m.v. CO in synthetic air and five bottles with synthetic air atmospheres.
The control experiment to determine gas leakages (18 bottles) included six different types of liquids and headspace compositions: (A) 50 mL MilliQ water and 200 mL headspace of pure helium. (B) Sterile floating polycarbonate filter on 50 mL of 1/10 diluted NMS medium without EDTA and 200 mL headspace with 2–5 p.p.m.v. CH4, H2, CO in synthetic air. (C) 50 mL of milliQ water and synthetic air. (D) 50 mL of 1/10 diluted NMS medium without EDTA and 2–5 p.p.m.v. CH4, H2, CO in synthetic air. (E) 50 mL of milliQ water and 2–5 p.p.m.v. CH4, H2, CO in synthetic air. (F) Empty glass bottle with 250 mL of 2–5 p.p.m.v. CH4, H2, CO in synthetic air.
To create the mentioned atmospheres, the bottles were sealed with halogenated bromobutyl rubber stoppers (DURAN, Merck, Darmstadt, Germany) and plastic screw caps under a sterile bench. Before usage, the rubber stoppers had been boiled ten times and then autoclaved. The sealed bottles were flushed for 15 min with ambient, high quality synthetic air (N2: 78%, O2: 21%, CO2: 400 p.p.m.v.) or pure helium by using a gassing manifold connected to sterile single-use needles. The final pressure in the bottles was adjusted to one bar absolute pressure. Afterwards, 1 mL of additional respective gases (CH4, CO and H2) was added by using a gas tight syringe (VICI AG International, Schenkon, Switzerland) to create the required atmospheres. To prevent contamination, each gassing step was carried out by interconnecting a sterile 0.2 µm cellulose acetate filter (VWR Collection, Lutterworth, UK) between needle and hose or syringe.
The prepared bottles were incubated at 20 °C for 57 days in the first experiment. The headspace concentrations of CH4, CO and H2 were measured the day after preparing the headspace atmospheres and after 7, 17 and 57 days. In the second experiment, the incubation lasted 145 h. In the control experiment, the incubations lasted 6, 54 or 70 days.
For combined measurements of CH4, CO and H2, 1 mL headspace gas was sampled with a gastight GC syringe (VICI AG International, Schenkon, Switzerland). The contained gas was injected manually into a gas chromatograph (ThermoScientific Trace 1310 with column TG-BOND Msieve 5A, ThermoFisher, Waltham, MA, USA). Detection was achieved by using a PDD detector. A high-quality gas containing 5 p.p.m.v. H2, 5 p.p.m.v. CH4, and 5 p.p.m.v. CO in N2 served as standard. To create standard curves, 2 × 0.1 mL, 2 × 0.5 mL, and 2 × 1 mL of the mentioned standard were injected on every measurement day. Bottle gas concentrations were calculated using the standard curve. Masses were calculated by applying the ideal gas law and adjusted for changes in bottle pressure due to gas removal.
2.4. Cell Quantification
Due to several layers of cells forming colonies on the filters after 9 months and 57 days of incubation, the number of cells on four filters per condition A–E in the gas uptake experiment could not be estimated reliably by cell counts. Instead, cell numbers were estimated using DNA extractions and comparison to a standard of DNA extraction yields for known cell numbers of M. gorgona MG08 on polycarbonate filters. Cells for a standard were prepared as follows. A 20 mL culture (1:10 (NMS:MilliQ) diluted liquid nitrate mineral salts (NMS) medium (pH 6.8) (DSMZ medium 921) without EDTA) with a headspace of 20% CH4 in air (20 mL 100% CH4 mixed with 80 mL of air) was incubated for 12 days, reaching several days (2–4) into the stationary phase. The culture was then exposed to atmospheric concentrations of CH4 for one additional day during which the number of cells in the culture was estimated using cell counts. Then, the cells were filtered out and the filters were prepared for extractions. With this approach, our aim was to extract DNA from cells in a slow growth state where the cells do not contain multiple partial genomes. Filters for the standard and those from the experiment incubation were cut into 12 pieces and put into 2 mL safelock tubes, after which DNA was extracted from the filters using DNA IQ™ Casework Pro Kit for Maxwell® 16 following the manufacturer’s instructions. In brief, we crushed the 12 filter pieces with a metal bead in a tissue lyser (Qiagen, Hilden, Germany) six times, 30 s each, applying liquid nitrogen prior to the first and between each round in the tissue lyser. Prior to extraction, the crushed pieces were spun down. After, 400 µL of extraction buffer containing proteinase K and thioglycerol followed by 400 µL lysis buffer was added. Then, the mixture was transferred into a cartridge of the Maxwell 16 system and processed. DNA extracts were quantified using Qubit (dsDNA HS Assay, Thermo Fisher, Waltham, MA, USA). Cell numbers on filters from the experiment were subsequently determined by comparing the extract yields per filter to those of the standard, converting the values from nanograms of DNA to cell numbers.
2.5. Contamination Tests and Microscopy
In addition to the start of the pre-incubation and after eight months, we inspected filters by fluorescence microscopy at the beginning and end of the first gas uptake experiment. At the end of the experiment, one filter from each of the five treatments was used for contamination tests and fluorescence microscopy. For the second gas uptake experiment, we inspected filters at the beginning and end of the pre-incubation after 4.5 months, in addition to contamination tests after the experiment. Preparation for fluorescence microscopy was carried out as described for the pre-incubation above. Contamination tests were carried out by cutting filters in pieces and placing them on TGYA plates for 15 days. TGYA plates contained 5 g tryptone, 2.5 g of yeast extract, 1 g of glucose, and 20 g of agar per 1 L of water. Presence and growth of heterotrophic microorganisms was then evaluated by visual inspection. The contamination tests were negative and cell sizes and cell shapes were the same as prior to the experiment, confirming the purity of the culture.
2.6. Plotting and Statistics
All plotting was performed using the R [23] package ggplot.2 [24].
2.7. Cell-Specific Oxidation Rates and Free Energy Yield Calculations
Oxidation rates were estimated by first order rate kinetics models. We log transformed the concentrations over time and fitted linear regression models to the transformed plots. The slope of the linear models corresponds to the rate constants. By multiplying the respective rate constant by the atmospheric concentrations of CH4, H2 and CO, we obtained the rates of oxidation at atmospheric gas concentrations. The fit of the linear models was evaluated and considered to have satisfactory coefficients of determination, giving the following values: R2 − CH4 = 0.93, R2 − H2 = 0.82, R2 − CO = 0.68 (Experiment 1), and R2 − CH4 = 0.99, R2 − H2 = 0.99, R2 − CO = 0.99 (Experiment 2). The oxidation rates of H2 and CO were subsequently corrected by adjusting for the abiotic gas leakages. Due to the first order rate kinetics nature of the CO (R2 of linear models = 0.77, n = 5) and H2 (R2 of linear models = 0.99, n = 5) leakages, their respective rate constants were estimated the same way as for the oxidation rates. The rate constants for the biological H2 and CO oxidation were subsequently adjusted by subtracting or adding the rate constants of the leaks of H2 (leakage out of the bottle) and CO (leakage into), respectively. For each condition A, B, C, D and E, cell numbers on three filters were estimated. Cell-specific oxidation rates were subsequently calculated by dividing the estimated oxidation rates by the corresponding cell numbers.
The expected maintenance requirements at 20 °C were calculated according to Tijhuis et al. [8]. The Gibbs free energy changes (ΔrG) were calculated for the following reactions and Gibbs free energies of formation ΔGf° (kJ/mol), assuming atmospheric concentrations and 20 °C: [CH4 + 2O2 → CO2 + 2H2O]; [2CO + O2 → 2CO2]; [2H2 + O2 → 2H2O]. CO: −137.16 kJ/mol, O2: 0 kJ/mol, CO2: −394.39 kJ/mol, H2: 0 kJ/mol, H2O: −237.13 kJ/mol, CH4: −50.6 kJ/mol. From the oxidation rates and free energy change of the reactions, we could estimate the amount of energy obtained per mol of biomass carbon per hour, applying the dry weight and carbon content of M. gorgona MG08 (see below) as previously shown [2,6]. All calculations, raw data and literature data needed for input in the calculations are provided in a detailed format. The data and calculations are provided as excel formulas that can be intuitively followed and inspected. These can serve as a template for those wishing to repeat our calculations for similar experiments and compare those to our data (See Supplementary dataset Table S1, tabs “Experiment 1”, “Experiment 2”, “Gibbs_free_energy” and “Maintenance_energy”).
2.8. Estimating Cell Dry Weight
In order to estimate the dry weight of M. gorgona MG08, we cultivated 10 × 10 mL of M. gorgona MG08 culture as described for a young stationary culture in the cultivation section above. This provided ten replicates from which 1 mL was used for cell counts and 9 mL for drying overnight at 110 °C. Based on this, we calculated that the weight of one M. gorgona MG08 cell is 8.8 × 10−14 g dry weight per cell (SD = 3.1 × 10−14). We assume that half of the cell consists of carbon [2]. This weight and size (length 1.2 µm and width 0.7 µm) makes M. gorgona MG08 the lightest known Alphaproteobacteria MOB, compared to previously weighed strains [2]. While the size of M. gorgona MG08 cells do not change when growing with air as its energy and carbon source, compared to higher CH4 concentrations (it retains its size of length ~1.2 µm and width ~0.7 µm), we acknowledge that the content, density and thus dry weight of the cells growing in air may differ from that of M. gorgona MG08 cultivated at high CH4 concentrations. Such differences could affect the estimates of kJ Cmol h−1. However, due to the low biomass, we were not able to estimate cell weights from filters cultivated in air.
3. Results and Discussion
Cells of M. gorgona MG08 were pre-incubated on filters under an atmosphere of ambient air (~1.84 p.p.m.v. CH4, ~0.39 p.p.m.v. CO and ~0.7 p.p.m.v. H2) for eight months. The filters floated on diluted mineral medium within bottles to enable optimal gas transfer. After four months, a subset of the cells had developed into microcolonies of more than 100 cells (Figure 1), while some cells had not divided. Visual inspection and counting showed that the number of generations in the majority of the colonies had surpassed seven (64 cells) (Figure 1C), matching previous observations [4]. Studies have shown that up to three generations can be supported by intracellular storages [4,25,26], confirming that the eight-month pre-incubation was sufficient to ensure growth with carbon and energy harvested solely from air. After nine months of pre-incubation, the filter-containing glass bottles were sealed with rubber stoppers before defined headspace atmospheres were created (Figure 2).
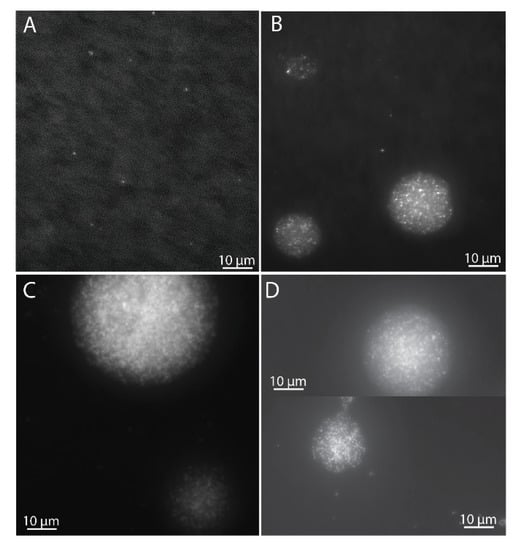
Figure 1.
Microcolony growth of M. gorgona MG08 at different times during pre-incubation and gas uptake experiments. (A) Cells after filtration on polycarbonate filters, prior to incubation. (B) Microcolonies after 4 months of pre-incubation under ambient air. (C) Microcolonies after 8 months of pre-incubation under ambient air. (D) Microcolonies after 9 months of pre-incubation under ambient air and 57 days of gas uptake experiment incubation under synthetic air ((D), upper panel), and synthetic air + 2–4 p.p.m.v. CH4, CO and H2 ((D), lower panel). For fixation, the filters were transferred to fresh-made 2% paraformaldehyde in 1× PBS in the refrigerator overnight. For staining, filters were transferred (side with bacteria up) on top of 200 μL droplets of 1000× SYBRgreen I (10× dilution of the stock concentration provided by Thermo Fisher Scientific, Invitrogen, Molecular probes) and incubated for 10 min, washed, and air dried.
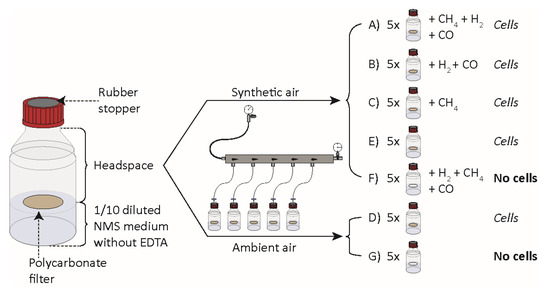
Figure 2.
Experimental setup for experiment 1 to determine gas uptake by M. gorgona MG08. NMS: Nitrate mineral salts. Synthetic air containing less than 0.2 p.p.m.v. of CH4, CO and H2. Ambient air containing 1.84 p.p.m.v. CH4, 0.4 p.p.m.v. CO and 0.7 p.p.m.v. H2. All injections of CH4, CO and H2 resulted in a final concentration between 2 and 4 p.p.m.v of the respective gas.
In a 57-day experiment, we could show that M. gorgona MG08 is able to oxidize ambient air to sub-atmospheric concentrations of CH4 and H2 (Figure 3). In several of the incubations, CH4 and H2 concentrations reached below atmospheric levels already after 7 days, while CO concentrations did not decrease below ~0.45 p.p.m.v., which is close to the atmospheric levels in urban areas [16,17,18,19] and similar to the average ambient air concentrations measured in the laboratory (0.39 p.p.m.v.). Furthermore, the CH4 oxidation rates did not depend on the CO and H2 concentrations (Figure 3A,C,D), while CO and H2 oxidation proceeded without the presence of CH4 (Figure 3B).
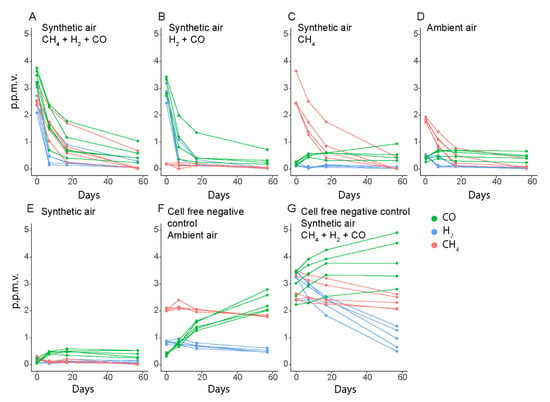
Figure 3.
Oxidation of CH4, CO, and H2 by M. gorgona MG08. (A–E) contained polycarbonate filters with M. gorgona MG08 floating on nitrate mineral salts medium. (F,G) contained sterile filters. The lower H2 concentrations at the start of the ambient air incubations with cells (D) compared to those without cells (F) are due to leaving prepared bottles overnight before measuring the first time point T0, allowing some oxidation to already occur. All gases used were of the highest commercially available quality, 6.0 (99.9999% purity). The multiple data points in each color represent different biological replicates from the same condition. Synthetic air contained less than 0.2 p.p.m.v. of CH4, CO and H2.
The stabilization of the CO concentrations around 0.45 p.p.m.v. in bottles with M. gorgona MG08 cells, irrespective of the start concentrations, was unexpected (Figure 3A,B,D,E). In the cell-free controls, we observed increasing CO concentrations, reaching 2 p.p.m.v. during 57 days of incubation (Figure 3F,G), suggesting these observations to be due to an abiotic CO source. To identify the CO source, we measured CO, H2 and CH4 in several cell-free controls, including empty bottles (Figure 4). CO accumulation was detected in these liquid-free bottles, and even in headspaces exposed to only glass and rubber stoppers. Thus, we concluded that CO was released from the rubber stoppers, meaning that the stabilization of CO concentrations at ~0.45 p.p.m.v. in the presence of M. gorgona MG08 was a confirmation of continuous CO oxidation at near ambient air concentrations of ~0.45 p.p.m.v. The decreasing or increasing CH4 and H2 concentrations in abiotic controls (Figure 3F,G and Figure 4A–F), on the other hand, could be explained by leakage during long incubation times, as previously observed [27,28]. In addition to the fact that such long-term leakages have been observed previously, the rationale for this conclusion is that the concentrations inside the bottles always increased when the outside concentrations were higher, and decreased when the outside concentrations were lower.
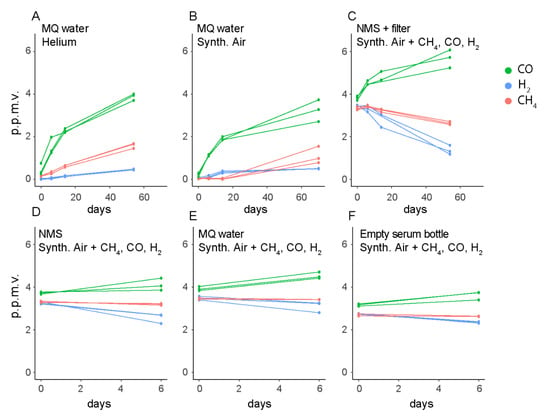
Figure 4.
Gas leakage and other abiotic gas sources. (A,B) Long incubations of cell-free controls were performed to identify signs of CO and CH4 leakage or release into or out of the bottles. (C–F) Short incubations of cell-free controls were considered sufficient to identify the same patterns of gas leakage and accumulation irrespective of bottle liquid and headspace composition. MQ: MilliQ. Synth. Air: Synthetic air. NMS: nitrate mineral salts. The multiple data points in each color represent different biological replicates from the same condition. Synthetic air contained less than 0.2 p.p.m.v. of CH4, CO and H2.
Cell-Specific Oxidation Rates and Energy Yield
In order to estimate the CH4, CO and H2 uptake rates by M. gorgona MG08 at atmospheric concentrations, we tested and confirmed that the rate change of CH4 uptake fulfilled the assumptions of first order rate kinetics (linear regression through the natural logarithm (LN) of rates over time R2 = 0.93, n = 15). As microcolonies contained more than one layer of cells, filter cell counts were not possible. Instead, we performed DNA extractions and cell quantification with a DNA to cell count standard for a subset of the incubation bottles, showing that all but one floating filter contained between 38.1 and 68 million cells (Table S1). Based on these estimates, we calculated the cellular CH4 oxidation rates at atmospheric CH4 concentrations to 0.7–2.8 × 10−18 mol cell−1 h−1 at 20 °C (n = 9). Similarly, the H2 and CO oxidation rates at atmospheric concentrations (average H2 linear model R2 = 0.82, n = 10, CO R2 = 0.68, n = 10) were estimated to be 0.17–0.36 and 0.20–0.34 × 10−18 mol cell−1 h−1, respectively (n = 6). These rates were corrected for the leakage and release of H2 and CO, respectively. The CH4 oxidation rates reflect the lower end of the atmospheric oxidation rates estimated for conventional MOB, which range from 0.2 to 17 × 10−18 mol cell−1 h−1 at 25 °C [6]. However, the rates of conventional MOB forming the basis for these estimates were measured at high CH4 concentrations and thus do not necessarily represent the rates that would be obtained at atmospheric conditions.
Based on the specific affinity estimated from oxidation rates at higher than atmospheric concentrations, the expected cell-specific CH4 oxidation rate at atmospheric concentrations can be calculated [2]. From the oxidation rates measured at concentrations between 823 p.p.m.v. and 6% [4], we found that M. gorgona MG08 would have a cell specific CH4 oxidation rate of 10 × 10−18 mol cell−1 h−1 when growing at atmospheric concentrations. However, these estimates are more than five times higher than the actual rate measurements at atmospheric concentrations provided in our current study. Thus, M. gorgona MG08 may not sustain the same catalytic properties or amount of particulate methane monooxygenase (pMMO) enzymes per cell during growth in air. At concentrations between 823 p.p.m.v. and 6%, we estimated the Km(app) of M. gorgona MG08 to be 4.905 [4], similar to that of various, presumed low affinity, MOB [2]. Although this indicated that M. gorgona MG08 has a low affinity for CH4, the dependency of Km(app) on the Vmax prevents us from determining the exact affinity unless we perform comparative kinetic experiments for purified enzymes from several MOB strains, or compare the CH4 oxidation rates and pMMO concentrations of several different MOB. Thus, it seems plausible that life at atmospheric CH4 concentrations can be sustained by low affinity enzymes, but it is still uncertain whether this is the case.
Based on the specific affinity estimated from oxidation rates at higher than atmospheric concentrations, the expected cell-specific CH4 oxidation rate at atmospheric concentrations can be calculated [2]. From the oxidation rates measured at concentrations between 823 p.p.m.v. and 6% [4], we found that M. gorgona MG08 would have a cell specific CH4 oxidation rate of 10 × 10−18 mol cell−1 hour−1 when growing at atmospheric concentrations. However, these estimates are more than five times higher than the actual rate measurements at atmospheric concentrations provided in our current study. Thus, M. gorgona MG08 may not sustain the same catalytic properties or amount of particulate methane monooxygenase (pMMO) enzymes per cell during growth in air. At concentrations between 823 p.p.m.v. and 6%, we estimated the Km(app) of M. gorgona MG08 to be 4.905 [4], similar to that of various, presumed low affinity, MOB [2]. Although this indicated that M. gorgona MG08 has a low affinity for CH4, the dependency of Km(app) on the Vmax prevents us from determining the exact affinity unless we perform comparative kinetic experiments for purified enzymes from several MOB strains, or compare the CH4 oxidation rates and pMMO concentrations of several different MOB. Thus, it seems plausible that life at atmospheric CH4 concentrations can be sustained by low affinity enzymes, but it is still uncertain whether this is the case.
The energy yields per mol of CH4, CO and H2 under the provided experimental conditions (ambient air at 20 °C) were approximately −814, −522 and −472 kJ/mol, respectively, assuming the following reactions: [CH4 + 2O2 → 2H2O + CO2], [2CO + O2 → 2CO2], [2H2 + O2 → 2H2O]. Based on the measured rates, we find that M. gorgona MG08 is able to conserve approximately 0.47 kJ Cmol h−1 from the combined oxidation of CH4, H2 and CO during growth in air at 20 °C (see data and full calculation in Table S1). This calculation implements our dry weight estimates for M. gorgona MG08 (Table S1). Interestingly, these estimates show that M. gorgona MG08 cells (length ~1.2 µm and width ~0.7 µm) are three times lighter (0.88 × 10−13 gDW cell−1) than the lightest reported MOB [2]. The mass of a cell could affect its energy budget, as more energy might be needed to supply maintenance in a heavier cell, leaving less for growth. Additionally, a heavier cell might require more energy for growth. Thus, the low weight of M. gorgona MG08 may reflect a strategy to reduce energy costs for growth. In contrast to our findings, the previously reported soil CH4 oxidation rates of 800 × 10−18 mol cell−1 h−1 [6] would provide 179 kJ Cmol h−1. In order to examine this contradiction and test the validity of our data, we repeated parts of our experiment (condition with 5 p.p.m.v. of each gas CH4, H2 and CO) with shorter pre-incubation (4.5 months) to minimize growth stagnation and the possibility for the accumulation of dead cells on the floating filters.
Furthermore, we shortened the gas uptake experiment to less than one week of incubation to prevent gas leakage effects on rate estimates. This new setup provided highly precise estimates of CH4, H2 and CO uptake rates (Figure 5), which confirmed their first order nature (linear regression through LN of rates over time; CH4 R2 = 0.99, n = 5; H2 R2 = 0.99; CO R2 = 0.99). Within this timeframe, the leakage of H2 and CH4 was negligible (Figure 5B,C), but the release of CO from the rubber stoppers occurred and was corrected for. Interestingly, similar but slightly lower cellular oxidation rates at atmospheric gas concentrations were estimated. The CH4 uptake ranged from 0.32 to 1.3 × 10−18 mol cell−1 h−1, the H2 uptake from 0.63 to 2.1 × 10−18 mol cell−1 h−1, and the CO uptake from 0.24 to 0.69 × 10−18 mol cell−1 h−1 at 20 °C. We suspect the differences were due to the higher accuracy of the uptake rates, and higher cell number estimates (average 9.54 × 107; Table S1), despite shorter incubation time (4.5 months). The number of initial cells that form colonies on the filters may vary between experiments, possibly due to small differences in the physiological state of the inoculum. This, and the possibility that colonies of a certain size reach a growth stagnation phase due to limiting concentrations of CH4, H2 and CO around the colony, can explain how additional pre-incubation time in the first experiment did not lead to higher cell numbers per filter than in the second experiment.

Figure 5.
Oxidation of CH4, CO, and H2 by M. gorgona MG08. (A) filters with M. gorgona MG08 floating on nitrate mineral salts medium. (B,C) sterile filters floating on nitrate mineral salts medium. All gases used were of the highest commercially available quality, 6.0 (99.9999% purity). The multiple data points in each color represent different biological replicates from the same condition.
With these new numbers, we find that M. gorgona MG08 is able to conserve approximately 0.38 kJ Cmol h−1 (close to the 0.48 kJ Cmol h−1 estimated from the first experiment) during growth in air at 20 °C (Table S1). This is more than seven times lower than the estimated average maintenance requirement of a bacterial population at 20 °C (2.8 kJ Cmol h−1) [8]. However, this amount of energy is apparently sufficient to sustain the growth of M. gorgona MG08. Thus, we question whether the average maintenance requirement of bacteria [8] appropriately describes the constraints for growth on atmospheric trace gases by M. gorgona MG08 and other atmMOB.
With our numbers, the 2.8 kJ Cmol h−1 minimum requirement mentioned above could be achieved if approximately 14 million out of the ~100 million cells on a filter were active [8]. Alternatively, if cell density and thus weight (cell sizes do not seem to vary with CH4 concentration) were lower during growth in air, an energy yield of 2.8 kJ Cmol h−1 or higher, could be achieved. The reason is that the same amount of energy would be distributed on less cellular mass than assumed for our empirical estimate of 0.38 kJ Cmol h−1. Regardless, the rates of M. gorgona MG08 are substantially lower than those in high upland soils, where CH4 oxidation rates of up of 800 × 10−18 mol cell−1 h−1, almost 500 times faster than M. gorgona MG08, were measured. However, these were based on soil cell numbers estimated from DNA extractions and pmoA qPCR [6]. Thus, the numbers have possibly been underestimated, as DNA extractions from soils may provide less than 100% yields, qPCR quantification can be inhibited by soil-derived impurities in the DNA extract, and primer mismatches can result in an underrepresentation of copy numbers. Methanogenic archaea and acetogenic bacteria were recently found to require much less maintenance energy (0.2 kJ Cmol h−1) than previously believed (9.8 kJ Cmol h−1) [29]. According to the authors, the low maintenance energy was based on the low growth rates of these organisms, a feature that had not previously been taken into account. If true, low maintenance requirements at low growth rates could also explain how M. gorgona MG08 can sustain its slow growth at a limited energy budget.
We conclude that M. gorgona MG08 oxidizes the atmospheric trace gases CH4, CO and H2 to harvest energy for growth in air. The ability of M. gorgona MG08 to grow using air as its only source of energy and carbon relies not only on this metabolic flexibility, but also on its low energy requirements. Our findings suggest that a high CH4 affinity is not a prerequisite to live on atmospheric CH4.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2607/9/1/153/s1, Table S1: Supplementary data and calculations.
Author Contributions
A.T.T. conceived the study with input from T.S., M.M.S. and A.G.H. A.G.H. and M.M.S. established methods for filter cultivation and performed strain cultivation with input from A.T.T. T.S., M.L. and A.T.T. established methods for gas uptake experiments. T.S. performed gas uptake experiments with assistance from M.L. A.D. established and performed method for cell quantification. A.T.T. and T.S. analyzed the data. A.T.T. wrote the manuscript with input from all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Research Council of Norway FRIPRO Mobility Grant Project Time and Energy (251027/RU) to ATT, co-founded by ERC under Marie Curie Grant 606895, and Tromsø Research Foundation starting grant project Cells in the Cold (16_SG_ATT) to ATT.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Van Amstel, A. Methane. A review. J. Integr. Environ. Sci. 2012, 9, 5–30. [Google Scholar] [CrossRef]
- Knief, C.; Dunfield, P.F. Response and adaptation of different methanotrophic bacteria to low methane mixing ratios. Environ. Microbiol. 2005, 7, 1307–1317. [Google Scholar] [CrossRef] [PubMed]
- Dunfield, P.F. The Soil Methane Sink. Greenhouse Gas. Sinks; Reay, D.S., Hewitt, C.N., Smith, K.A., Grace, J., Eds.; CABI: Wallingford, UK, 2007; pp. 152–170. [Google Scholar]
- Tveit, A.T.; Hestnes, A.G.; Robinson, S.L.; Schintlmeister, A.; Dedysh, S.N.; Jehmlich, N.; Von Bergen, M.; Herbold, C.; Wagner, M.; Richter, A.; et al. Widespread soil bacterium that oxidizes atmospheric methane. Proc. Natl. Acad. Sci. USA 2019, 116, 8515–8524. [Google Scholar] [CrossRef] [PubMed]
- Knief, C. Diversity and habitat preferences of cultivated and uncultivated aerobic methanotrophic bacteria evaluated based on pmoA as molecular marker. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef]
- Kolb, S.; Knief, C.; Dunfield, P.F.; Conrad, R. Abundance and activity of uncultured methanotrophic bacteria involved in the consumption of atmospheric methane in two forest soils. Environ. Microbiol. 2005, 7, 1150–1161. [Google Scholar] [CrossRef]
- Bender, M.; Conrad, R. Kinetics of CH4 oxidation in oxic soils exposed to ambient air or high CH4 mixing ratios. FEMS Microbiol. Lett. 1992, 101, 261–270. [Google Scholar] [CrossRef]
- Tijhuis, L.; Van Loosdrecht, M.C.M.; Heijnen, J.J. A thermodynamically based correlation for maintenance gibbs energy requirements in aerobic and anaerobic chemotrophic growth. Biotechnol. Bioeng. 1993, 42, 509–519. [Google Scholar] [CrossRef]
- Cordero, P.R.F.; Bayly, K.; Leung, P.M.; Huang, C.; Islam, Z.F.; Schittenhelm, R.B.; King, G.M.; Greening, C. Atmospheric carbon monoxide oxidation is a widespread mechanism supporting microbial survival. ISME J. 2019, 13, 2868–2881. [Google Scholar] [CrossRef]
- Ji, M.; Greening, C.; VanWonterghem, I.; Carere, C.R.; Bay, S.K.; Steen, J.A.; Montgomery, K.; Lines, T.; Beardall, J.; Van Dorst, J.; et al. Atmospheric trace gases support primary production in Antarctic desert surface soil. Nature 2017, 552, 400–403. [Google Scholar] [CrossRef]
- Greening, C.; Carere, C.R.; Rushton-Green, R.; Harold, L.K.; Hards, K.; Taylor, M.C.; Morales, S.E.; Stott, M.B.; Cook, G.M. Persistence of the dominant soil phylum Acidobacteria by trace gas scavenging. Proc. Natl. Acad. Sci. USA 2015, 112, 10497. [Google Scholar] [CrossRef]
- Greening, C.; Berney, M.; Hards, K.; Cook, G.M.; Conrad, R. A soil actinobacterium scavenges atmospheric H2 using two membrane-associated, oxygen-dependent [NiFe] hydrogenases. Proc. Natl. Acad. Sci. USA 2014, 111, 4257. [Google Scholar] [CrossRef] [PubMed]
- Greening, C.; Biswas, A.; Carere, C.R.; Jackson, C.J.; Taylor, M.C.; Stott, M.B.; Cook, G.M.; Morales, S.E. Genomic and metagenomic surveys of hydrogenase distribution indicate H2 is a widely utilised energy source for microbial growth and survival. ISME J. 2016, 10, 761–777. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.E.; Zhang, E.; Terauds, A.; Ji, M.; Kong, W.; Ferrari, B.C. Soil Microbiomes With the Genetic Capacity for Atmospheric Chemosynthesis Are Widespread Across the Poles and Are Associated With Moisture, Carbon, and Nitrogen Limitation. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- World Meteorological Organization. The State of Greenhouse Gases in the Atmosphere Using Global Observations through 2018. WMO GHG Bullet. 2019, 15, 1–8, ISSN 2078-0796. [Google Scholar]
- Khalil, M.A.K.; Rasmussen, R.A. The global cycle of carbon monoxide: Trends and mass balance. Chemosphere 1990, 20, 227–242. [Google Scholar] [CrossRef]
- Novelli, P.C.; Masarie, K.A.; Lang, P.M. Distributions and recent changes of carbon monoxide in the lower troposphere. J. Geophys. Res. Atmos. 1998, 103, 19015–19033. [Google Scholar] [CrossRef]
- Chi, X.; Winderlich, J.; Mayer, J.-C.; Panov, A.V.; Heimann, M.; Birmili, W.; Heintzenberg, J.; Cheng, Y.; Andreae, M.O. Long-term measurements of aerosol and carbon monoxide at the ZOTTO tall tower to characterize polluted and pristine air in the Siberian taiga. Atmos. Chem. Phys. 2013, 13, 12271–12298. [Google Scholar] [CrossRef]
- Petrenko, V.V.; Martinerie, P.; Novelli, P.; Etheridge, D.M.; Levin, I.; Wang, Z.; Blunier, T.; Chappellaz, J.; KaiseriD, J.; Lang, P.; et al. A 60 yr record of atmospheric carbon monoxide reconstructed from Greenland firn air. Atmos. Chem. Phys. 2013, 13, 7567–7585. [Google Scholar] [CrossRef]
- Novelli, P.C.; Lang, P.M.; Masarie, K.A.; Hurst, D.F.; Myers, R.; Elkins, J.W. Molecular hydrogen in the troposphere: Global distribution and budget. J. Geophys. Res. Atmos. 1999, 104, 30427–30444. [Google Scholar] [CrossRef]
- Schmitz, R.A.; Pol, A.; Mohammadi, S.S.; Hogendoorn, C.; Van Gelder, A.H.; Jetten, M.S.M.; Daumann, L.J.; Camp, H.J.M.O.D. The thermoacidophilic methanotroph Methylacidiphilum fumariolicum SolV oxidizes subatmospheric H2 with a high-affinity, membrane-associated [NiFe] hydrogenase. ISME J. 2020, 14, 1223–1232. [Google Scholar] [CrossRef]
- Hakobyan, A.; Zhu, J.; Glatter, T.; Paczia, N.; Liesack, W. Hydrogen utilization by Methylocystis sp. strain SC2 expands the known metabolic versatility of type IIa methanotrophs. Metab. Eng. 2020, 61, 181–196. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-project.org/ (accessed on 23 January 2020).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Available online: https://ggplot2.tidyverse.org (accessed on 23 January 2020).
- Pieja, A.J.; Sundstrom, E.R.; Criddle, C.S. Poly-3-Hydroxybutyrate Metabolism in the Type II Methanotroph Methylocystis parvus OBBP. Appl. Environ. Microbiol. 2011, 77, 6012–6019. [Google Scholar] [CrossRef] [PubMed]
- Ratcliff, W.C.; Kadam, S.V.; Denison, R.F. Poly-3-hydroxybutyrate (PHB) supports survival and reproduction in starving rhizobia. FEMS Microbiol. Ecol. 2008, 65, 391–399. [Google Scholar] [CrossRef]
- Lange, S.F.; Allaire, S.E.; van Bochove, E. Transfer of CO2, N2O and CH4 to butyl rubber (polyisobutylene) septa during storage. J. Environ. Monit. 2008, 10, 775–777. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, T. A method for equilibration chamber sampling and gas chromatographic analysis of the soil atmosphere. Plant Soil 1989, 120, 39–47. [Google Scholar] [CrossRef]
- Bonk, F.; Popp, D.; Weinrich, S.; Sträuber, H.; Becker, D.; Kleinsteuber, S.; Harms, H.; Centler, F. Determination of Microbial Maintenance in Acetogenesis and Methanogenesis by Experimental and Modeling Techniques. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).